Sex Hormones and Androgen Receptor:Risk Factors of Coronary Heart Disease in Elderly Men△
Jian Cao*,Hui Zou,Bing-po Zhu,Hao Wang,Jian Li,Yu Ding,and Xiao-ying Li
The First Geriatric Cardiology Department,Chinese Peoples’ Liberation Army General Hospital,Beijing 100853,China
IT has been demonstrated in previous studies that the incidence of cardiovascular diseases in male is significantly higher than that in female.The morbidity and mortality of coronary heart disease (CHD)in premenopausal female are significantly lower than those in male.
However,it has not been determined whether these differences were related to risk factors of atherosclerosis such as vascular endothelial function,macrophages,or lipid load.1Accumulating studies show that there is a clear correlation between vascular lesion and the significant difference in sex hormonal control mechanism between male and female.It has been proved that androgen could improve the combination of monocytes to umbilical vein endothelial cells (UVECs) in male,but not in female.2Furthermore,animal experiments revealed that testosterone could inhibit the thickening of endomembrane in proximal aortic arch in emasculated male rabbit,but had no effect on oophorectomized female rabbit.3Results from observational studies in which female subjects received estrogen replacement therapy suggested some benefits of this therapy.4However,large-scale randomized controlled trials demonstrated that estrogen replacement therapy had no protective effects for female subjects.5In 2000,a famous study demonstrated that androgen level in male patients with CHD was significantly lower than that in male with normal coronary arteries.6Malkin et al7found that testosterone replacement therapy for 1 month could mitigate myocardial ischemia and angina pectoris in male patients with CHD and hypogonadism.
The main androgens in human body are testosterone and dihydrotestosterone (DHT).Testosterone exists in blood in three forms,free testosterone (FT,2%),albumin-bound testosterone (Alb-T,68%),and sex hormone binding globulin (SHBG) testosterone (30%).The total amount is called total testosterone (TT).The concentrations of these sex steroid hormones vary with age.The TT level does not change much,but the FT level decreases significantly.In males of 50 and 70 years,the FT level could decrease by 50% and 80%,respectively.The incidence of hypogonadism,also called low testosterone,a medical term for a defect of the gonads,in the total population is 7%,but the figure is 20% in elderly male.8Hence,we presumed that the protection of coronary artery may have less or even no effect in elderly male due to the decreased level of androgen.In order to provide evidences for clinical prevention and treatment of CHD,we investigated the role of sex hormone and its receptor in the development of CHD by monitoring the levels of serum sex hormones and androgen receptor (AR) in elderly male CHD patients.
SUBJECTS AND METHODS
Subjects
Altogether 539 elderly (≥60 years old) males were enrolled and divided into CHD group (139 patients diagnosed with CHD) and control group (400 healthy subjects).The study sample is derived from a cross-sectional survey on the prevalence of cardiovascular risk factors in a representative sample of elderly men from 7 residential districts and 7 cadre sanatoriums in Wanshoulu area of Beijing from January 2005 to January 2007.WHO criteria for CHD was applied in the diagnosis;while the healthy group was determined based on the domestic criteria established by Geriatric Medicine Branch of Chinese Medical Association in 1995.
Data collection
The basic information of subjects,including age,smoking history,hypertension,and diabetes mellitus,etc,was recorded.The systolic blood pressure (SBP) and the diastolic blood pressure (DBP) were measured three consecutive times in resting condition and mean value of the results was recorded.Other indices measured were body weight (BW),height,waist circumference (WC),hip circumference (HC),waist-to-hip ratio (WHR),and body mass index (BMI).
Laboratory test
After 12-hour fasting,4 mL of non-anticoagulated and 1 mL of ethylenediamine tetraacetic acid (EDTA) anticoagulated venous blood were drawn from ulnar vein of each subject at 07:30-08:30 am.The non-anticoagulated venous blood was centrifuged at 600×gfor 10 minutes and the supernate was taken.The serum,stored in a deep freezer at-80°C,was used for the measurement of dehydroepiandrosterone sulfate (DHEAS),TT,FT,estradiol (E2),SHBG,luteinizing hormone (LH),and follicle-stimulating hormone(FSH).The EDTA anticoagulated venous blood was used for detection of AR.
Enzyme-linked immunosorbent assay (kit produced by DSL company,USA) was applied to measure DHEAS,TT,FT,and SHBG.The intra-assay coefficient of variation was 4.8%-6.8%,5.4%-7.5%,3.7%-6.3%,and 4.5%-6.3%,respectively;and the inter-assay coefficient of variation was 2.8%-4.9%,0.22%-6.18%,5.0%-8.8%,and 2.6%-4.2%,respectively.Chemiluminescence (kit produced by DPC company,USA) was used to detect E2,LH,and FSH.The intra-assay coefficient of variation was 8.3%,4.8%,and 3.0%,respectively;and the inter-assay coefficient of variation was 8.6%,6.6%,and 4.6%,respectively.The white blood cells derived from the EDTA anticoagulated blood of the 139 patients in the CHD group were labeled with immunofluorescence indirectly,and the AR fluorescence intensity of the labeled white blood cells was detected with flow cytometry (FCM,BD Company,USA),and the results were compared with those of 102 healthy males randomly selected from the control group.
Statistical analysis
All the data were recorded in Excel forms and later analyzed with statistical software SPSS 14.The continuous data were expressed in the form of mean±SD and processed with one-way analysis of variance (ANOVA) to determine inter-group variation.The interrelation between the variables was calculated with Pearson correlation analysis and binary logistic regression analysis.The level of statistical significance was determined atP<0.05.
RESULTS
Anthropometric parameters in CHD and control groups
The age,BW,BMI,WC,HC,WHR,and SBP in CHD group were significantly higher than those in control group(P<0.01).No significant difference was found in DBP between the CHD group and the control group (Table 1).
Sex hormones profile and AR in CHD and control groups
The levels of DHEAS,TT,FT,and SHBG were significantly lower in CHD group than those in control group (P<0.05),and the levels of E2 and FSH in CHD group were significantly higher (P<0.01).In CHD patients,E2/T was higher than that in healthy controls,but the difference was not statistically significant.The fluorescence intensity of AR in CHD group was significantly lower than that in healthy group (P<0.01) (Table 1).
Correlations between sex hormones,AR fluorescence intensity,and risk factors of CHD
TT was negatively correlated with DBP (r=-0.25,P=0.01)and age (r=-0.28,P=0.00);FT was negatively correlated with age (r=-0.17,P=0.01);DHEAS was negatively correlated with SBP (r=-0.14,P=0.02);SHBG was negatively correlated with BMI (r=-0.15,P=0.01) and positively correlated with age (r=0.14,P=0.04);E2 was negatively correlated with SBP (r=-0.16,P=0.03),and positively correlated with age (r=0.33,P=0.00) and BW (r=0.24,P=0.00);AR fluorescence intensity was negatively correlated with SBP (r=-0.12,P=0.01).No other significant correlations were found between sex hormones,AR fluorescence intensity,and risk factors of CHD.
Binary logistic regression analysis between sex hormones,AR,anthropometric parameters and CHD
The binary logistic regression analysis with CHD as the dependent variable and sex hormones,AR,and anthropometric parameters as covariant variables showed that age,WC,TT,SHBG,and AR might be independent risk factors or predictors of CHD (Table 2).
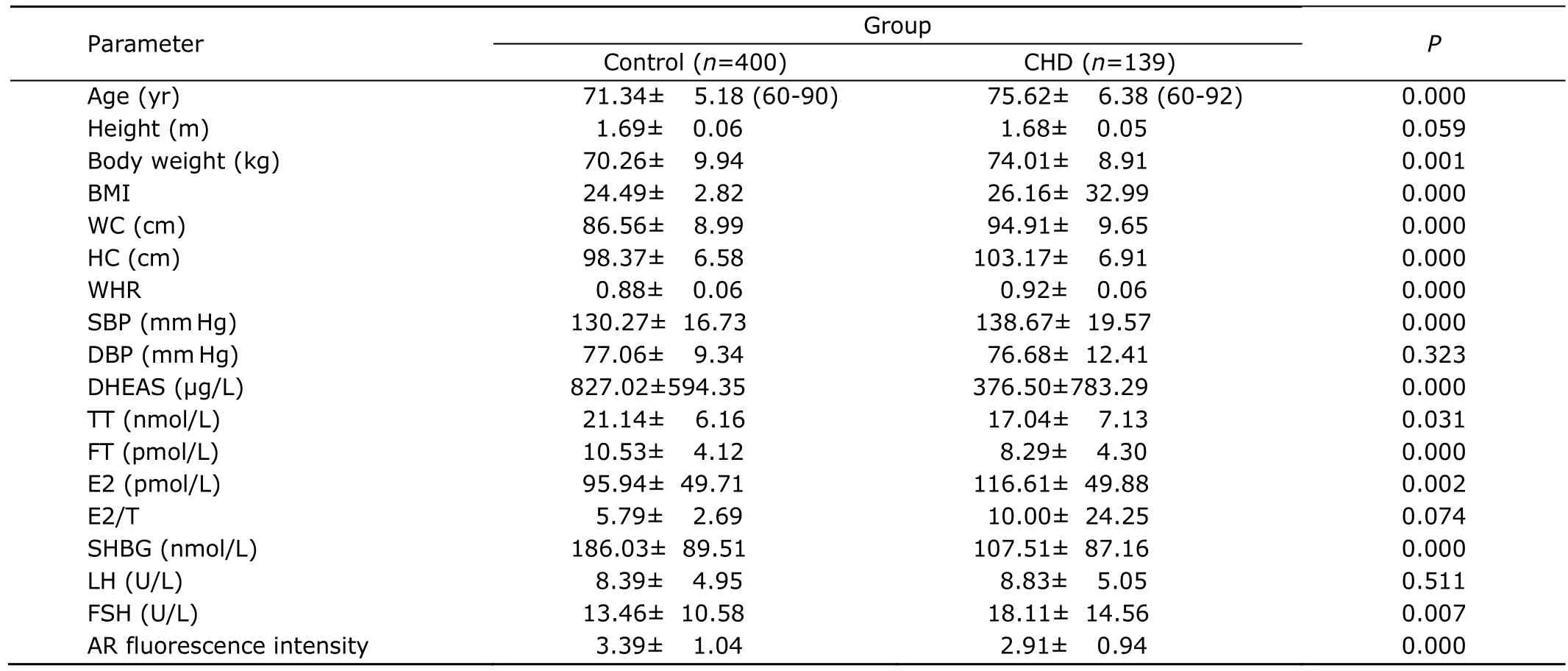
Table 1.Anthropometric parameters,sex hormones profile,and androgen receptor in CHD and control groups§
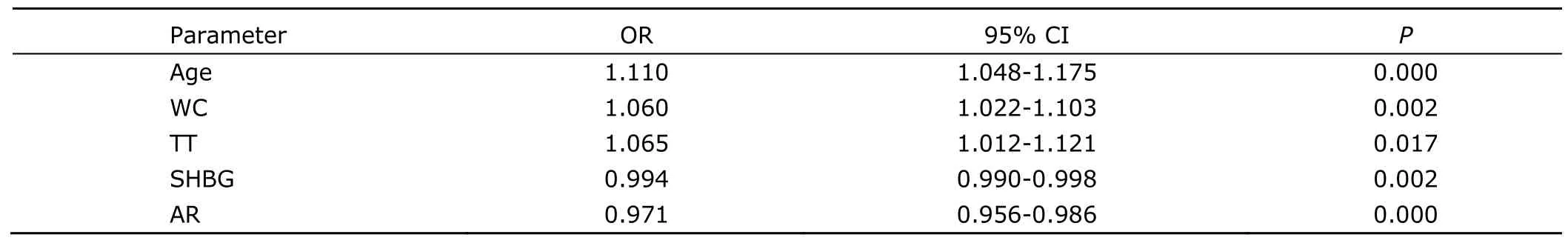
Table 2.Binary logistic regression analysis between sex hormones,AR,anthropometric parameters and CHD
DISCUSSION
In the present study,the serum levels of DHAES,TT,SHBG,and FT in elderly male patients with CHD were significantly lower than those in healthy controls (P<0.05).This finding was shown to be consisted with the study of Turhan et al.9Also,in our study,CHD group demonstrated a higher E2/T,which was similar with the finding of Dunajska et al.10Furthermore,the multiple regression analysis showed that TT,SHBG,and AR might be the independent risk factors or predictors of CHD.
Clinical observations showed that the incidence of CHD in male was significantly higher than that in premenopausal female,and the long-term treatment with androgen had a negative regulatory effect on lipids.11These results might imply that androgen had a harmful effect on cardiovascular system.However,recent studies have demonstrated that physiological dose of testosterone could reduce the serum levels of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c),while no significant alteration in the level of high-density lipoprotein cholesterol (HDL-c) was observed.12Previous trials suggest that testosterone could protect patients against CHD.It was reported that low dose testosterone could reverse myocardial ischemia induced by exercise in male CHD patients.Moreover,administration of testosterone through vein is shown to have protective effect against myocardial ischemia.13Administration of physiological dose of testosterone in coronary arteries of CHD patients could increase the blood supply of affected coronary arteries by relaxing the vessel,which is related to the increase of nitric oxide (NO) in vascular endothelium.14The direct relaxation of peripheral resistance vessels by testosterone might be partially mediated by endothelium-derived NO,as well as by ATP-sensitive K+channels on the membrane of vascular smooth muscle cells.15-17However,testosterone could induce a rapid and repeat calcium channel reaction in human macrophages,endotheliocytes,and smooth muscle cells.Recently,English et al15demonstrated that testosterone could induce vascular dilation through inhibiting the calcium-dependent vasoconstriction in the anterior descending branch in rats.This finding suggests that testosterone possesses an antagonistic potential on calcium channels.
However,the specific mechanism of the protective effect of testosterone against atherosclerosis is still unclear,while some hypotheses were suggested.Firstly,testosterone could directly dilate coronary arteries through the metabolic pathway.Secondly,testosterone could be transferred by aromatase in peripheral adipose tissue to estrogen,which has a protective effect on blood vessels,and thereby slow down the process of atherosclerosis in male.11Thirdly,not only could low level of FT induce hyperinsulinism,which could develop to insulin resistance,low level of SHBG associated with hyperinsulinism also plays a role in decreasing TT and DHEAS levels.18-20Studies showed that testosterone level is negatively correlated with insulin,fibrinogen,and plasminogen activator inhibitor-1(PAI-1) in male CHD patients.21-24Relatively hypercoagulable state caused by low testosterone level could also contribute to the development of atherosclerosis.In artery trials,administration of testosterone after endothelium injury could decrease plaque load and increase the level of mRNA,which could induce AR in tissues.These findings suggested that the protective effects of testosterone on coronary artery might be mediated by AR.25The results of these studies suggest that the reduction of plasma testosterone level in elderly male might be a risk factor of CHD.In the present study,the AR fluorescence intensity in CHD group is significantly lower than that in control group(P<0.01),consistent with the findings described above.
It has been demonstrated by a number of studies that hypertension is a risk factor of CHD.After studying 1 132 males aged 30-79 years with hypertension,Khaw et al26found that the level of testosterone in patients with BP≥160/95 mm Hg was lower than that in non-hypertensives.In the total population,SBP and DBP were shown to be negatively correlated with testosterone level.The serum level of testosterone in male hypertension patients decreased significantly.This finding implies that androgen has a protective effect against the development of hypertension in male.In the present study,TT was negatively correlated with DBP,while SBP was negatively correlated with DHEAS,E2,and AR fluorescence intensity.These data reveal that androgen and its receptor are involved in the development of hypertension.It was noted that testosterone could directly stimulate cultured atrial and ventricular muscle cells of rat to synthesize and secrete atrial natriuretic factor (ANF),hence the reduction of ANF due to deficiency of androgen may play a role in the development and persistence of hypertension.10Furthermore,the decreased level of testosterone may contribute to the expression of β-myosin heavy chain (MHC) isozyme in ventricular muscle,and β-MHC predominance is associated with hypertension and myocardial hypertrophy.27On the other hand,in vitrocell culture showed that testosterone could inhibit the generation of prostacyclin (PGI2) in vascular smooth muscle cells from the aortic rings of female rats,suggesting that high level of testosterone in female may have atherogenic function by modulating PGI2 synthesis by vascular tissues.28These changes could further accelerate the development of CHD.
In the present study,age,BW,BMI,WC,and WHR in CHD group were significantly higher than those in control group.We also found that SHBG had a negative correlation with BMI.Dunajska et al10reported that TT,T/E2 ratio,and SHBG level had a negative correlation with the parameters of obesity.This finding implied that low androgen level was a precipitating factor of increased fat proportion in viscera.The possible explanation is that hyperinsulinism,low SHBG level,and increased transformation of androgen to estrogen in adipose tissue in obesity patients could lead to decrease of TT level.29Other studies suggested a correlation with the inhibitive effect of leptin to hypothalamic-pituitary-gonadal axis.30Dunajska et al10found that the median level of SHBG in CHD patients was higher than that in control group,which is inconsistent with the result of the present study.We attribute this discrepancy to the difference in the design of study.
In summary,the serum levels of DHAES,TT,SHBG,and FT in the studied elderly male patients with CHD were significantly lower than those in control group.Testosterone,SHBG,and AR are deemed as independent risk factors or predictors of CHD.The levels of sex hormone and its receptor in elderly males are significantly correlated with CHD.
1.Schreiner PJ,Niemela M,Miettinen H,et al.Gender differences in recurrent coronary events;the FINMONICA MI register.Eur Heart J 2001;22:762-8.
2.Death AK,McGrath KC,Sader MA,et al.Dihydrotestosterone promotes vascular cell adhesionmolecule-1 expression in male human endothelial cellsviaa nuclear factor-B-dependent pathway.Endocrinology 2004;145:1889-97.
3.Bruck B,Brehme U,Gugel N,et al.Gender-specific differences in the effects of testosterone and estrogen on the development of atherosclerosis in rabbits.Arterioscler Thromb Vasc Biol 1997;17:2192-9.
4.Alexandersen P,Karsdal MA,Christiansen C.Long-term prevention with hormone-replacement therapy after the menopause:which women should be targeted? Womens Health (Lond Engl) 2009;5:637-47.
5.Prentice RL,Manson JE,Langer RD,et al.Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause.Am J Epidemiol 2009;170:12-23.
6.English KM,Mandour O,Steeds RP,et al.Men with coronary artery disease have lower levels of androgens than men with normal coronary angiograms.Eur Heart J 2000;21:890-4.
7.Malkin CJ,Pugh PJ,Morris PD,et al.Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life.Heart 2004;90:871-6.
8.Vermeulen A,Kaufman JM.Ageing of the hypothalamo-pituitary-testicular axis in men.Horm Res 1995;43:25-8.
9.Turhan S,Tulunay C,Güle? S,et al.The association between androgen levels and premature coronary artery disease in men.Coron Artery Dis 2007;18:159-62.
10.Dunajska K,Milewicz A,Szymczak J,et al.Evaluation of sex hormone levels and some metabolic factors in men with coronary atherosclerosis.Aging Male 2004;7:197-204.
11.Nathan L,Shi W,Dinh H,et al.Testosterone inhibits early atherogenesis by conversion to estradiol:critical role of aromatase.Proc Natl Acad Sci U S A 2001;98:3589-93.
12.Zgliczynski S,Ossowski M,Slowinska-Srzednicka J,et al.Effect of testosterone replacement therapy on lipids and lipoproteins in hypogonadal and elderly men.Atherosclerosis 1996;121:35-43.
13.Rosano GM,Leonardo F,Pagnotta P,et al.Acute antiischemic effect of testosterone in men with coronary artery disease.Circulation1999;99:1666-70.
14.Channer KS,Jones TH.Cardiovascular effects of testosterone:implications of the“male menopause”? Heart 2003;89:121-2.
15.English KM,Jones RD,Jones TH,et al.Testosterone acts as a coronary vasodilator by a calcium antagonistic action.J Endocrinol Invest 2002;25:455-8.
16.Platz EA,Rimm EB,Willett WC,et al.Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals.J Natl Cancer Inst 2000;92:2009-17.
17.H?rk?nen K,Huhtaniemi I,M?kinen J,et al.The polymorphic androgen receptor gene CAG repeat,pituitary-testicular function and andropausal symptoms in ageing men.Int J Androl 2003;26:187-94.
18.Kaufman JM,Vermeulen A.The decline of androgen levels in elderly men and its clinical and therapeutic implications.Endocrin Rev 2005;26:833-76.
19.Simon D,Charles MA,Nahoul K,et al.Association between plasma total testosterone and cardiovascular risk factors in healthy adult men:The Telecom Study.J Clin Endocrinol Metab 1997;82:682-5.
20.Stellato RK,Feldman HA,Hamdy O,et al.Testosterone,sex hormone-binding globulin,and the development of type 2 diabetes in middle-aged men:prospective results from the Massachusetts male aging study.Diabetes Care 2000;23:490-4.
21.Phillips GB,Pinkernell BH,Jing TY.The association of hypotestosteronemia with coronary artery disease in men.Arterioscler Thromb 1994;14:701-6.
22.Yang XC,Jing TY,Resnick LM,et al.Relation of hemostatic risk factors to other risk factors for coronary heart disease and to sex hormones in men.Arterioscler Thromb 1993;13:467-71.
23.Caron P,Bennet A,Camare R,et al.Plasminogen activator inhibitor in plasma is related to testosterone in men.Metabolism 1989;38:1010-5.
24.Glueck CJ,Glueck HI,Stroop D,et al.Endogenous testosterone,fibrinolysis,and coronary heart disease risk in hyperlipidemic men.J Lab Clin Med 1993;122:412-20.
25.Hanke H,Lenz C,Hess B,et al.Effect of testosterone on plaque development and androgen receptor expression in the arterial vessel wall.Circulation 2001;103:1382-5.
26.Khaw KT,Barrett-Connor E.Blood pressure and endogenous testosterone in men:an inverse relationship.J Hypertens 1988;6:329-32.
27.Morano I,Gerstner J,Rüegg JC,et al.Regulation of myosin heavy chain expression in the hearts of hypertensive rats by testosterone.Circ Res 1990;66:1585-90.
28.Wakasugi M,Noguchi T,Kazama YI,et al.The effects of sex hormones on the synthesis of prostacyclin (PGI2) by vascular tissues.Prostaglandins 1989;37:401-10.
29.Schneider G,Kirschner M,Berkowitz R,et al.Increased estrogen production in obese men.J Clin Endocrinol Metab 1979;48:633-8.
30.Cohen PG.The hypogonadal-obesity cycle:role of aromatase in modulating the testosterone-estradiol shunt--a major factor in the genesis of morbid obesity.Med Hypotheses 1999;52:49-51.
 Chinese Medical Sciences Journal2010年1期
Chinese Medical Sciences Journal2010年1期
- Chinese Medical Sciences Journal的其它文章
- Comparison between Ophthalmologists and Community Health Workers in Screening of Shallow Anterior Chamber with Oblique Flashlight Test△
- Factors Influencing Pleural Effusion after Fontan Operation:an Analysis with 95 Patients
- Relationship between Carotid Atherosclerosis and Cerebral Infarction
- Expression of FLICE-inhibitory Protein in Synovial Tissue and Its Association with Synovial Inflammation in Juvenile Idiopathic Arthritis△
- A Case of Large“Silent”Extra-adrenal Retroperitoneal Paraganglioma Resected Laparoscopically
- Revascularization for Iliac-femoral Artery Pseudoaneurysm with Greater Saphenous Vein
