Liquid-solid Equilibria in Quinary System Na+, K+, Mg2+//Cl-,?at 25 °C*
HUANG Xueli (黃雪莉) and LI Songwan (李松菀)
?

HUANG Xueli (黃雪莉)**and LI Songwan (李松菀)
College of Chemistry and Chemical Engineering, Xinjiang University, Urumqi 830046, China
The solubilities of the quinary system Na+, K+, Mg2+//Cl-, NO-3-H2O and its two quaternary subsystems, Na+, K+, Mg2+//NO-3-H2O and K+, Mg2+//Cl-, NO-3-H2O, were studied by isothermal method at 25°C and their phase diagrams were plotted. In the equilibrium phase diagram of quaternary system Na+, K+, Mg2+//NO-3-H2O, there are one invariant point, three univariant curves and three regions of crystallization with one salt: NaNO3, KNO3and Mg(NO3)2·6H2O. In the equilibrium phase diagram of quaternary system K+, Mg2+//Cl-, NO-3-H2O, there are three invariant points, seven univariant curves and five regions of crystallization with one salt: KNO3, KCl, Mg(NO3)2·6H2O, MgCl2·6H2O and KCl·MgCl2·6H2O. In the equilibrium phase diagram of the quinary system Na+, K+, Mg2+//Cl-, NO-3-H2O, there are four invariant points, and seven regions of crystallization with one salt: NaCl, KCl, NaNO3, KCl·MgCl2·6H2O, KNO3, MgCl2·6H2O and Mg(NO3)2·6H2O.
quinary system, phase equilibrium, nitrate, solubility
1 INTRODUCTION

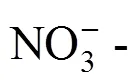
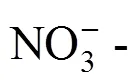
2 EXPERIMENTAL
All reagents used are analytically pure. Conductivity of distilled water is less than 10-4S·m-1, with pH 6.6.
A certain amount of solute and water is added into a bottle of about 100 ml. A stirrer is used to promote the establishment of equilibrium. The bottle is kept in a thermostat maintained at ±0.1°C.
Experiments show that the equilibria are attained in 6, 7 days at 25°C with stirring. After reaching the equilibrium, the stirrer is dismantled, the solid particles are allowed to settle down and the liquid is clarified. The liquid is sampled with a dried and weighed pipette that is modified so that the lower part of the tube has to be inserted into the body to about two-thirds height to form an annular space to hold the solution that is drawn in. This ensures that the solution will not pour out if the finger is to be removed from the top. The mass of the solution is calculated from the total mass of the pipette and the solution sample [9, 11]. The liquid sample is transferred into a 250 ml volumetric flask and diluted up to the mark for analysis.

The concentration of chloride ions are determined by Mohr’s method (with a mass fraction uncertainty of 0.4%), that of nitrate ions by potassium dichromate oxidization (with a mass fraction uncertainty of 0.6%) or evaluated according to ion balance, that of magnesium ions by ethylene diamine tetraacetic acid (EDTA) titration (with a mass fraction uncertainty of 0.4%) and that of potassium ions by gravimetric method (with a mass fraction uncertainty of 0.6%). Sodium ion concentration is evaluated according to ion balance [12]. The components of the solid phase are estimated by the wet residue method and are further identified by stereomicroscopy, differential thermal analysis and X-ray diffraction.

Table 1 Solubilities of the system Na+, K+, Mg2+//-H2O at 25 °C
① Nit: Mg(NO3)2·6H2O.
3 RESULTS AND DISCUSSION
3.1 Na+, K+, Mg2+//-H2O system
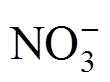



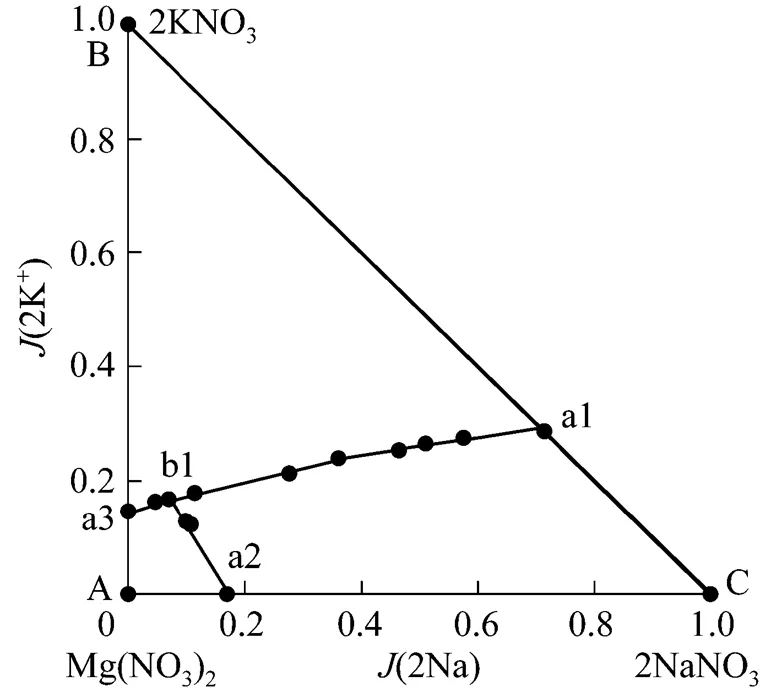
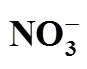
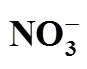
It is seen from Table 1 and Fig. 1 that in the phase diagram, there are one invariant point, three univariant curves and three regions of crystallization. Three regions of crystallization are KNO3(a3-B-a1-b1), NaNO3(a2-b1-a1-C), and Mg(NO3)2·6H2O(A-a3-b1-a2). Three univariant curves are a1-b1 (saturated with KNO3and NaNO3), a2-b1 [saturated with NaNO3and Mg(NO3)2·6H2O], and a3-b1 [saturated with KNO3and Mg(NO3)2·6H2O]. One invariant point is b1 saturated with Mg(NO3)2·6H2O, KNO3and NaNO3.
According to the phase rule,-+2, the degrees of freedom,, of the point, curves and regions in Fig. 1 can be determined. Here,is 3 (not include water), pressure and temperature are constant. For point b1,3-3+2-2=0; for the curves,3-2+2-21; for the regions,3-1+2-22.
3.2 K+, Mg2+//Cl-,?-H2O system

Table 2 Solubilities of system K+, Mg2+//Cl-,?-H2O at 25 °C
① Nit: Mg(NO3)2·6H2O;② Bis: MgCl2·6H2O;③ Car: KCl·MgCl2·6H2O.
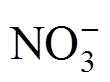
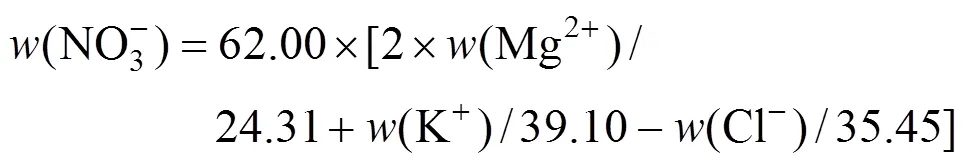
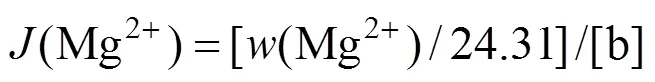




The index values obtained are listed in Table 2. The corresponding dry salt phase diagram and water diagram are plotted in Figs. 3 and 4, respectively.
It is seen from Table 2 and Fig. 3 that the equilibrium diagram consists of three invariant points, seven univariant curves and five regions of crystallization. Five regions of crystallization are Mg(NO3)2·6H2O (b2-a3-C-a7-b4), KNO3(a4-B-a3-b2-b3), MgCl2·6H2O (a6-b4-a7-D), KCl·MgCl2·6H2O(a5-b3-b2-b4-a6), and KCl (A-a4-b3-a5). Seven univariant curves are a4-b3 (saturated with KCl and KNO3), b2-b3 (saturated with KNO3and KCl·MgCl2·6H2O), a3-b2 (saturated with KNO3and Mg(NO3)2·6H2O), a5-b3 (saturated with KCl and KCl·MgCl2·6H2O), a6-b4 (saturated with KCl·MgCl2·6H2O and MgCl2·6H2O), and a7-b4 (saturated with Mg(NO3)2·6H2O and MgCl2·6H2O). Three invariant points are b2, b3, and b4, where b2 is saturated with KNO3, Mg(NO3)2·6H2O and KCl·MgCl2·6H2O; b3 is saturated with KCl, KNO3and KCl·MgCl2·6H2O; and b4 is saturated with KCl·MgCl2·6H2O, Mg(NO3)2·6H2O and MgCl2·6H2O.
3.3 Na+, K+, Mg2+//Cl-,?-H2O system
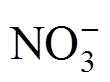
The experimental results are given in Table 3. The ion J?necke index values are calculated according to the following correlations:

Table 3 Solubilities of the Na+, K+, Mg2+//Cl-,?-H2O system at 25 °C
① Nit: Mg(NO3)2·6H2O;② Hal: NaCl;③ Car: KCl·MgCl2·6H2O;④ Bis: MgCl2·6H2O.
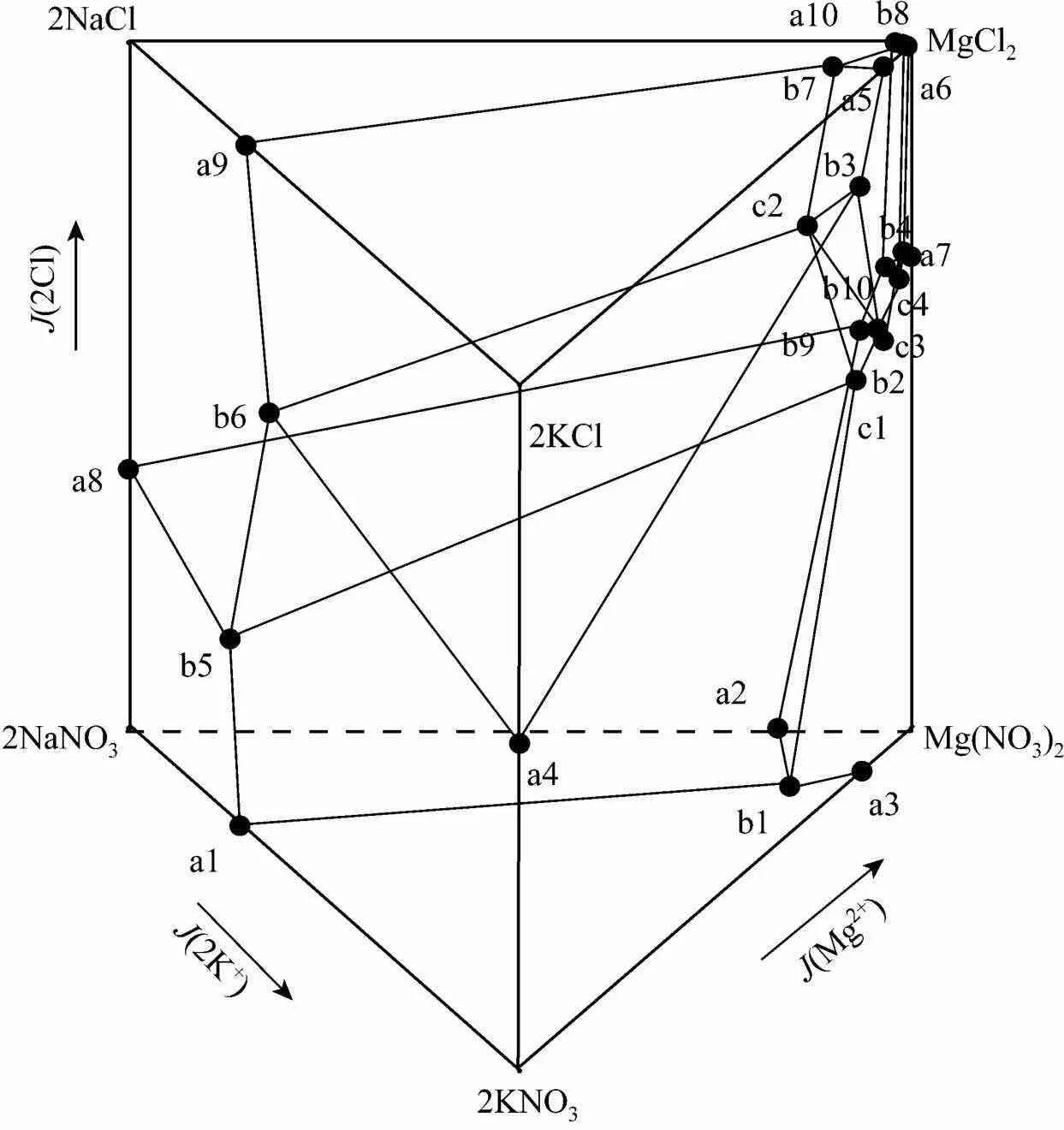
Figure 6 Enlargement of part of Fig. 5

(some points are plotted out of proportion to experimental results)

Figure 6 Enlargement of part of Fig. 5
Figure 6 Enlargement of part of Fig. 5


(out of proportion to Fig. 5)

Letting
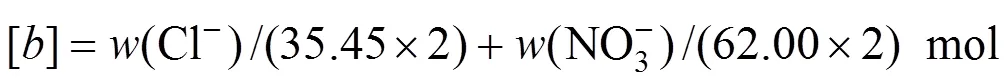
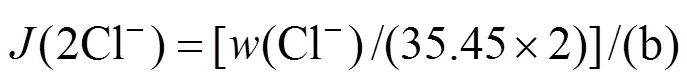
In Fig. 5, there are seven regions of crystallization with one salt and four invariant points. The seven crystallization regions are NaCl, NaNO3, KNO3, MgCl2·6H2O, KC·MgCl2·6H2O, KCl ?and ?Mg(NO3)2·6H2O. The shapes of the seven crystallization regions are shown in Fig. 7. The four invariant points are c1, c2, c3, and c4, where c1 is saturated with NaNO3, NaCl, KNO3and Mg(NO3)2·6H2O; c2 is saturated with KCl, NaCl, KNO3and KCl·MgCl2·6H2O; c3 is saturated with KNO3, Mg(NO3)2·6H2O, NaCl and KCl·MgCl2·6H2O; c4 is saturated with NaCl, MgCl2·6H2O, Mg(NO3)2·6H2O and KCl·MgCl2·6H2O.
4 CONCLUSIONS
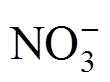
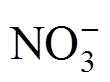
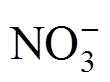
1 Su, Y.G., Lü, B.L., Wang, X.R., The Study on Phase Diagram of Inorganic Chemical Production (1) Theoretical Foundation, Chemical Industry Press, Beijing, 223-257(1985). (in Chinese)
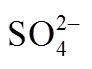
3 Huang, X.L., Lü, B.L., “Study on phase diagram of manufacture of KNO3from saltpeter (1)”,..., 28 (1), 9-11(2001). (in Chinese)
4 Huang, X.L., Gao, F., Hu, K.L., Minawaer, “Study on phase diagram of manufacture of KNO3from saltpeter (2)”,.., 32 (5), 37-40 (2001). (in Chinese)
5 Huang, X.L., “Advances of research and technology with nitrate in xinjiang Lop Nur”,, 26 (7), 10-12 (2006). (in Chinese)
6 Huang, X.L., Ma, F.Y., Hu, Z.Z., “Study on the preparation of KNO3from the brine”,., 62 (12), 1-4 (2002). (in Chinese)
7 Huang, X.L., Zhang, J.S., Hu, Z.Z., Ma, F.Y., “Study on preparation of KNO3from the brine in Xinjiang Lop Nur(2)”,., 33 (8), 6-7 (2004). (in Chinese)
8 Huang, X.L., Zhang, J.S., Hu, Z.Z., Ma, F.Y., “Study on preparation of KNO3from the brine in Xinjiang Lop Nur(3)”,., 33 (10), 13-15 (2004). (in Chinese)
10 Silcok., H., Solubililies of Inorganic and Organic Compounds, Pergamon Press, New York (1979).
11 Lü, B.L., “The correlation of activity coefficients for the electrolyte-nonelectrolyte mixed solution—The thermodynamics of the liquid solid phase equilibria in (NH2)2CO-NaCl-NH4-Cl-H2O system”,, 44 (4), 283-293 (1986).
12 Analytical Room of Qinghai Institute of Salt-lake, Academia Sinica in China, The Analyses of Brines and Salts (China), Science Press, Beijing (1988).
13 Samant, K.D., Ng., Ka M., “Representation of high-dimensional solid-liquid phase diagrams of ionic systems”,, 47 (4), 861-879 (2001).
** To whom correspondence should be addressed. E-mail: xuelih@163.com
2010-07-08,
2010-10-15.
the National Natural Science Foundation of China (20466003, 20866008).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Dynamic Simulation and Analysis of Industrial Purified TerephthalicAcid Solvent Dehydration Process*
- Preparation of p-Hydroxybenzaldehyde by Hydrolysis of DiazoniumSalts Using Rotating Packed Bed*
- Pervaporation Separation of Butanol-Water Mixtures UsingPolydimethylsiloxane/Ceramic Composite Membrane*
- Reaction Kinetics of Biodiesel Synthesis from Waste Oil Using a Carbon-based Solid Acid Catalyst
- Enzyme-catalyzed Synthesis of Vitamin E Succinate Using aChemically Modified Novozym-435*
- Alkylation of p-Cresol with tert-Butanol Catalyzed by NovelMultiple-SO3H Functioned Ionic Liquid*
