Properties of Bio-oil from Fast Pyrolysis of Rice Husk*
GUO Xiujuan (郭秀娟), WANG Shurong(王樹榮), WANG Qi(王琦), GUO Zuogang (郭祚剛) and LUO Zhongyang (駱仲泱)
?
Properties of Bio-oil from Fast Pyrolysis of Rice Husk*
GUO Xiujuan (郭秀娟), WANG Shurong(王樹榮)**, WANG Qi(王琦), GUO Zuogang (郭祚剛) and LUO Zhongyang (駱仲泱)
State Key Laboratory of Clean Energy Utilization, Zhejiang University, Hangzhou 310027, China
Physicochemical properties of bio-oil obtained from fast pyrolysis of rice husk were studied in the present work. Molecular distillation was used to separate the crude bio-oil into three fractions. light fraction, middle fraction and heavy fraction. Their chemical composition was analyzed by gas chromatograph and mass spectrometer (GC-MS). The thermal behavior, including evaporation and decomposition, was investigated using thermogravimetric analyzer coupled with Fourier transform infrared spectrometer (TG-FTIR). The product distribution was significantly affected by contents of cellulose, hemicellulose and lignin. The bio-oil yield was 46.36% (by mass) and the yield of gaseous products was 27% (by mass). The chemicals in the bio-oil included acids, aldehydes, ketones, alcohols, phenols, sugars,. The light fraction was mainly composed of acids and compounds with lower boiling point temperature, the middle and heavy fractions were consisted of phenols and levoglucosan. The thermal stability of the bio-oil was determined by the interactions and intersolubility of compounds. It was found that the thermal stability of bio-oil was better than the light fraction, but worse than the middle and heavy fractions.
biochemical engineering, bio-oil, fast pyrolysis, decomposition, distillation
1 INTRODUCTION
Biomass is an interesting answer to the growing demand for renewable energy [1]. They are usually the by-products in various industries,.. agricultural, food, wood processing and paper industry or they can be purposely grown for energy utilization [2, 3]. Thermochemical conversion of biomass is a promising route for energy and fuel production [4], which is also considered as the easiest process to adapt to current energy infrastructure [5].
More attentions have been focused on fast pyrolysis for bio-oil production as the substituent for transported fuels [6]. The feedstock ranges from bark, wood, agricultural wastes/residues, nuts, seeds, algae, grasses, and forestry residues to energy crops such as miscanthus and sorghum. Wood materials are widely used as they can give high liquid yields, up to 80% (by mass) on dry feed basis at 500-520 °C with residence time not more than 1 s [7]. However, this high yield can not be achieved in the related studies. Luo. [8] conducted the fast pyrolysis of wood feedstock,,and, in a fluidized bed reactor and the highest bio-oil yield was 56% (by mass) at 500 °C. Park. [9] obtained a bio-oil yield 50% (by mass) from fast pyrolysis of Radiata pine sawdust at 400-450 °C in a bubbling fluidized bed. In addition to wood residues, agricultural waste and algae are also used as raw materials. The high bio-oil yield of 55% (by mass) was obtained from fast pyrolysis of rice husk and cotton stalk in a fluidized bed at 465 °C and 510 °C, and the product could be directly used as fuel oils for combustion in a boiler or a furnace based on the analysis of heating value, stability, miscibility, and corrosion [10, 11]. Miao. [12] investigated the fast pyrolysis of microalgae in a fluidized bed operated at 500 °C with a vapour residence time of 2-3 s. The yield of bio-oil was 18% (by mass) and 24% (by mass) forandrespectively. The two bio-oils were considered as more suitable fuel oils than bio-oils from lignocellulosic materials, as they had a high heating value of 29 MJ·kg-1, a density of 1.16 kg·L-1and a viscosity of 0.10 Pa·s.
Currently, a great number of applications are focused on bio-oil, though it has the disadvantage of low heating value, high moisture content, instability and corrosiveness [13]. It can be adopted as boiler fuel for stationary power and heat production, or for chemical extraction. After upgraded, it may be used as substitution for transportation fuel [4]. All these applications require better understanding of physicochemical properties of bio-oil. The objective of the present paper is to report properties of bio-oil obtained from fast pyrolysis of rice husk in a fluidized bed. Moreover, thermal stability is studied using the novel combination of molecular distillation technique, gas chromatograph and mass spectrometer analysis (GC-MS), and a thermogravimetric analyzer coupled with a Fourier transform infrared spectrometer (TG-FTIR).
2 EXPERIMENTAL
2.1 Fast pyrolysis of biomass for bio-oil production
A continuous fluidized bed reactor with a capacity 5 kg·h-1was established and described previously [14]. The important operation conditions were pyrolysis temperature (450-550 °C), gas residence time (less than 1 s), and condenser system (one spray condenser with L-isopar as the medium and two indirect condensers using cooling water). The pictures of rice husk and bio-oil are shown in Fig. 1.

Figure 1 Pictures of rice husk and bio-oil from fast pyrolysis of rice husk
The ground, air-dried particles of rice husk with the size of 0.45-1 mm were used in all experiments. Table 1 summarizes the proximate, ultimate and compositional analysis of samples. Rice husk has a high ash content in proximate analysis and corresponding high acid-insoluble ash content in compositional analysis. The contents of cellulose and lignin are lower than wood materials, but the content of hemi-cellulose is higher.
2.2 Measurements of bio-oil properties
In view of the methods for bio-oil characterization adopted in IEA (International Energy Agency), the moisture and oxygen content of bio-oil were measured using a Karl-Fisher moisture analyzer and a Flash EA-1112 elemental analyzer, respectively. The pH value, viscosity, and the content of insoluble solid were measured by PHB-1 acidimeter, capillary viscosimeter, and the combination of vacuum pump, filter and collection bottles with the standard of IEA-EU, respectively. The heating value was analyzed by the automatic calorimetry according to the rule of GB/T384-81.
The composition of samples was separated and identified with a Voyager GC-MS system using a 30 m×0.25 mm×0.25 mm Agilent DB-WAXetr capillary column. The oven temperature was controlled at 35 °C for 1 min, heated at a rate of 8 °C?min-1to 240 °C, and then kept at 240 °C for 21 min. Data was acquired with Xcalibur software with a Gateway computer with the NBS mass spectra library database. The quantitative method was the relative area normalization method. Samples (0.1 μl) of the bio-oil fractions were injected directly into the GC column to minimize the loss of oil components and avoid the obstruction of capillary column.
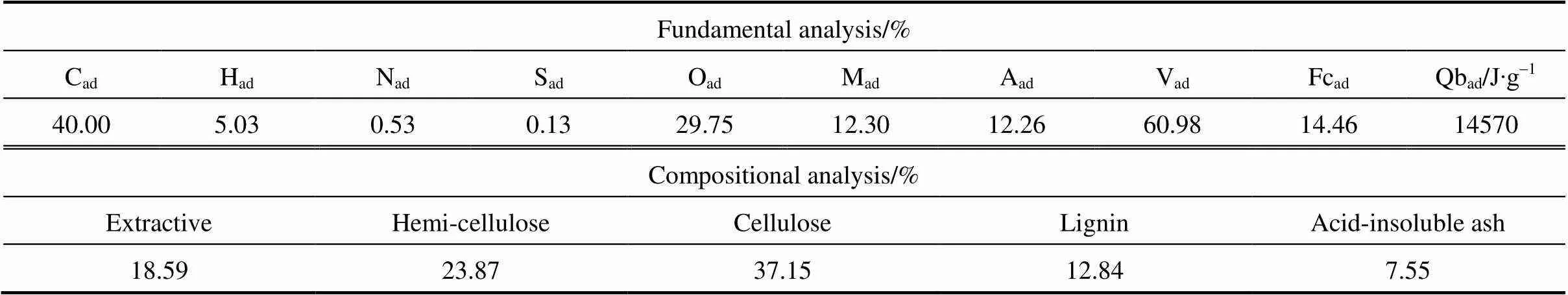
Table 1 Fundamental analysis of rice husk sample
2.3 Thermal stability analysis of bio-oil
The bio-oil was first separated into three fractions. light, middle and heavy fractions by the molecular distillation with a KDL-5 modular instrument manufactured by the UIC Corporation, Germany. The operation details were depicted in our previous study [15]. The volatilization process of the three fractions at low heating rate was investigated using TG-FTIR (a Mettler-Toledo TGA/SDTA851e thermogravimetric analyzer coupled with a Nicolet NETXUS 670 FTIR spectrometer). Experiments were carried out with a heating rate of 10 °C?min-1within the temperature range of 20-650 °C, with a nitrogen flow rate 30 ml?min-1. The weight of samples was 5-10 mg to ensure an accurate kinetic analysis and a sufficient amount of volatiles. The released volatiles were detected with a deuterated triglycine sulfate pyroelectric detector, which has a rapid response and low noise. The spectrum scope was 400-4000 cm-1and resolution factor was 1 cm-1.
3 RESULTS AND DISCUSSION
3.1 Product distribution
The product distribution in the fast pyrolysis of rice husk is shown in Fig. 2, which is significantly affected by the contents of cellulose, hemicellulose and lignin. Fast pyrolysis of cellulose produces more sugars such as levoglucosan and D-glucopyranose, while lignin is apt to generate solid chars.
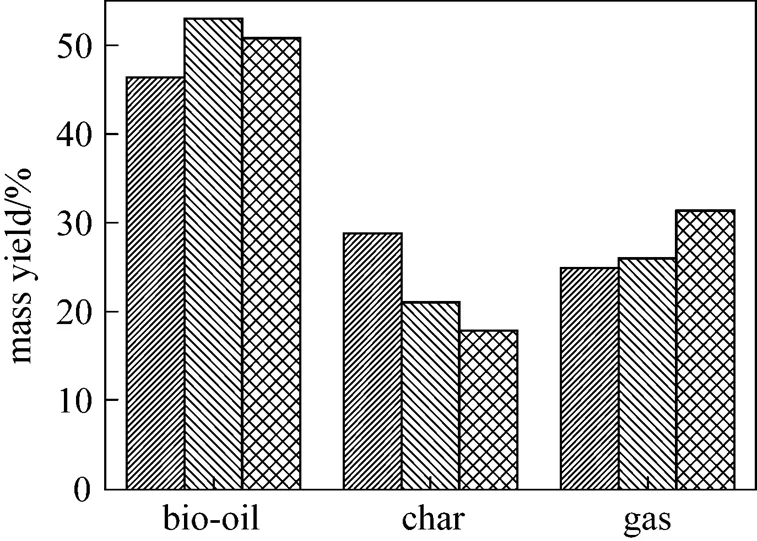
Figure 2 Product distribution in fast pyrolysis of rice husk (operation temperature: 520 °C)

A bio-oil yield of 50.8% (by mass) was obtained in the fast pyrolysis of pine with cellulose content of 47.68% [15], while the rice husk gives lower yields of bio-oil and gases and higher char yield due to the catalytic effect of the ash contained. The extractives are usually less than 10% and their effect is usually ignored [16, 17], but the influence of extractives on product distribution can not be neglected here due to the high content of 18.59%. The bio-oil yield of 46.36% (by mass) from this rice husk pyrolysis is different from the yield of 53% (by mass) from the rice husk pyrolysis in a fluidized bed reactor at 510 °C [10], because of different flow field in reactor, operation temperature, raw materials,.
3.2 Physical properties of bio-oil
The bio-oil can be directly used as combustion fuels, but its application is limited in other areas due to its high viscosity, resulting in difficulties in chemicals extraction and production of liquid fuel. The physical properties of crude bio-oil are shown in Table 2, which are in accordance with the previous study [10]. The lower viscosity corresponds to higher temperature, which is consistent with that the viscosity was 120 mm2·s-1and 40 mm2·s-1, measured at the temperature 20 °C and 60 °C respectively [10]. The high viscosity makes the study on bio-oil difficult, especially for the molecular distillation tests. Therefore, the bio-oil was pretreated before its injection into the molecular distillation equipment to decrease its viscosity, to avoid the sudden blocking of bio-oil and reduce the load on the vacuum system.
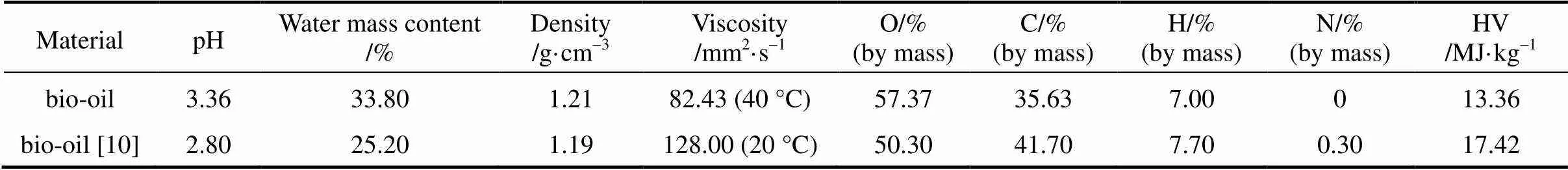
Table 2 Physical properties of crude bio-oil from fast pyrolysis of rice husk
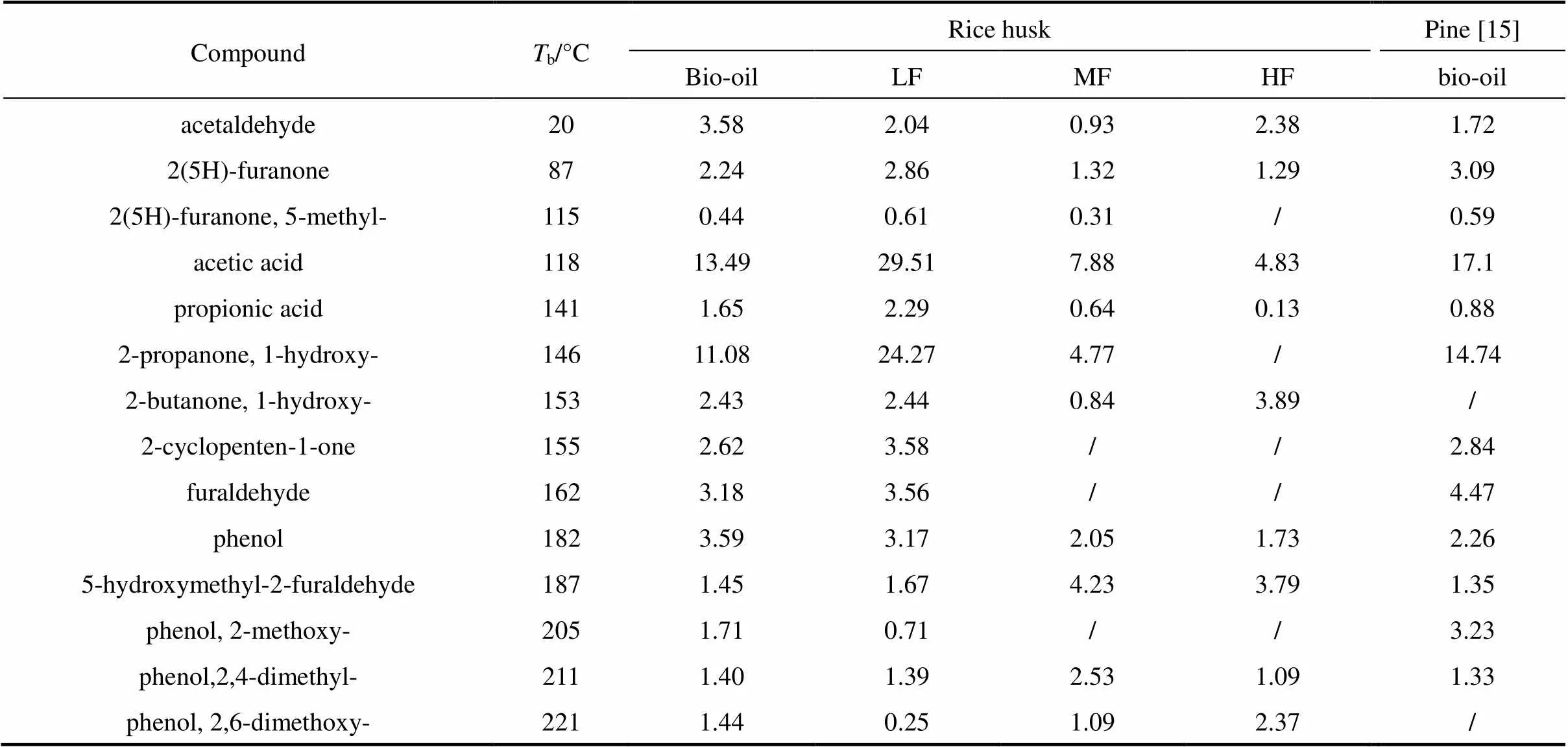
Table 3 Yields of main chemical composition of bio-oil and its fractions (%)
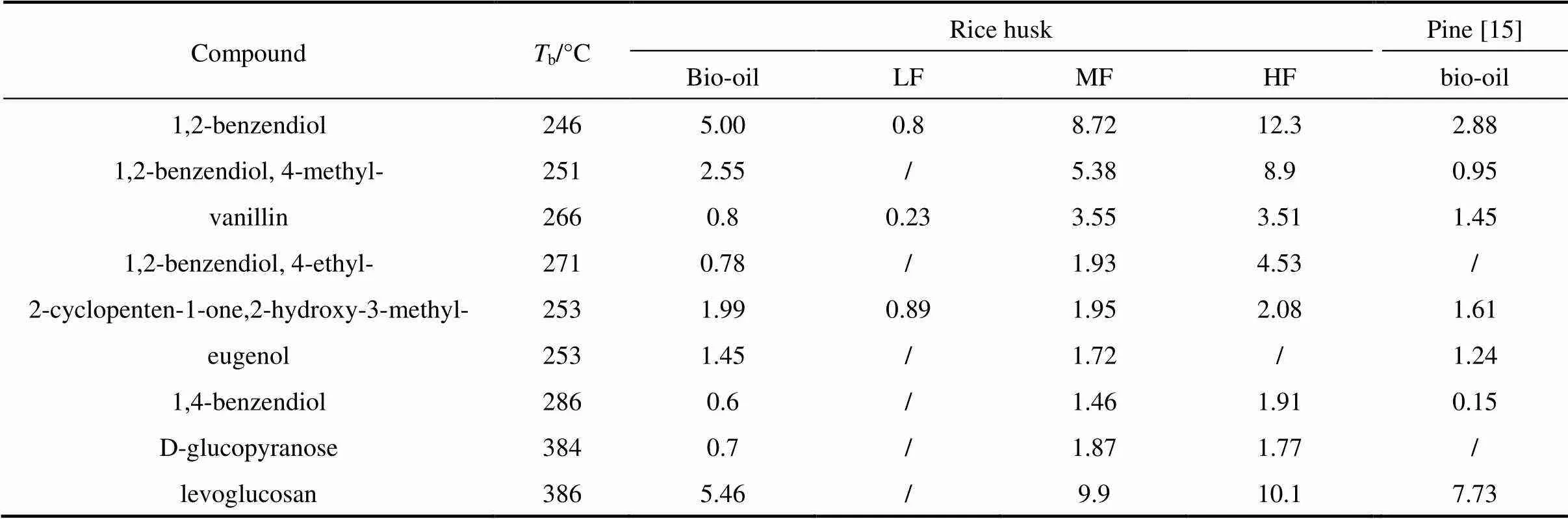
Table 3 (Continued)
3.3 Thermal stability of rice husk bio-oil
3.3.1
A few qualitative analyses were performed with bio-oils and their fractions obtained from fast pyrolysis of different materials. The composition of bio-oil in the present study agrees well with that from other researches [13, 18, 19]. Many compounds have been identified and divided into several chemical categories such as acids, phenols, aldehyde, alcohols, sugars, esters, ketones,.
Molecular distillation was used to separate the bio-oil into three fractions. light fraction (LF), middle fraction (MF) and heavy fraction (HF). The yields of LF, MF and HF are 58.9% (by mass), 17.9% (by mass) and 19.5% (by mass), respectively. The compounds in the bio-oil and its fractions are given in Table 3 in increasing order ofb(boiling point temperature). LF contains more acidic compounds including acetic acid and propionic acid and ketones such as 1-hydroxy-2- propanone, 1-hydroxy-2-butanone and 2-cyclopenten- 1-one. MF and HF are consisted of phenols and sugars, such as 2,4-dimethyl-phenol, 1,2-benzendiol, 4-methyl- 1,2-benzendiol, vanillin and levoglucosan. The composition of rice husk bio-oil and pine bio-oil is similar except for the absence of 2,6-dimethoxy-phenol due to the single guaiacyl lignin unit in pine [20].
3.3.2
As shown in Fig. 3, the bio-oil and its fractions have a rapid mass loss rate in the temperature range of 25-200 °C due to their high moisture contents. The bio-oil and LF begin to evaporate at 25 °C, complete evaporation at about 130 °C, and achieve their maximum mass loss rate at 69 °C and 95 °C, respectively, which are lower than the boiling point temperature of water. It can be explained by that water would evaporate below its boiling point temperature at a slow heating rate [21]. The char yield is 20% (by mass) for the bio-oil and 30%-40% for the MF and HF. No char is formed in LF. The kinetic parameters, activation energy and pre-exponential factor, are calculated based on Arrhenius theory using the integral Coats-Redfern method. The activation energy of the bio-oil and LF is 51.39 and 52.72 kJ?mol-1, respectively, at 25-120 °C. The activation energy of MF and HF is 35.29 and 36.71 kJ?mol-1at 25-200 °C, and it is 57.83 and 55.42 kJ?mol-1, respectively, at 300-500 °C.
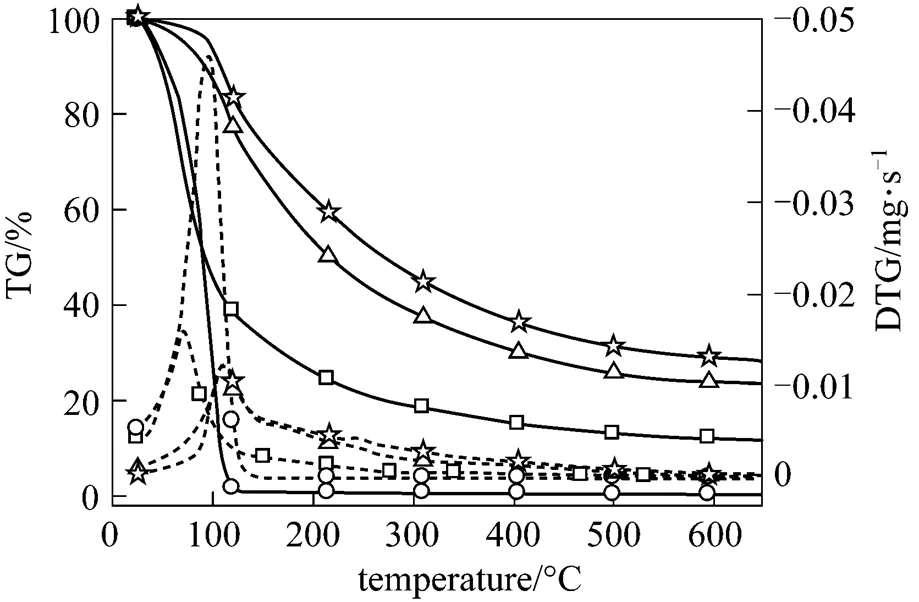
Figure 3 TG (black line) and DTG (dot line) curves of bio-oil and its fractions (heating rate of 10 °C?min-1)
□?bio-oil;○?LF;△?MF;☆?HF
3.3.3
The separation of bio-oils into LF, MF and HF is for comprehensive analysis of thermal stability. Products released from the pyrolysis of bio-oil and its fractions are studied to deduce the reason of instability. The original infrared spectrum is shown in Fig. 4, from which species and intensity of released product are analyzed. The band at 3500-3964 cm-1in the spectrum indicates the formation of water and the bands at 2217-2391 cm-1and 586-726 cm-1characterize the generation of CO2[22]. CO2is a typical product of bio-oil and water is obviously present in LF pyrolysis. CO, characterized by the dual bands at 2180 and 2112 cm-1, and CH4, characterized by the band at 2850-3200 cm-1, are not observed at this maximal release intensity, because they are secondary cracking products. The identification of organic compounds is complex due to their multi characteristic bands. For example, carbonyl group contained in different chemical families has different vibration range and intensity. Main pyrolysis products of bio-oil and its fractions are acetic acid, 1-hydroxy-2-propanone and ethyl alcohol. The related infrared curve of ethyl alcohol are shown in Fig. 4, in which acetic acid and 1-hydroxy-2-propanone are characterized by the band at 1710-1846 cm-1and bands at 2870-2996, 1600-1800 and 1107-1400 cm-1, respectively. 1-hydroxy-2-propanone and acetic acid are evaporated from the bio-oil and LF, while ethyl alcohol is mainly produced from the decomposition of MF and HF.
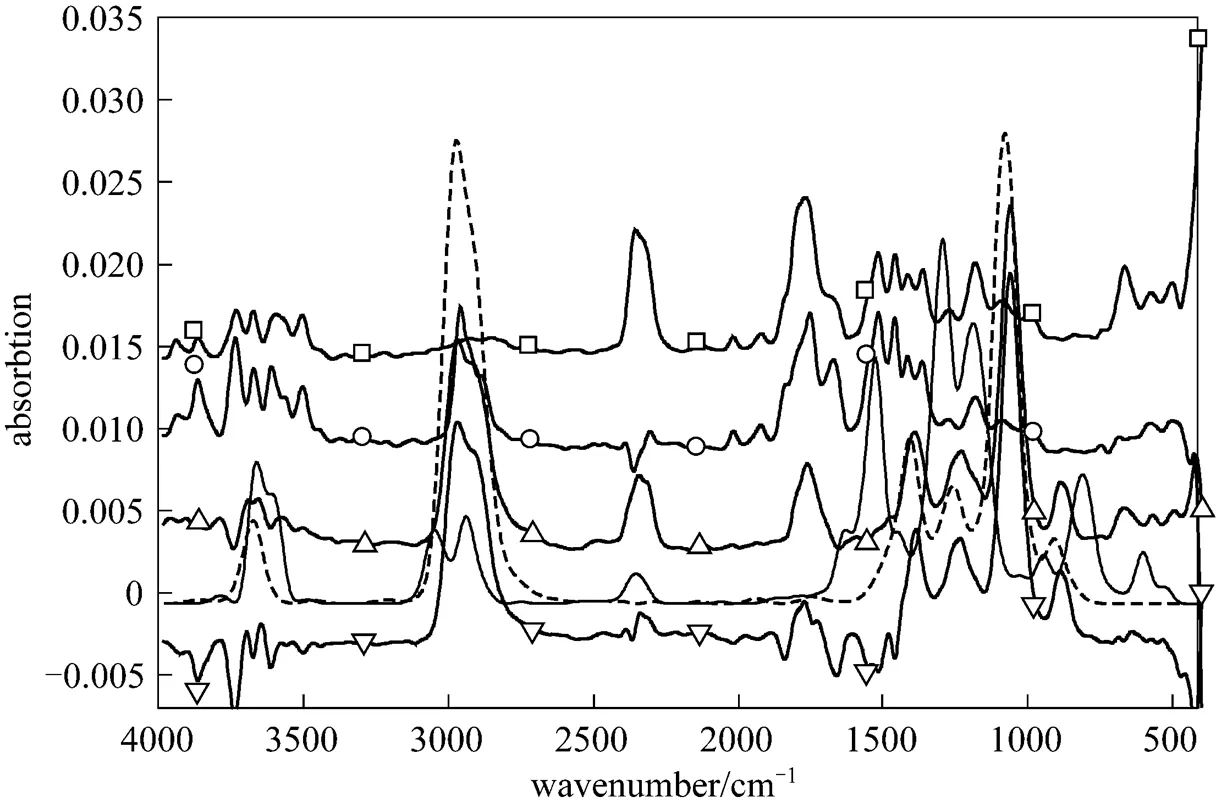
Figure 4 Infrared spectra of bio-oil and its fractions at maximal release intensity

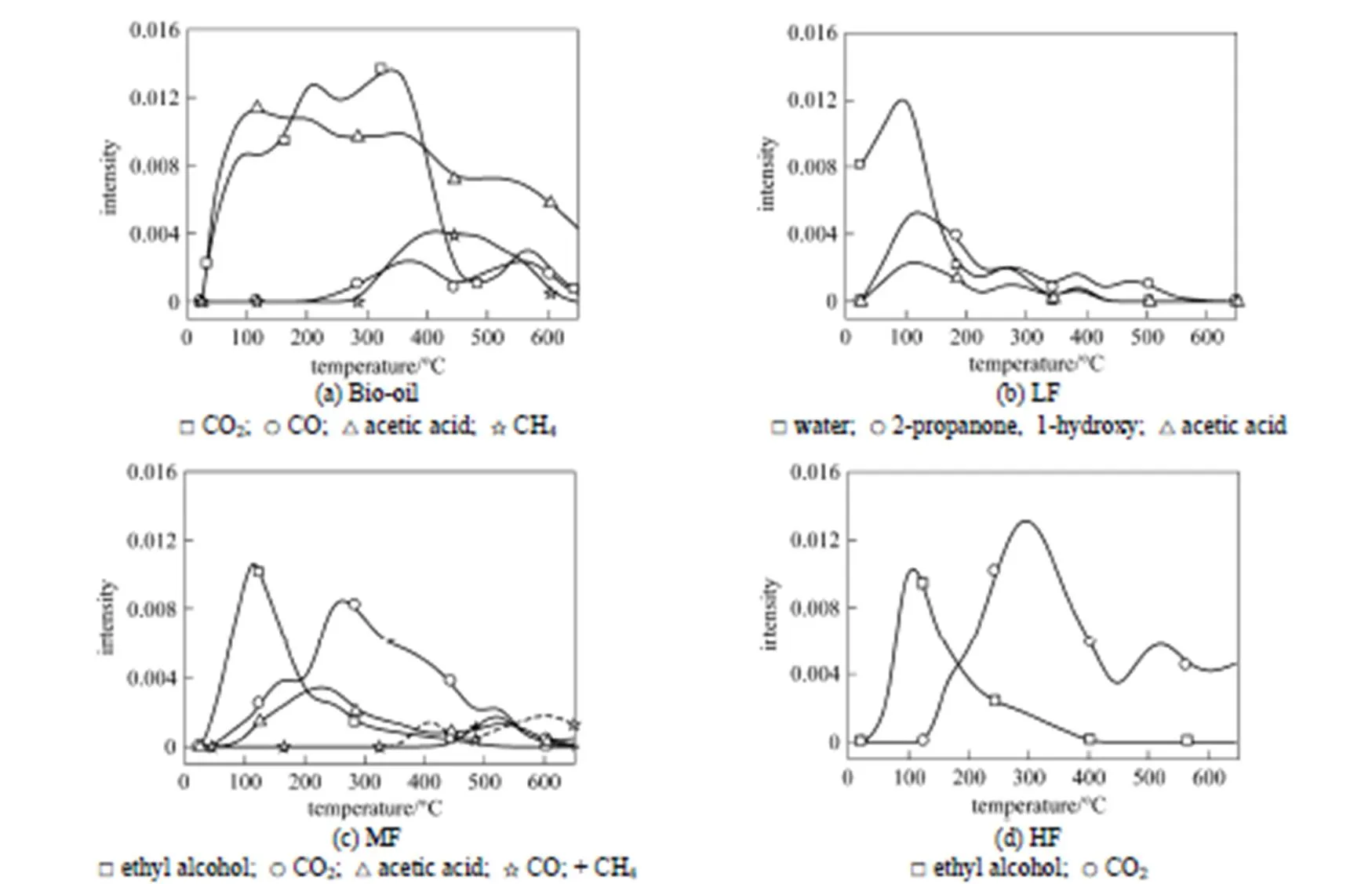
Figure 5 Product release during pyrolysis of bio-oil and its fractions


Thermal stability is mainly affected by the constituents. Water is the main product of LF pyrolysis and will evaporate completely at temperatures lower than 200 °C, so LF has poor thermal stability. MF and HF have good thermal stability and their constituents will be decomposed into ethyl alcohol and gases such as CO2, CO and CH4at high temperature. The interactions of compounds can not be ignored, especially the catalytic effect and the intersolubility of acetone and phenol.
4 CONCLUSIONS
In this study, fast pyrolysis of rice husk gives a bio-oil yield of 46.36% (by mass) and a solid char yield of 30% (by mass). The composition of bio-oil includes several chemical categories such as acids, phenols, aldehyde, alcohols, sugars, esters, ketones,. The crude bio-oil is separated into three fractions using molecular distillation. The constituents of LF begin to evaporate at low temperature with high release intensity, while MF and HF, with decomposition of oxygenated compounds, produce ethyl alcohol and gases such as CO2, CO and CH4. Competition of decomposition and evaporation is dominant during bio-oil pyrolysis. Thermal stability is complex and mainly affected by the compounds and their interactions.
1 Mohan, D., Pittman, C.U., Steele, P.H., “Pyrolysis of wood/biomass for bio-oil: A critical review”,, 20, 848-889 (2006).
2 Strezov, V., Patterson, M., Zymla, V., Fisher, K., Evans, T.J., Nelson, P.F., “Fundamental aspects of biomass carbonization”,..., 79, 91-100 (2007).
3 Xu, X.B., Lin, J.P., Cen, P.L., “Advanced in the research and development of acrylic acid production from biomass”,...., 14 (4), 419-427 (2006).
4 Huber, G.W., Iborra, S., Corma, A., “Synthesis of transportation fuels from biomass: Chemistry, catalysts and engineering”,.., 106, 4044-4098 (2006).
5 Budarin, V.L., Clark, J.H., Lanigan, B.A., Shuttleworth, P., Breeden, S.W., Wilson, A.J., Macquarrie, D.J., Milkowski, K., Jones, J., Bridgeman, T., Ross, A., “The preparation of high-grade bio-oils through the controlled, low temperature microwave activation of wheat straw”,.., 100, 6064-6068 (2009).
6 Lédé, J., Broust, F., Ndiaye, F.T., Ferrer, M., “Properties of bio-oils produced by biomass fast pyrolysis in a cyclone reactor”,, 86, 1800-1810 (2007).
7 Bridgwater, A.V., Meier, D., Radlein, D., “An overview of fast pyrolysis of biomass”,.., 30, 1479-1493 (1999).
8 Luo, Z.Y., Wang, S.Y., Liao, Y.F., Zhou, J.S., Gu, Y.L., Cen, K.F., “Research on biomass fast pyrolysis for liquid fuel”,, 26, 455-462 (2004).
9 Park, H.J., Park, Y.K., Kim, J.S., “Influence of reaction conditions and the char separation system on the production of bio-oil from radiata pine sawdust by fast pyrolysis”,.., 89, 797-802 (2008).
10 Zheng, J.L., “Bio-oil from fast pyrolysis of rice husk: Yields and related properties and improvement of the pyrolysis system”,..., 80, 30-35 (2007).
11 Zheng, J.L., Yi, W.M., Wang, N.N., “Bio-oil production from cotton stalk”,., 49, 1724-1730 (2008).
12 Miao, X., Wu, Q., Yang, C., “Fast pyrolysis of microalgae to produce renewable fuels”,..., 71, 855-863 (2004).
13 ?zbay, N., Uzun, B.B., Varol, E.A., Pütün, A.E., “Comparative analysis of pyrolysis oils and its subfractions under different atmospheric conditions”,.., 87, 1013-1019 (2006).
14 Qi, W., Qian, L., Bo, H., Wang, S.Y., Luo, Z.Y., Cen, K.F., “Experimental research on biomass flash pyrolysis for bio-oil in a fluidized bed reactor”,..., 29, 885-892 (2008).
15 Guo, X.J., Wang, S.R., Guo, Z.G., Luo, Z.Y., Cen, K.F., “Pyrolysis characteristics of bio-oil fractions separated by molecular distillation”,., 87, 2892-2898 (2010).
16 Ranzi, E., Cuoci, A., Faravelli, T., Frassoldati, A., Migliavacca, G., Pierucci, S., Sommariva, S., “Chemical kinetics of biomass pyrolysis”,, 22, 4292-4300 (2008).
17 Wang, G., Li, A.M., “Thermal decomposition and kinetics of mixtures of polylactic acid and biomass during copyrolysis”,..., 16 (6), 923-933 (2008).
18 Branca, C., Giudicianni, P., Blasi, C.D., “GC/MS characterization of liquids generated from low-temperature pyrolysis of wood”,...., 42, 3190-3202 (2003).
19 Branca, C., Blasi, C.D., Elefante R., “Devolatilization and heterogeneous combustion of wood fast pyrolysis oils”,...., 44, 799-810 (2005).
20 Caballero, J.A., Font, R., Marcilla, A., “Kinetic study of the secondary thermal decomposition of kraft lignin”,..., 38, 131-152 (1996).
21 Branca, C., Blasi, C.D., Elefante, R., “Devolatilization of conventional pyrolysis oils generated from biomass and cellulose”,, 20, 2253-2261 (2006).
22 Fu, P., Hu, S., Xiang, J., Sun, L.S., Yang, T., Zhang, A.C., Zhang, J.Y., “Mechanism study of rice straw pyrolysis by Fourier transform infrared technique”,...., 17 (3), 522-529 (2009).
23 Das, D., Lee, J.F., Cheng, S., “Selective synthesis of bisphenol-A over mesoporous MCM silica catalysts functionalized with sulfonic acid groups”,.., 223, 152-160 (2004).
** To whom correspondence should be addressed. E-mail: srwang@zju.edu.cn
2010-07-05,
2010-10-13.
the International Science and Technology Cooperation Program of China (2009DFA61050), the National High Technology Research and Development Program of China (2009AA05Z407), and the National Natural Science Foundation of China (50676085, 90610035).
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Boundary Layers on Polycrystalline Silicon Chemical Vapor Deposition in a Trichlorosilane and Hydrogen System*
- Experimental and CFD Study on the Role of Fluid Flow Pattern onMembrane Permeate Flux
- Separation of Eu3+ Using a Novel Dispersion Combined LiquidMembrane with P507 in Kerosene as the Carrier*
- Fabrication of SPES/Nano-TiO2 Composite Ultrafiltration Membrane and Its Anti-fouling Mechanism*
- Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
- Induction of Recombinant Uridine Phosphorylase and Its Application in Biosynthesis of Pyrimidine Nucleosides*
