QTL mapping of starch granule size in common wheat using recombinant inbred lines derived from a PH82-2/Neixiang 188 cross
Nn Feng,Zhonghu He,b,Yong Zhng,Xinchun Xi,Yn Zhng,*
aInstitute of Crop Science,National Wheat Improvement Center/The National Key Facility for Crop Gene Resources and Genetic Improvement, Chinese Academy of Agricultural Sciences(CAAS),Beijing 100081,China
bInternational Maize and Wheat Improvement Center(CIMMYT)China Office,c/o CAAS,Beijing 100081,China
QTL mapping of starch granule size in common wheat using recombinant inbred lines derived from a PH82-2/Neixiang 188 cross
Nan Fenga,Zhonghu Hea,b,Yong Zhanga,Xianchun Xiaa,Yan Zhanga,*
aInstitute of Crop Science,National Wheat Improvement Center/The National Key Facility for Crop Gene Resources and Genetic Improvement, Chinese Academy of Agricultural Sciences(CAAS),Beijing 100081,China
bInternational Maize and Wheat Improvement Center(CIMMYT)China Office,c/o CAAS,Beijing 100081,China
A R T I C L E I N F O
Article history:
Received 25 February 2013
Received in revised form 15 May 2013
Accepted 28 May 2013
Available online 10 July 2013
Triticum aestivum
QTL
Starch granule size distribution
A-type starch granule
B-type starch granule
Starch is a crucial component determining the processing quality of wheat(Triticum aestivum L.)-based products.Wheat starch generally contains A-type and B-type starch granules,having different effects on starch properties and end-use qualities.In the present study,240 recombinant inbred lines(RILs)derived from a PH82-2/Neixiang 188 cross were grown in Anyang,Henan,China,during three cropping seasons.A-type and B-type granule contents were determined using a laser diffraction particle size analyzer,defined as the percentage of totalstarch volume.A total of195 SSR and STS markers were used to construct a genetic map.QTL analysis was performed by composite interval mapping.Three QTL for A-type starch granule content were mapped on chromosomes 1DL,7BL and 4AL,explaining 5.6%,5.2%and 3.8%of the phenotypic variation,respectively.These results provide useful information for improving starch quality in common wheat.
?2013 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.All rights reserved.
1.Introduction
Starch,a major component of wheat(Triticum aestivum L.) endosperm,accounts for 65–75%of the dry weight of the mature grain and is highly related to end-use quality of wheat-based products[1,2].Generally,wheat endosperm contains A-type and B-type starch granules,showing a bimodal granule size distribution.A-type granules are bigger(10–35 μm)and disk-or lenticular-shaped,accounting for 3%of total wheat starch by number and more than 70%by weight,whereas B-type granules are smaller(<10 μm)and spherical or angular,making up over 90%by number and less than 30%by weight[3–6].
In wheat,A-type starch granules begin to form 3 d postanthesis,whereas B-type starch granules occur 15 d post-anthesis[2,7],resulting in differences in the molecular organization of amylose and amylopectin fractions and the molecular architecture of amylopectin[8–11].A-type granules have higher gelatinization enthalpy,peak viscosity,minimum viscosity,breakdown viscosity,final viscosity,setback and lower gelatinization onset,peak temperature,and amylopectin content,whereas B-type granules have higher amylose/lipid complex enthalpy and lower onset temperature[2,12–16].
Differences in chemical and structural properties of A-type and B-type starch granules lead to different functionalities. It was reported that higher proportions of smaller granules increased dough elastic properties[17].B-type granules bind more water,which likely increases dough stiffness and reduces the elasticity[18].The processing ability and the qualities of both dried and cooked starch noodles made from small-sized granule fractions are much better than those made from large-sized granule fractions[19],but small A-type granules (about 12 μm)can increase bread weight[20].When A-type and B-type starch granules were remixed in various proportions,the optimum proportion of B-type granules for superior bread quality was 25–35%by weight[21].On the other hand, environmental factors influenced starch size distribution,but cultivars played a major role[22].Therefore,it is necessary to study the genetic factors influencing starch size distribution.
A few QTL studies of starch granules have been done in Triticeae crops.A major QTL was identified on chromosome 4S of Ae.peregrina for the content of B-type starch granules, accounting for 44.4%of the phenotypic variation[23].A QTL for A:B ratio was detected on wheatchromosome 4B[24].AQTL was found on barley chromosome 2(2H),affecting A-type granules and the mean F-shape of B-type granules,and two others on chromosomes 4(4H)and 7(5H)affected the mean F-shape of B-type granule and the mean maximum diameter of A-type granules,respectively[25].In addition,QTL were localized for granules<5.0 μm,5.1–10.0 μm and>28.0 μm on chromosome 4DS and for granules 10.1–15.0 μm on 7AS and 1BL[26].
However,there is no consistent major QTL controlling starch granule size or distribution,and no study on QTL mapping of starch granule size distribution in Chinese wheat cultivars has been carried out.Thus any association ofstarch granule type and Chinese dry noodle properties remains unknown.The aim of the present study was to map QTL for differences in wheat starch granules using a RIL population derived from a PH82-2/Neixiang 188 cross,and to identify closely linked molecular markers. PH82-2,a hard wheat released in Shandong,China,is suitable for making Chinese noodles and steamed bread,whereas Neixiang 188,a soft wheat released in Henan,is known for its broad adaptation.Both ofthemhave wild type non-waxy protein genes.
2.Materials and methods
2.1.Plant materials and field trials
The 240 recombinant inbred lines(RILs)generated from a PH82-2/Neixiang 188 cross were used for QTL mapping of starch granule size distribution.Field trials were conducted in a latinized alpha lattice design[27]with three partial replications at Anyang,Henan,China,in the 2005–2006,2010–2011 and 2011–2012 cropping seasons.In total,390 plots were assigned to a 13 row×30 column array at each location, among which 60 RILs were randomly selected and then planted with three replications,whereas the other 180 RILs were planted in single replications in the 2005–2006 season.In 2010–2011 and 2011–2012 seasons,320 plots were assigned to a 10 row×32 column array at each location,among which the 60 RILs randomly selected in the 2005–2006 season were planted with two replications,and the other 180 RILs were planted as a single replication.The two parents were included as check cultivars with 10 to 15 replications in each field trial across seasons for error estimation.
2.2.Milling
Grain hardness was measured on 300-kernel samples with a Perten Single Kernel Characterization System(SKCS)4100 (Perten Instruments,Springfield,IL,USA).The tested samples were tempered overnight to 14.5%,15.5%and 16.5%moisture for soft,medium,and hard wheats,respectively.Grain samples of 100 g from each line were milled using a Brabender Quadrumat Junior Mill(Brabender Inc.,Duisberg,Germany).
2.3.Starch isolation and granule size determination
Starch was extracted according to Liu et al.[28]and Park et al. [29]with minor modifications,in which the tailings were centrifuged twice and all the starch was pooled together.To separate gluten from starch,dough was prepared by mixing 6 g offlourwith 4 g ofdistilled water,stood for 10 min,and then washed with 60 mL of water.The gluten was washed twice with 20 mL of water to ensure collection of all the starch.The combined starch suspensions were filtered through a nylon bolting cloth(75 μm openings)to remove impurities.The starch suspension was centrifuged at 2,500×g for 15 min,and the supernatant was discarded.The precipitate was divided into two portions and the upper gray-colored tailings were moved to another tube.Water(3 mL g?1of starch)was added into the lower light-colored portions and slurries were centrifuged again. These steps were repeated until there were no gray-colored tailings on top of the starch.The tailings that gathered from each repeat were re-suspended and centrifuged twice.Then, the top layer was discarded as described above.The upper and lower portions were combined,frozen,lyophilized and ground lightly with a mortar and pestle to pass a 100-mesh sieve.
A-type and B-type starch granule contents were determined using a Sympatec Helos/Rodos laser diffraction particle size analyzer(Sympatec GmbH,Clausthal-Zellerfeld,Germany), and the data were calculated as the percentage of total starch volume.Granules with sizes of<10.0 μm and 10.1–35.0 μm in diameter were classified as B-type and A-type starch granules, respectively[6].Granules with diameters>35.0 μm were considered to be impurities or starch polymers.Each sample was measured twice,and the differences between two repeats of B-type granule contents were less than 0.5%.
2.4.Statistical analysis
All traits were separately analyzed by fitting an appropriate spatial model with rows and columns[30,31].The best linear unbiased predictions from the best-fit model were used forsubsequent analysis[30].Analysis of variance,correlation coefficients and other computations were conducted by the Statistical Analysis System(SAS)v9.0(SAS Institute Inc.,Cary, NC,USA).
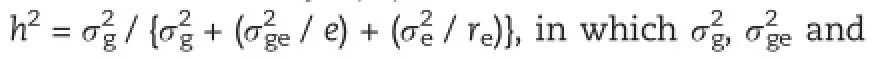
2.5.QTL analysis
The linkage map and marker data for the RIL population were described in a previous study[31].A total of 195 SSR and STS markers were used to construct the linkage map.QTL were detected by composite interval mapping(CIM)based on 1,000 permutation tests and a LOD score of 2.0 with the software QTL Cartographer v2.5.Map distances in centiMorgan units were calculated from recombination values using the Kosambi mapping function.

Table 2–Mean,rangeandheritabilities(h2)ofthecontentsof A-type and B-type starch granules among PH82-2/Neixiang 188 RILs and parents based on phenotypic data averaged over three years.
3.Results
3.1.Phenotypic variation and broad-sense heritability
The correlation coefficients of A-type and B-type starch granule contents across three cropping seasons are presented in Table 1.The contents of A-type starch granules or B-type starch granules among different years were positively correlated,with the correlation coefficients in the ranges of 0.35–0.46 and 0.53–0.66,respectively.The contents of A-type and B-type starch granules in the same years were negatively correlated, with correlation coefficients of–0.72,–0.78 and–0.46 in 2006, 2011 and 2012,respectively.
The mean contents of A-type starch granules of PH82-2 and Neixiang 188 were 79.9%and 82.6%,whereas the mean contents of B-type starch granules were 17.4%and 16.9%, respectively(Table 2).The mean contents of A-type and B-type starch granules in the RIL population were 79.0%and 18.1%,with ranges of 65.7–89.0%and 11.9–28.2%,respectively.Although there were no obvious differences between PH82-2 and Neixiang 188,variation among RILs was significant with transgressive segregation observed in the RIL population(Fig.1),indicating polygenic inheritance.
The analysis of variance for the 240 RILs showed that genotypes,years and their interaction had significant variances,and genotypes contributed to the largest component. Broad-sense heritabilities(h2)estimated for A-type and B-type starch granules were 81.2%and 87.3%,respectively.
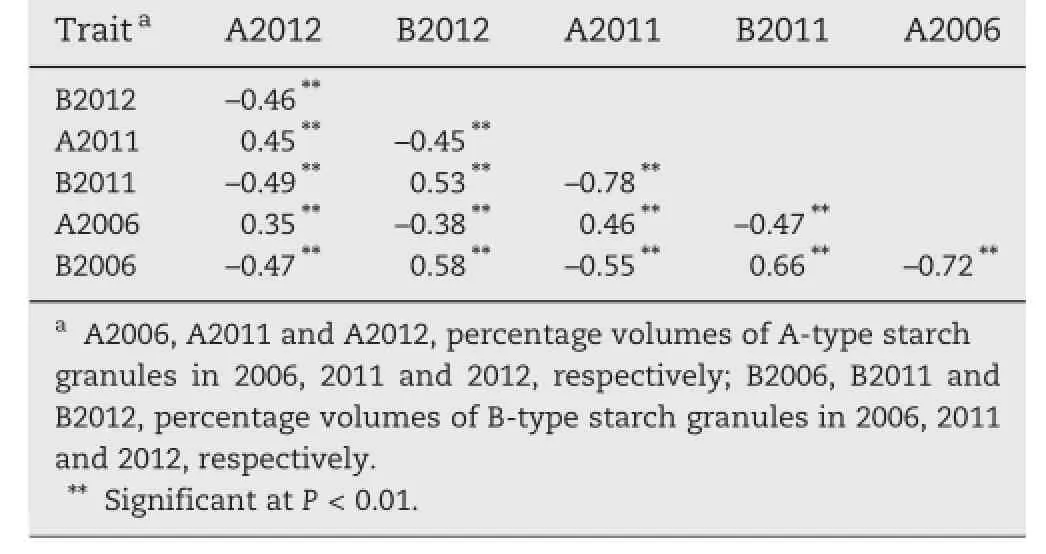
Table 1–Correlation coefficients of contents of A-type and B-type starch granules for PH82-2/Neixiang 188 RILs across three years.
3.2.QTL for starch granule size
Three QTL for content of A-type starch granules were detected in the population(Table 3 and Fig.2).Two QTL on chromosomes 1DL and 7BL were found in the 2012 trial,explaining 5.6 and 5.2%of phenotypic variation,with the increasing allele effects from Neixiang 188 and PH82-2,respectively.One QTL with the increasing allele effect from PH82-2 was located on chromosome 4AL in the 2006 trial,explaining 3.8%of the phenotypic variation.
The LOD threshold for significance was 2.0.LOD scores are shown on the horizontal axes,and molecular markers and genetic distances(cM)are shown on the vertical axes.
4.Discussion
4.1.Location of QTL for relative starch granule types
In previous studies,a major QTL for starch granule size distribution was mapped on group 4 chromosomes in Triticeae [23–26].Although Qga.caas-4AL was located near the Wx-B1 locus,both parents in the current cross have the wild type non-waxy Wx-B1 allele,thus suggesting a different locus.No QTL was previously found on chromosomes 1DL,4AL and 7BL in common wheat,implying that the present QTL for content of A-type starch granules are new.However,the QTL were not consistently detected across environments and thus other populations or materials should be used in QTL or association mapping to validate these findings.
It was concluded that A-type and B-type starch granules were controlled by different genes[32].Although the relative quantity reflects the granule size distribution and is relatively easy to estimate.Percentage volume is not a suitable parameter for direct comparison of QTL conferring the two types of granules. Therefore,the specific diameters,numbers and weights of A-type and B-type starch granules should be examined in the future.
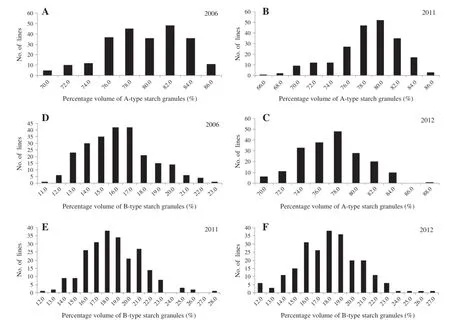
Fig.1–Frequency distribution of percentage volumes of A-type and B-type starch granules in the RIL population from PH82-2/ Neixiang 188 in three years.
4.2.QTL for starch properties
Starch granule size and RVA parameters are important factors in determining starch function.In a previous study,RVA parameters were mapped with the same RIL population[31]. Compared to the previous results,Qga.caas-1DL was located near QTL for sedimentation value and mixograph parameters and the marker for Dx5+Dy10,where a QTL for palate, stickiness and smoothness of Chinese dry noodle was also mapped[33],indicating that these parameters are related to each other and may have pleiotropic effects on noodle quality.The QTL for both starch properties and dough tolerance may contribute to quality improvement.In addition,Batey et al.[24]mapped a QTL for peak viscosity on chromosome 7BL in the same interval as Qga.caas-7BL. Therefore,content of A-starch granules is closely related to RVA parameters.

Table 3–QTL for content of A-type starch granules detected by composite interval mapping(CIM)in the RIL population from PH82-2/Neixiang 188.
4.3.Relationship of identified QTL with starch biosynthesis enzymes
Many enzymes are involved in starch biosynthesis.The genes for the key enzyme involved in amylose synthesis,granulebound starch synthase I(GBSS I),were identified on chromosomes 7AS,4AL and 7DS[34].It was reported that partially waxy and waxy wheats had less A-type starch granules and more B-type starch granules than non-waxy wheats[35].GBSS I was found to be responsible for the ratio of A-type to B-type starch granules[1].In this study,however,both PH82-2 andNeixiang 188 have wild type Wx-A1,Wx-B1 and Wx-D1 alleles and no QTL was found at these loci.
Soluble starch synthase may control starch granule size distribution in the early stage of grain filling[1].SS III and SS IV(soluble starch synthase)genes were located on common wheat homoeologous group 1 chromosomes[36,37],and it was reported that SS IV affected starch granule formation in Arabidopsis thaliana[38].In addition,the genes for ADP-glucose pyrophosphorylase low subunit,SS I and SS II,and branching enzymes(SBE I and SBE II)were located on homoeologous group 7 chromosomes[39–42].Starch branching enzymes were associated with A-type starch granules[7].Mutation of an isoamylase gene on barley chromosome 7H was found to have a dramatic effect on the number,structure and initiation of starch granules[43].However,map positions of these genes have not been determined.The possibility that these genes are candidate genes for Qga.caas-1DL and Qga.caas-7BL remains unknown.To understand the synthesis of starch granules, more traits,such as diameter,number and weight of starch granules should be examined.
Starch granule development can be divided into two stages,formation of the starch granule nuclei and development of the nuclei into A and B granules[7].The enzymes mentioned above may have different functions in the two phases,or there may be other enzymes regulating starch granule initiation and development.This should be verified by expression analysis of starch biosynthesis enzymes combined with dynamic changes during granule development.Exploring the mechanism of starch granule formation and the driving key enzymes will help develop cultivars with desirable quality characteristics through genetic engineering and markerassisted selection.
The isolation method has a significant effect on starch granules.We dried wet starch by 40°C treatment and lyophilization.Compared to high temperature drying,lyophilization produced more starch(1–35 μm)up to 90%or even 100%, with less peaks beyond 35 μm.The latter may be caused by aggregation of small starch granules that are difficult to separate after drying.
Despite significant environmental effects,starch granule size distribution can be genetically determined.Fine mapping and discovering novelgenes are feasible and fundamentalfor further study and eventually for breeding high quality cultivars.
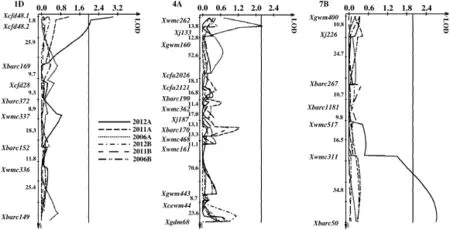
Fig.2–Logarithm of odds(LOD)contours obtained by composite interval mapping(CIM)for QTL on chromosomes 1D,4A and 7B for content of A-type starch granules in the PH82-2/Neixiang 188 RIL population.
Acknowledgments
The study was supported by the National Natural Science Foundation of China(31171547)and China Agriculture Research System(CARS-3-1-3).
R E F E R E N C E S
[1]C.H.Zhang,D.Jiang,F.L.Liu,J.Cai,T.B.Dai,W.X.Cao,Starch granules size distribution in superior and inferior grains of wheatis related to enzyme activities and their gene expressions during grain filling,J.Cereal Sci.51(2010)226–233.
[2]Y.A.Yin,J.C.Qi,W.H.Li,L.P.Cao,Z.B.Wang,Formation and developmental characteristics of A-and B-type starch granules in wheat endosperm,J.Integr.Agric.11(2012)73–81.
[3]A.D.Evers,The size distribution among starch granules in wheat endosperm,Starch-Starke 25(1973)303–304.
[4]H.S.Kim,K.C.Huber,Channels within soft wheat starch A-and B-type granules,J.Cereal Sci.48(2008)159–172.
[5]W.R.Morrison,H.Gadan,The amylose and lipid contents of starch granules in developing wheat endosperm,J.Cereal Sci. 5(1987)263–275.
[6]M.Peng,M.Gao,E.S.M.Abdel-Aal,P.Hucl,R.N.Chibbar, Separation and characterization of A-and B-type starch granules in wheat endosperm,Cereal Chem.76(1999) 375–379.
[7]M.Peng,M.Gao,M.Baga,P.Hucl,R.N.Chibbar, Starch-branching enzymes preferentially associated with A-type starch granules in wheat endosperm,Plant Physiol. 124(2000)265–272.
[8]D.B.Bechtel,I.Zayas,L.Kaleikau,Y.Pomeranz, Size-distribution of wheat starch granules during endosperm development,Cereal Chem.67(1990)59–63.
[9]L.Copeland,J.Blazek,H.Salman,M.C.Tang,Form and functionality of starch,Food Hydrocolloid 23(2009)1527–1534.
[10]M.L.Parker,The relationship between A-type and B-type starch granules in the developing endosperm of wheat, J.Cereal Sci.3(1985)271–278.
[11]S.V.Shinde,J.E.Nelson,K.C.Huber,Soft wheat starch pasting behavior in relation to A-and B-type granule content and composition,Cereal Chem.80(2003)91–98.
[12]B.P.Geera,J.E.Nelson,E.Souza,K.C.Huber,Composition and properties ofA-and B-type starch granules ofwild-type,partial waxy,and waxy soft wheat,Cereal Chem.83(2006)551–557.
[13]H.S.Kim,K.C.Huber,Physicochemical properties and amylopectin fine structures of A-and B-type granules of waxy and normal soft wheat starch,J.Cereal Sci.51(2010)256–264.
[14]S.Sahlstr?m,A.B.B?vre,E.Br?then,Impact of starch properties on hearth bread characteristics:I.Starch in wheat flour,J.Cereal Sci.37(2003)275–284.
[15]S.Sahlstr?m,A.B.B?vre,E.Br?then,Impact of starch properties on hearth bread characteristics:II.Purified A-and B-granule fractions,J.Cereal Sci.37(2003)285–293.
[16]H.N.Soh,M.J.Sissons,M.A.Turner,Effect of starch granule size distribution and elevated amylose content on durum dough rheology and spaghetti cooking quality,Cereal Chem. 83(2006)513–519.
[17]N.M.Edwards,J.E.Dexter,M.G.Scanlon,Starch participation in durum dough linear viscoelastic properties,Cereal Chem. 79(2002)850–856.
[18]Y.C.Huang,H.M.Lai,Noodle quality affected by different cereal starches,J.Food Eng.97(2010)135–143.
[19]Z.Chen,H.A.Schols,A.G.J.Voragen,Starch granule size strongly determines starch noodle processing and noodle quality,J.Food Sci.68(2003)1584–1589.
[20]S.Sahlstr?m,E.Br?then,P.Lea,K.Autio,Influence of starch granule size distribution on bread characteristics,J.Cereal Sci.28(1998)157–164.
[21]A.B.Soulaka,W.R.Morrison,The bread baking quality of six wheat starches differing in composition and physical properties,J.Sci.Food Agric.36(1985)719–727.
[22]H.Dengate,P.Meredith,Variation in size distribution of starch granules from wheat grain,J.Cereal Sci.2(1984)83–90.
[23]T.Howard,N.A.Rejab,S.Griffiths,F.Leigh,M.Leverington-Waite, J.Simmonds,C.Uauy,K.Trafford,Identification of a major QTL controlling the content of B-type starch granules in Aegilops,J. Exp.Bot.62(2011)2217–2228.
[24]I.L.Batey,M.J.Hayden,S.Cai,P.J.Sharp,G.B.Cornish,M.K. Morell,R.Apples,Genetic mapping of commercially significant starch characteristics in wheat crosses,Aust.J. Agr.Res.52(2001)1287–1296.
[25]A.Borém,D.E.Mather,D.C.Rasmusson,R.G.Fulcher,P.M. Hayes,Mapping quantitative trait loci for starch granule traits in barley,J.Cereal Sci.29(1999)153–160.
[26]G.Igrejas,B.Faucher,D.Bertrand,D.Guilbert,P.Leroy,G. Branlard,Genetic analysis of the size of endosperm starch granules in a mapped segregating wheat population,J.Cereal Sci.35(2002)103–107.
[27]Y.Zhang,Z.H.He,A.M.Zhang,M.van Ginkel,R.J.Pe?a,G.Y. Ye,Pattern analysis on protein properties of Chinese and CIMMYT spring wheat cultivars sown in China and CIMMYT, Aust.J.Agr.Res.57(2006)811–822.
[28]Q.Liu,Z.Gu,E.Donner,I.Tetlow,M.Emes,Investigation of digestibility in vitro and physicochemical properties of A-and B-type starch from soft and hard wheat flour,Cereal Chem.84(2007)15–21.
[29]S.H.Park,O.K.Chung,P.A.Seib,Effects of varying weight ratios of large and small wheat starch granules on experimental straight-dough bread,Cereal Chem.82(2005)166–172.
[30]A.R.Gilmour,B.R.Cullis,A.Verbyla,Accounting for natural and extraneous variation in the analysis of field experiments, J.Agric.Biol.Environ.Stat.2(1997)269–293.
[31]Y.L.Zhang,Y.P.Wu,Y.G.Xiao,J.Yan,C.X.Ma,X.C.Xia,Z.H. He,QTL mapping for milling,gluten quality,and flour pasting properties in a recombinant inbred line population derived from a Chinese soft×hard wheat cross,Crop Pasture Sci.60 (2009)587–597.
[32]F.L.Stoddard,Genetics of wheat starch B-granule content, Euphytica 112(2000)23–31.
[33]J.L.Zhao,M.S.Chen,Y.M.Ma,R.J.Li,Y.P.Ren,Q.Q.Sun,S.S.Li, QTL mapping for quality traits of Chinese dry noodle,Agric. Sci.China 8(2009)394–400.
[34]T.Nakamura,M.Yamamori,H.Hirano,S.Hidaka,Identification of three Wx proteins in wheat(Triticum aestivum L.),Biochem. Genet.31(1993)75–86.
[35]A.C.Bertolini,E.Souza,J.E.Nelson,K.C.Huber,Composition and reactivity of A-and B-type starch granules of normal, partial waxy,and waxy wheat,Cereal Chem.80(2003) 544–549.
[36]Z.Li,G.Mouille,B.Kosar-Hashemi,S.Rahman,B.Clarke,K.R. Gale,R.Appels,M.K.Morell,The structure and expression of the wheat starch synthase I gene.Motifs in the expressed gene define the lineage of the starch synthase III gene family, Plant Physiol.123(2000)613–624.
[37]M.Leterrier,L.D.Holappa,K.E.Broglie,D.M.Beckles,Cloning, characterisation and comparative analysis of a starch synthase IV gene in wheat:functional and evolutionary implications,BMC Plant Biol.8(2008)98.
[38]I.Roldán,F.Wattebled,M.Mercedes Lucas,D.Delvallé,V. Planchot,S.Jiménez,R.Pérez,S.Ball,C.D'hulst,Mérida,The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation,Plant J. 49(2007)492–504.
[39]C.Ainsworth,M.Tarvis,J.Clark,Isolation and analysis of a cDNA clone encoding the small subunit of ADP-glucose pyrophosphorylase from wheat,Plant Mol.Biol.23(1993)23–33.
[40]Z.Y.Li,X.S.Chu,G.Mouille,L.L.Yan,B.Kosar-Hashemi,S.Hey, J.Napier,P.Shewry,B.Clarke,R.Appels,M.Morell,S.Rahman, The localization and expression of the class IIstarch synthases of wheat,Plant Physiol.120(1999)1147–1155.
[41]K.F.McCue,W.J.Hurkman,C.K.Tanka,O.D.Anderson, Starch-branching enzymes Sbe1 and Sbe2 from wheat (Triticum aestivum cv.Cheyenne):molecular characterization, development expression,and homoeologue assignment by differential PCR,Plant Mol.Biol.Rep.20(2002)191–192.
[42]M.Yamamori,T.Endo,Variation of starch granule proteins and chromosome mapping of their coding genes in common wheat,Theor.Appl.Genet.93(1996)275–281.
[43]R.A.Burton,H.Jenner,L.Carrangis,B.Fahy,G.B.Fincher,C. Hylton,D.A.Laurie,M.Parker,D.Waite,S.van Wegen,T. Verhoeven,K.Denyer,Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity, Plant J.31(2002)97–112.
*Corresponding author.Tel.:+86 10 82108741.
E-mail address:zhangyan07@caas.cn(Y.Zhang).
Peer review under the responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
- The Crop Journal的其它文章
- Identification and functional analysis of miRNAs in developing kernels of a viviparous mutant in maize
- Enhanced tolerance to drought in transgenic rice plants overexpressing C4photosynthesis enzymes
- Molecular approaches unravel the mechanism of acid soil tolerance in plants
- Enhanced tolerance to drought in transgenic rice plants overexpressing C4 photosynthesis enzymes
- HrcQ is necessary for Xanthomonas oryzae pv.oryzae HR-induction in non-host tobacco and pathogenicity in host rice
- Isolation and characterization of an isoamylase gene from rye

