Polyphenolic extract of Sorghum bicolor grains enhances reactive oxygen species detoxificatio in N-nitrosodiethylamine-treated rats
Tofeek O.Ajioye Yesirt O.Komolfe Oyelol B.Oloyee Simit M.Ogunoe Morim D.Aeoye Irhim O.Aulslmi Quri O.Nurueen
a Antioxidants,Free Radicals and Toxicology Research Laboratory,Biochemistry and Nutrition Unit,Department of Chemical Sciences,Fountain University,Osogbo,Nigeria
b Nutritional Biochemistry Research Laboratory,Biochemistry and Nutrition Unit,Department of Chemical Sciences,Fountain University,Osogbo,Nigeria
c Industrial and Environmental Chemistry Unit,Department of Chemical Sciences,Fountain University,Osogbo,Nigeria
d Phytomedicine,Toxicology and Reproductive Biochemistry Research Laboratory,Department of Biochemistry,University of Ilorin,Ilorin,Nigeria
Abstract Reactive oxygen species detoxificatio potentials of Sorghum bicolor polyphenolic extract was investigated in the liver of N-nitrosodiethylaminetreated rats.Male rats,weighing (135±5.5)g were completely randomized into 7 groups (A-G) of fie rats each.Rats in C,D,E and F were administered orally once daily at 24-h interval for 7 d with 500,125,250 and 500 mg/kg body weight of polyphenolic extract of S.bicolor,respectively.Group G was given 100 mg/kg body weight of vitamin C.On the sixth day,groups B,D,E,F and G were administered with 100 mg/kg body weight N-nitrosodiethylamine (NDEA).Group A,which served as the control was treated like the test groups except,that the animals received distilled water only.Reactive oxygen species detoxifying enzymes(superoxide dismutase,catalase,glutathione peroxidase,glutathione reductase and glucose 6-phosphate dehydrogenase) activities were significantl (P <0.05) induced by S.bicolor. These inductions significantl(P <0.05) attenuated the NDEA-mediated decrease in reactive oxygen species detoxifying enzymes and compared favourably with vitamin C.NDEA-mediated elevation in the concentrations of oxidative stress biomarkers;malondialdehyde,conjugated dienes,lipid hydroperoxides,protein carbonyl and percentage DNA fragmentation were significantl (P <0.05)lowered by S.bicolor polyphenolic extract.Overall,the results obtained from this study revealed that the polyphenolic extract of S.bicolor grains enhanced the detoxificatio of reactive oxygen species in NDEA-treated rats.The polyphenols also prevented the peroxidation of lipid,oxidation of proteins as well as fragmentation of DNA component in the liver of rats and hence gave the evidence of possible prophylactic potentials of S.bicolor grains.
Keywords:Antioxidant;Sorghum bicolor;Reactive oxygen species;N-nitrosodiethylamine;Detoxification Polyphenols
1.Introduction
N-nitrosodiethylamine (NDEA) is a potent hepatocarcinogenic nitrosamine present in tobacco smoke,ground water with high level of nitrates,cheddar cheese,cured and fried meals,soya beans,alcoholic beverages,occupational settings,cosmetics,agricultural chemicals and pharmaceutical agents[1,2].In the liver,cytochrome P450(CYP2E1) activatesNnitrosodiethylamine [3]to form electrophilic and reactive oxygen species[4],which causes oxidative damage leading to cytotoxicity,carcinogenicity and mutagenicity[5,6].
Oxygen-derived radicals known as reactive oxygen species(ROS)include the highly reactive superoxide(O2-·),hydroxyl(·OH) and peroxyl (RO2·) as well as non-radicals such as hydrogen peroxide (H2O2) and peroxynitrite (ONOO-) [7].The productions of these reactive species(O2-·,·OH,RO2·,H2O2and ONOO-) are usually in response to endogenous and exogenous stimulus[8].Regardless of the origin,increased ROS production or oxidative stress results to either activation of specifi signal transduction pathways or damage to cellular components resulting to adaptive and maladaptive molecular responses,respectively [9].Cell death arising from carbonylation of protein,peroxidation of lipids and fragmentation of DNA are consequential effects of ROS-induced oxidative stress[10].Furthermore,excessive ROS production has been reported to stimulate oncogenesisviaalterations in redox regulated signaling pathways suggesting that the redox state plays a critical role in signal transduction,cellular proliferation,differentiation and apoptosis[11,12].
Antioxidant defense arsenal in liver cells is responsible for the detoxificatio of ROS and repair damage resulting from ROS[13].Thus,catastrophic free radical events such as lipid peroxidation,protein oxidation and fragmentation of DNA are rarely the cause of cell death in realisticin vivocondition[14].However,when the antioxidant defense arsenals are overwhelmed,ROS cause direct damage to proteins,lipids,and nucleic acids,leading to cell death[15].Consumptions of dietary antioxidants complement the cellular defense system to prevent oxidative damage to cellular macromolecules.Recently,we have reported the antioxidants and cytoprotective activities of some dietary medicinal plants and alluded the protective role to the polyphenolic and flvonoid constituents of the plants[16-19].Sorghum bicolor grains represent one of the common cereals that is widely consumed in Nigeria because of the good amount of antioxidant,carbohydrate and protein contents.
Sorghum(S.bicolor(L.)Moench)is an important staple food in developing countries of the semi-arid tropics.It is the world’s fift most important cereal,with higher protein content than corn [20].It is particularly important as human food resource and folk medicine in Asia and Africa.Studies have shown that sorghum has antioxidant activity,anti-carcinogenic effects,antimutagenic effects,cholesterol-lowering effects and can reduce the risk of cardiovascular disease[21,22].Most of these activities have been shown to be due to the presence of numerous flvonoids,phenolics and anthocyanins in sorghum.Recently,Ajiboye et al.[23]reported thatS.bicolorgrains extract protected NDEA-induced oxidative stress in rat microsomesin vitro[23].
Phytochemical constituents of sorghum include phenolic compounds,polyflvonols and thiols,anthocyanins and tannins.Several flvonoids have been identifie and characterized in sorghum over the years.Recently,3-deoxyanthocyanidin,flvone,and flvanone levels were reported in red/black sorghum genotypes [22].Despite these myriad studies onS.bicolor,there is little or no literature that describes the effects of the polyphenolic rich extract ofS.bicolorgrains on ROS detoxifying enzymesin vivo.This study thus investigates the capability of polyphenolic rich-extract ofS.bicolorgrains to promote ROS detoxificatio in the liver of NDEA-treated rats.
2.Materials and methods
2.1.Materials
2.1.1.Plant materials
Red variety ofS.bicolorgrains were obtained from Igbona market,Osogbo,Nigeria and was authenticated by Prof.F.A.Oladele of the Department of Plant Biology,University of Ilorin,Ilorin,Nigeria,where a voucher specimen was deposited in the herbarium.
2.1.2.Experimental animals
Two-month-old,healthy male albino rats (Rattus norvegicus) of Wistar strain,weighing (135±5.5)g were obtained from Animal House of the Department of Veterinary Physiology,Biochemistry and Pharmacology,University of Ibadan,Nigeria.They were kept in clean plastic cages contained in wellventilated house conditions with free access to feeds(Capfeed Ltd.,Osogbo,Nigeria) and tap water.The animals were used according to the Guidelines of National Research Council Guide for the Care and Use of Laboratory Animals[24]and in accordance with the principles of Good Laboratory Procedure(GLP)[25].
2.1.3.Chemicals and assay kits
Diphenylamine 5,5′-Dithio-bis(2-nitrobenzoic acid),guanidine hydrochloride,andN-ethyl-maleimide (NEM) were procured from Research Organics,Cleveland,Ohio,USA.Superoxide dismutase (SOD),glutathione peroxidase (GSHPx),glutathione reductase(GSH-Red)and glucose 6-phosphate dehydrogenase(Glc 6-PD)were products of Randox Laboratories Ltd.Co.,Antrim,United Kingdom.All other reagents used were supplied by Sigma-Aldrich Inc.,St.Louis,USA.
2.2.Methods
2.2.1.Preparation of polyphenolic rich-extract of Sorghum bicolor grains
Finely groundS.bicolorgrains were defatted exhaustively in hexane for 24 h with constant shaking.The residue was re-extracted exhaustively with methanol for 48 h.The filtere extract was concentrated under reduced pressure using rotatory evaporator(R-200,BUCHI,Flawil,Switzerland)and kept frozen till further use.
2.2.2.Animal treatment
Thirty-fie male rats were completely randomized into seven groups (A-G) of 5 animals each.Rats in groups C,D,E and F were administered orally once daily at 24-h interval for 7 d with 500,125,250 and 500 mg/kg bodyweight of polyphenolic extract ofS.bicolorgrains,respectively.Group G was given 100 mg/kg bodyweight of vitamin C.On the sixth day,groups B,D,E,F and G were administered with 100 mg/kg bodyweight of NDEA.Group A,which served as the control was treated like the test groups except,that the animals received distilled water only.
2.2.3.Preparation of serum and tissue homogenates
The rats were sacrifice 24 h after their last daily doses using the anesthetic method described by Yakubu et al.[26].Under diethyl ether anesthesia,rats were made to bleed through their cut jugular veins(slightly displaced to prevent blood from being contaminated with interstitial fluid into centrifuge tubes.The blood samples were allowed to clot for 15 min and centrifuged at 33.5×gfor 15 min to obtain the sera.The sera were frozen and used within 12 h of preparation for the biochemical assay.Liver excised from the animals were blotted in tissue paper,cut thinly with sterile scalpel blade and then homogenized in icecold 0.25 mol/L sucrose solution (1:5,w/v).The homogenates were centrifuged at 800×gat 4°C for 10 min to obtain the supernatant that was kept frozen at-20°C before being used for the various biochemical assays.
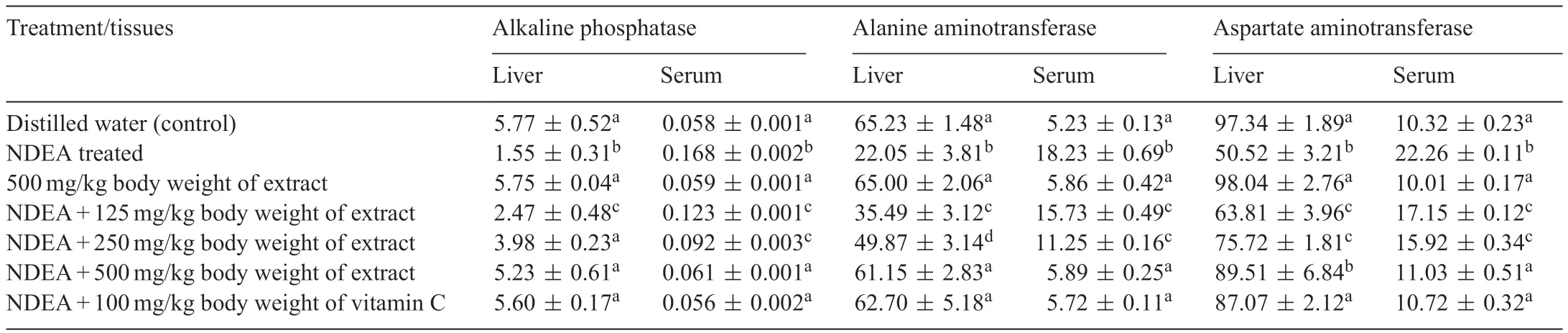
Table 1 Specifi activities of hepatic marker enzymes following the administration of polyphenolic extract of Sorghum bicolor to N-nitrosodiethylamine-treated rats.
2.2.4.Biochemical assay
The activities of alkaline phosphatase (ALP),alanine and aspartate aminotrasferases(ALT and AST)were determined as described by Wright et al.[27]and Bergmeyer et al.[28,29],respectively.SOD,Catalase,GSH-Px,GSH-red and Glc 6-PD activities were assayed according to the procedures described by Misra and Fridovich[30],Beers and Sizers[31],Rotruck et al.[32],Mavis and Stellwagen [33]and Kornberg and Horecker[34],respectively.The concentration of protein carbonyl in the liver homogenates was determined according to the procedure described by Levine et al.[35].The concentrations of conjugated dienes,lipid hydroperoxides and malondialdehyde were assessed according to the procedure described by Bus et al.[36].The quantity of fragmented DNA was quantifie according to the procedure described by Burton[37].
2.2.5.Statistical analysis
Results were expressed as the mean of fie determinations±SD.Analysis of variance (ANOVA) followed by Tukey-Kramer test for differences between means was used to detect any significan differences (P<0.05) between the treatment groups in this study using StatPlus,2011(AnalystSoft Inc.,Alexandria,VA,USA).
3.Results
3.1.Hepatocellular enzymes
Administration of NDEA alone significantl (P<0.05)reduced the activities of ALP,ALT and AST in the liver with corresponding increase in the activities of these enzymes(ALP,ALT and AST)in the serum(Table 1).This trend was reversed when the polyphenolic extract ofS.bicolorgrains at various doses were administered to NDEA-treated rats,as the activities of the liver and serum enzymes compared favourably(P>0.05)with that of the control and vitamin C pretreated groups(Table 1).
3.2.Reactive oxygen detoxifying enzymes
ROS detoxifying enzymes(SOD,CAT,GSH-Px,GSH-Red and Glc 6-PD) were significantl (P<0.05) reduced following the administration of 100 mg/kg body weight of NDEA(Table 2).In addition to the increase in the activities of ROS detoxifying enzymes following the administration of the polyphenolic extract ofS.bicolorgrains alone,the extract completely attenuated NDEA-mediated decrease in these enzymes(Table 2).
3.3.Non-enzymatic antioxidants
The level of the non-enzymatic antioxidant glutathione reduced (GSH) was significantl reduced following the administration of NDEA.While the concentration of peroxidised glutathione (GSSG) in the liver increased significantl(P<0.05),GSH:GSSG ratio decreased significantl followingthe administration of NDEA (Table 3).The polyphenolic rich extract ofS.bicolorgrains significantl (P<0.05)reversed the NDEA-mediated alterations in the levels of these non-enzymatic antioxidants(Table 3).
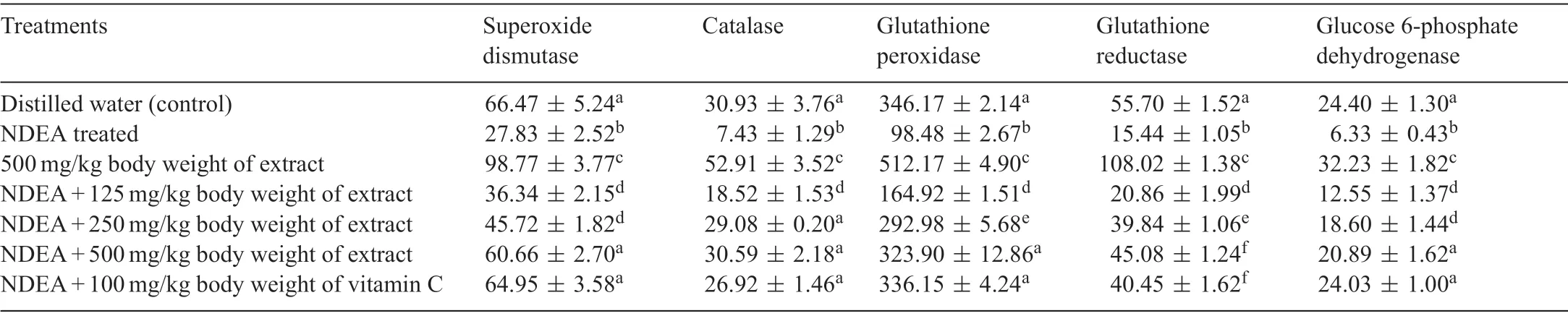
Table 2 Specifi activities of antioxidant enzymes following the administration of polyphenolic extract of Sorghum bicolor to N-nitrosodiethylamine-treated rats.

Table 3 Levels of non-enzymic antioxidants following the administration of polyphenolic extract of Sorghum bicolor to N-nitrosodiethylamine-treated rats.
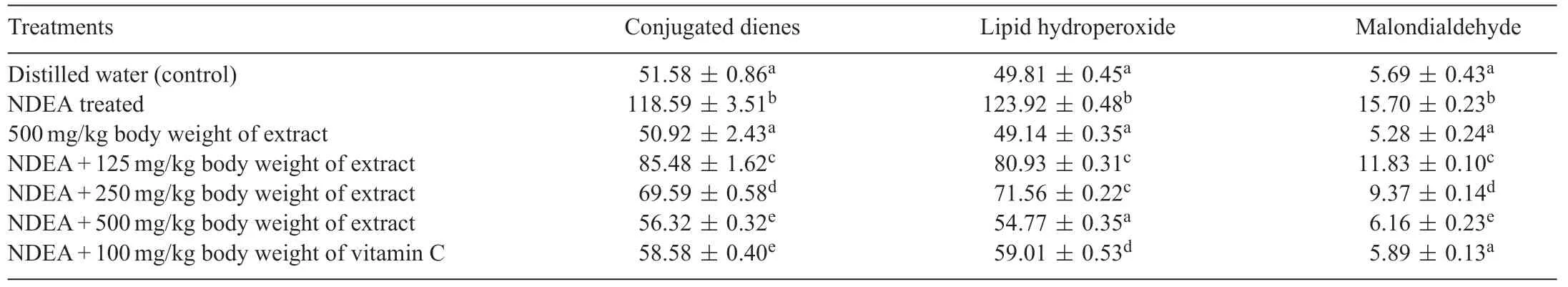
Table 4 Levels of lipid peroxidised products following the administration of polyphenolic extract of Sorghum bicolor to N-nitrosodiethylamine-treated rats.
3.4.Lipid peroxidation
NDEA administration resulted to significan (P<0.05)increase in the levels of lipid peroxidation products(conjugated dienes,lipid hydroperoxides and malondialdehyde)in the liver of rats(Table 4).The NDEA-mediated increase in the lipid peroxidation products were significantl (P<0.05)reduced in the liver of rats by the polyphenolic extract ofS.bicolorgrains.
3.5.Protein oxidation
Carbonylation of protein resulting from the oxidation of liver protein significantl (P<0.05)increased in the liver of NDEAtreated rats.Although,treatment of rats with only polyphenolic rich extract ofS.bicolorgrains produced no change in the level of protein carbonyl,it significantl (P<0.05)reduced the protein carbonyl level in the liver of NDEA-treated rats(Table 5).
3.6.DNA fragmentation
The extent of DNA damage in the liver as assessed by the measurement of DNA fragmentation in NDEA-treated rats increased significantl (P<0.05).S.bicolorsignificantl(P<0.05)reversed the NDEA-mediated increase in DNA fragmentation and it compared significantl (P<0.05) with the control and vitamin C treated groups(Table 5).
4.Discussion
Dietary antioxidants elicit protective activities by interacting with biomolecules at cellular and molecular levels to induce cytoprotective enzymes or inhibits/inactivates those involve in carcinogen activation.In this study,the capability of the polyphenolic extract ofS.bicolorgrains to enhance reactive oxygen species detoxificatio in the liver of NDEA-treated rats was investigated.
4.1.Hepatocellular enzymes
Alkaline phosphatase is a marker enzyme for plasma membrane such that any alteration in the level of this enzyme showscompromise of the integrity of the plasma membrane[38].The significan reduction in specifi activity of ALPin the liver of rats with a corresponding increase in serum is an indication of loss ofintegrity in the liver plasma membrane.The decrease in specifi activity of ALP in the liver of rats might have resulted from peroxidation of polyunsaturated fatty acids of the plasma membrane by ROS (O2-·,·OH,RO2·,H2O2and ONOO-) generated during NDEA metabolism [4].The capability of polyphenolic extract ofS.bicolorgrains to prevent NDEA-mediated alteration in ALP could be attributed to free radical and ROS scavenging capability ofS.bicolormade possible by flvonoids,phenolics,anthocyanins and thiols present inS.bicolorgrain[21].
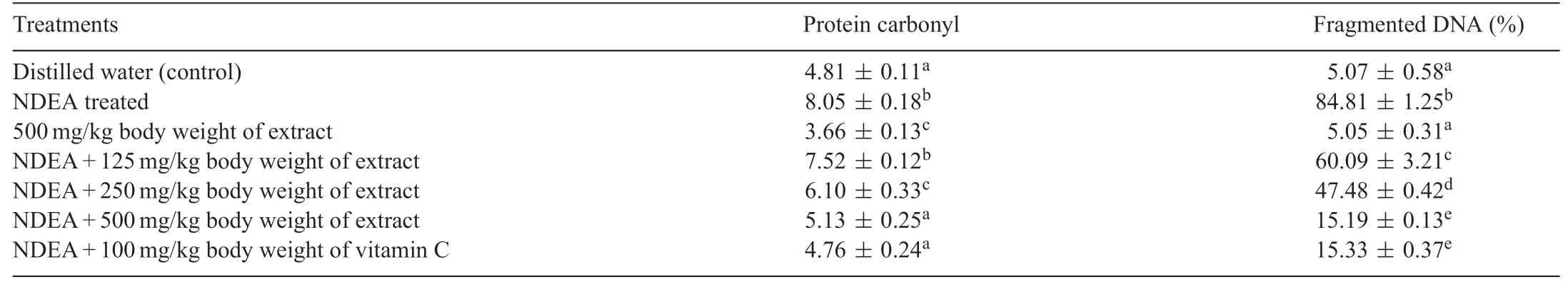
Table 5 Levels of protein carbonyl and fragmented DNA following the administration of polyphenolic extract of Sorghum bicolor to N-nitrosodiethylamine-treated rats.
The reduction in specifi activities of ALT (cytosolic) and AST(cytosolic and mitochondrial)in the liver of DEN treated rats is not surprising,as the pattern of alterations on ALP revealed that integrity of the plasma membrane had been compromised.Damage to plasma membrane will consequentially lead to leakage of cellular cytosolic content to the external milieu.The capability of the extract to reverse this trend in a manner similar to vitamin C suggests antioxidant potential of the extract.This amelioration may be adduced to capability of the extract to scavenge ROS (O2-·,·OH,RO2·,H2O2and ONOO-)generated during NDEA metabolism[4].
4.2.Reactive oxygen detoxifying enzymes
Oxidative damage to cellular macromolecules(lipid,protein,DNA,etc.) arising from redox imbalances is normally counteracted by ROS detoxifying enzymes (SOD,CAT,GSH-Px,GSH-Red and Glc 6-PD) [16].The reduction in the specifi activities of these ROS detoxifying enzymes could have resulted from the excessive mobilization of antioxidant enzymes towards the detoxificatio of ROS (O2-·,·OH,RO2·,H2O2and ONOO-) during NDEA carcinogenesis [4].These reductions could lead to uncontrolled oxidative attack on the cellular macromolecules resulting to oxidative damage and cell death.Similar reduction in the activities of these enzymes(SOD,CAT,GSH-Px and GSH-Red) were reported to be due to the excessive generation of ROS during NDEA hepatocarcinogenesis [1,39,40].Thus,the significan attenuation of NDEA-mediated reduction in specifi activities of ROS detoxifying enzymes(SOD,CAT,GSH-Px,GSH-Red and Glc 6-PD) by the polyphenolic rich extract ofS.bicolorgrains might have resulted from capability of the extract to scavenge ROS generated during NDEA metabolism.It might also have resulted from the capability ofS.bicolorto induce ROS detoxifying enzymes.Reports have shown attenuation of NDEA-mediated decrease in the antioxidant enzymes by medicinal plants and plant components[5,41].
4.3.Non-enzymatic Antioxidant
The significan (P<0.05) reduction in the level of GSH,a non-enzymatic antioxidant playing complementary role in prevention of oxidative damage resulting from ROS generated during NDEA metabolism might have resulted from the depletion of GSH-Px and GSH-Red,as they have direct relationship with GSH[42].Conversely,NDEA-mediated increase in the level of GSSG might have resulted from the oxidation of GSH or mobilization of GSH towards the production of GSH-Px.The reduction in GSH:GSSG ratio following the administration of NDEA indicates that the liver cell is prone to oxidative attack.Thus,the preservation of the levels of GSH,high GSH:GSSG and low GSSG in the liver of NDEA-treated rats by the polyphenolic rich-extract ofS.bicolorgrains shows the possible antioxidant potentials.
4.4.Lipid peroxidation
Elevation in the status of lipid peroxidation in liver during NDEA treatment has been reported [43,44].Thus,the significan increase in the levels of lipid peroxidation products(conjugated dienes,lipid hydroperoxides and malondialdehydes) shows indiscriminate oxidative assaults on the cellular lipids.These increase(most especially conjugated dienes)could result to mutation [45].The capability ofS.bicolorextract to reverse the NDEA-mediated increase in conjugated dienes,lipid hydroperoxide and malondialdehyde might have resulted from the ROS scavenging activity of the extract.It might have also resulted from the capability of the extract to promote the detoxificatio (through the induction of antioxidant enzymes)of ROS,which could cause the peroxidation of polyunsaturated fatty acids of plasma membrane.Pradeep et al.[6]also reported similar reduction in level of lipid peroxidised products following the administration of Silymarin to NDEA-treated rats.
4.5.Protein oxidation
Protein carbonyl content,an indicator ofirreversible oxidative damage leading to protein oxidation[46],may have lasting detrimental effects on cells and tissues [47].Thus,the signifi cant increase in protein carbonyl,a marker of protein oxidation in NDEA-treated rat could have resulted from the oxidation of protein by the free radicals and ROS generated during NDEA metabolism.The attenuation of NDEA-mediated increase in the level of protein carbonyl by the polyphenolic extract ofS.bicolorgrains further shows possible ROS scavenging and its capability to promote the detoxificatio of ROSviathe induction of antioxidant enzymes.
4.6.DNA fragmentation
Oxidative stress and accumulation of calcium ion have been reported to mediate DNA fragmentation [48].This damage,which usually results from OH.-,can lead to either arrest or induction of transcription,induction of signal transduction pathways,replication errors and genomic instability,all of which are associated with carcinogenesis [49].Thus,the significan increase in the level of fragmented DNA in the liver of NDEAtreated rat shows the genotoxicity arising from NDEA treatment.It also denotes possible initiation of carcinogenesis.The reduction in the level of fragmented DNA in the liver of NDEA-treated rat by the polyphenolic extract ofS.bicolorgrains shows the antioxidants and antigenotoxic role of the extract.
5.Conclusion
The results from this study show that the polyphenolic extract ofS.bicolorgrains enhanced the detoxificatio ofNnitrosodiethylamine possibly by enhancing the activities of reactive oxygen species detoxifying enzymes,thus preventing the oxidation and fragmentation of cellular macromolecules such as DNA,lipids and proteins.Hence,the consumption ofS.bicolorgrains as staple food is encouraged because ofits prophylactic potentials.
Acknowledgement
Part of this paper was presented at the 8th Congress of Toxicology in Developing Countries(8CTDC)under the auspices of International Union of Toxicology(IUTOX)September 10-13,2012:at Centara Grand at Central Ladprao,Bangkok,Thailand.
- 食品科學(xué)與人類健康(英文)的其它文章
- Black tea in chemo-prevention of cancer and other human diseases
- Changes in physicochemical properties of proteins in Kayserian Pastirma made from the M.semimembranosus muscle of cows during traditional processing
- Protective role of concomitant administration of fla lignan concentrate and omega-3-fatty acid on myocardial damage in doxorubicin-induced cardiotoxicity
- Epigenetic origins of metabolic disease:The impact of the maternal condition to the offspring epigenome and later health consequences
- GUIDE FOR AUTHORS
- Natural products for cancer prevention associated with Nrf2-ARE pathway

