Oxidative Desulfurization of Model Sulfur Compound by Potassium Ferrate in the Presence of Phosphomolybdic Acid Catalyst
Liu Yanxiu; Song Hua; Zhang Wenchao
(1. Provicial Key Laboratory of Oil and Gas Chemical Engineering, College of Chemistry and Chemical Engineering, Northeastern Petroleum University, Daqing 163318; 2. Daqing Petrochemical Engineering Co., Ltd., Daqing 163714)
Oxidative Desulfurization of Model Sulfur Compound by Potassium Ferrate in the Presence of Phosphomolybdic Acid Catalyst
Liu Yanxiu1; Song Hua1; Zhang Wenchao2
(1. Provicial Key Laboratory of Oil and Gas Chemical Engineering, College of Chemistry and Chemical Engineering, Northeastern Petroleum University, Daqing 163318; 2. Daqing Petrochemical Engineering Co., Ltd., Daqing 163714)
In this work, the removal of thiophene from simulated oil has been studied by using the adsorption, extraction and oxidation/adsorption methods, respectively. In the adsorptive desulfurization process, different commercial adsorbents were used to eliminate thiophene at ambient pressure and mild temperature, and the results showed that carbon powder had the best adsorption ability. In the extractive desulfurization process, the best desulfurization result was obtained when DMF is used. In the oxidative/adsorptive desulfurization procedure using synthesized potassium ferrate as the oxidant and phosphomolybdic acid solution as the catalyst, thiophene was oxidized and removed from hydrocarbons in combination with active carbon adsorption, and the residual sulfur content of simulated oil could be reduced to 15.3 mg/L from the original level of 200 mg/L, with the desulfurization rate reaching 92.3%.
oxidative desulfurization; phosphomolybdic acid; potassium ferrate
1 Introduction
In recent years, non-hydrogenation desulfurization technologies (NHDS) have been studied extensively. Removal of organosulfur compounds by extraction[1-2]or adsorption[3-4]is economically viable and simple in operation. In this paper the removal of thiophene from the simulated oil by different commercial solvents (absorbents) was investigated. The test result shows that it is difficult to achieve deep desulfurization. At present, the oxidative desulfurization (ODS)[5-7]was considered to be one of the promising deep desulfurization processes thanks to its mild reaction conditions. Sulfur compounds are known to be slightly more polar than hydrocarbons of similar structure. However, the oxidized sulfur compounds such as sulfones or sulfoxides are substantially more polar than sulfides. This phenomenon permits the selective removal of sulfur compounds from hydrocarbons by means a combination process of selective oxidation and solvent extraction or solid adsorption[8].
The Fe (VI) species are strong oxidizing agents which can be evidenced by the reduction potentials of reactions (1) and (2) in acidic and alkaline solutions, respectively[9]. However, K2FeO4does not dissolve in a majority of organic solvents and the oxidation of thiophene in a mixture of ferrate with organic compounds is extremely difficult. Studies show that potassium ferrate (VI) in conjunction with an appropriate heterogeneous catalyst in non-aqueous media can efficiently oxidize a number of organic compounds. For example, the oxidation of benzyl alcohol to benzaldehyde at 100% yield was achieved without over-oxidation to benzoic acid by K2FeO4in cyclohexane using K10 clay as a catalyst[10]. Our workgroup studied the catalyst on oxidizing benzyl alcohol by potassium ferrate in organic solvent and obtained certain results[11], showing that adding some aqueous acid solutions could efficiently catalyze the reaction. In this paper, this technology is thereby used in the oxidative desulfurization process.
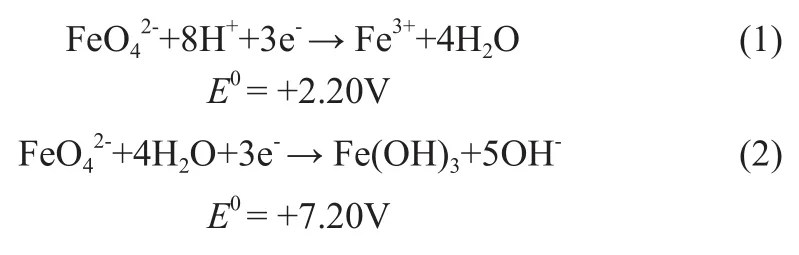
2 Experimental
2.1 Materials
Thiophene (Aldrich) was dissolved into cyclohexane to make a stock solution with a sulfur content of 200 mg/L. Potassium ferrate (K2FeO4) was synthesized by our laboratory. Activated carbon was obtained from the Tangfeng Industrial Protective Products Co., LTD (Tangshan, China). Silicone and zeolite Y were supplied by the Fushun No. 3 Catalyst Manufacturing Plant. DMF, methanol, ethanol and cyclohexane, which were all AR regents, were obtained from the Tianjin Zong-Hengxing Industry and Trade Co., Ltd.
2.2 Desulfurization Methods
2.2.1 Adsorption procedures
Five mL of sulfur-containing solution was added to one gram of adsorbent placed in a 20-mL vial. The vial was then placed in a thermostatic bath at 30 ℃ and shaken at 150 r/min for 20 min. In each case, a control vial with the same amount of solution but without any adsorbent was also placed in the shaker. The liquid was analyzed by gas chromatography and the amount of sulfur removed was calculated by comparing the sulfur content in the solution with that in the control vial.
2.2.2 Extraction procedures
Five mL of sulfur-containing solution was mixed with 5 mL of solvent, and the system was then placed in a thermostatic bath at 15 ℃ and shaken at 150 r/min for 20 min. Samples were taken and laid aside for 15 min. In each case, a control vial with the same amount of solution but without any solvent was also placed in the shaker. The liquid was analyzed by gas chromatography and the amount of sulfur removed was calculated by comparing the sulfur content in the solution with that in the control vial.
2.2.3 Oxidation/adsorption experiments
In a typical run, the water bath was first heated up and maintained at the desired reaction temperature. An appropriate volume of simulated oil sample was placed in a glass reactor equipped with a thermostatic bath, and a specified amount of potassium ferrate and phosphomolybdic acid solution were added into the reactor. After cooling and filtering, the simulated oil sample was taken out for conducting the adsorption procedure as described in section 2.2.1.
2.3 Analysis method
The sulfur compounds in the feed and product were analyzed by a Shimadzu GC-14C gas chromatograph equipped with a flame photometric detector (FPD). A capillary column PH-1 ms (60 m×0.25 mm of i.d.) was used, and the column temperature was at first maintained at 60 ℃ for 3 min and was then increased at a rate of 10 ℃/min to 270 ℃, which was again maintained for 5 min.
3 Results and Discussion
3.1 Adsorption procedures
Six commercial absorbents were used in this study. Table 1 compares the desulphurization effects achieved by the absorbents. It can be seen from Table 1 that the powdered activated carbon, having the biggest specific area and pore volume, had the best adsorption capacity for the sulfur after reducing the sulfur content to 44.4 mg/L from 200 mg/L, with the desulphurization rate reaching 77.8%. The adsorptive desulphurization activity of adsorbents decreased in the following order: powdered activated carbon>granular activated carbon>zeolite Y>silicone.
3.2 Extraction procedures
The desulphurization results for the different solvents are shown in Table 2. The best result was obtained when DMF was used in the experiments. The data indicate that the solubility decreased in the following order: DMF>methanol>ethanol>NaOH solution. It can be dis-covered that DMF is a kind of aprotic solvents, which is different from other solvents studied.
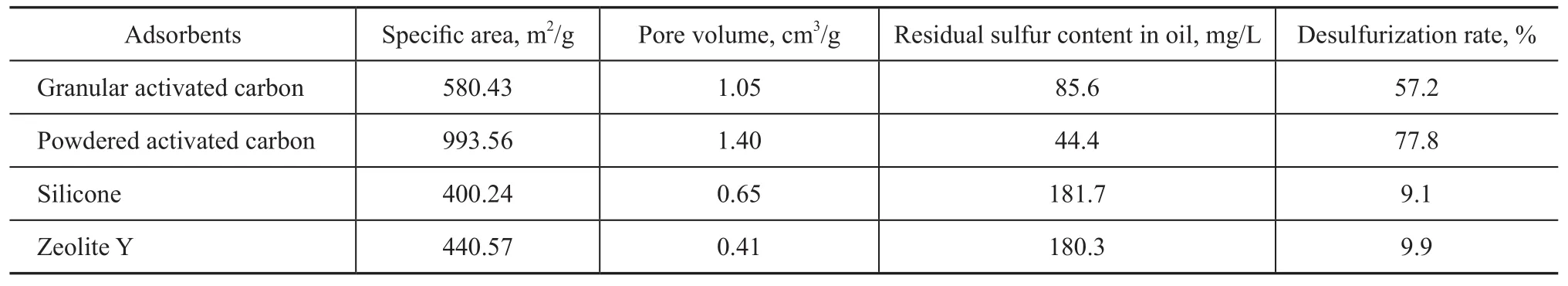
Table 1 Summary of thiophene capacities of various adsorbents studied
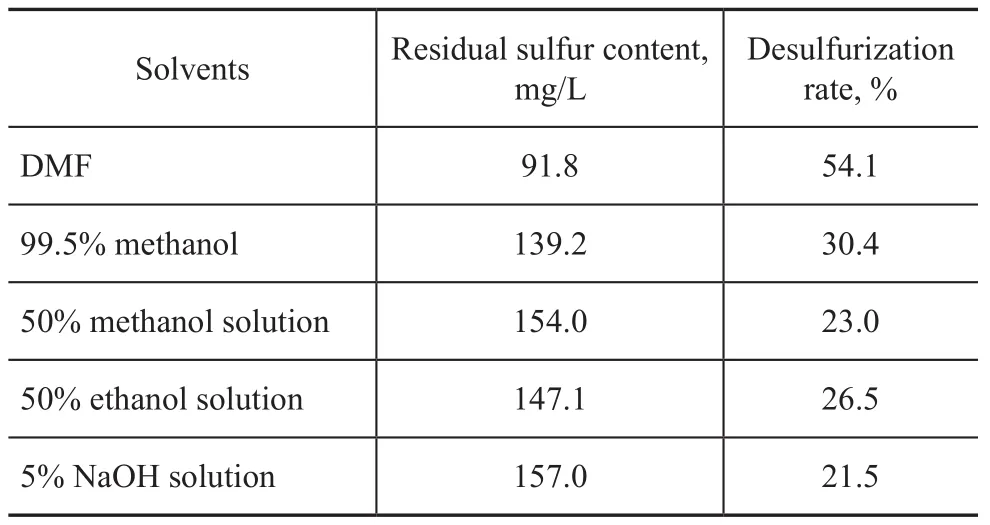
Table 2 Summary of thiophene capacities for various solvents studied
3.3 Oxidation/adsorption experiments
3.3.1 Synthesis and characterization of K2FeO4
Potassium ferrate (K2FeO4) was synthesized according to the procedures described in the literature[12]. Atomic absorption spectrometric analysis of the oil sample was conducted with a Shimadzu AA-670 spectrophotometer. The element content of the compound is shown in Table 3. The measured element contents are compared with their theoretical values in K2FeO4.
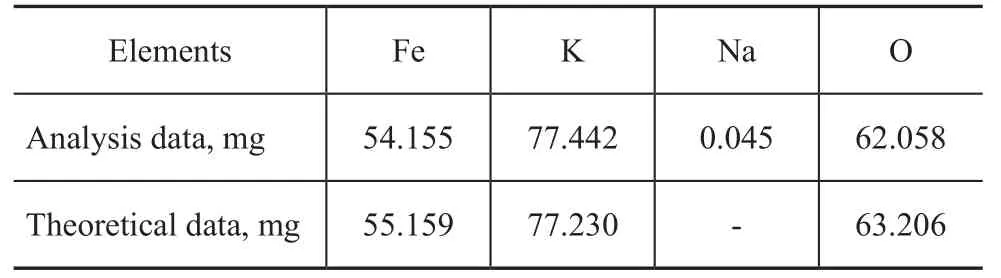
Table 3 ASS Analysis of Synthesized K2FeO4Sample
The SEM images of K2FeO4samples were obtained with a JEOL JSM-6360LA electron microscope. As shown in Figure 1, the morphology of K2FeO4crystals is of the thin fork-like shape. In the (010) crystal plane the length is twice the width and the overall size is 100×200 μm (Figure 1a). The surfaces of crystals are shiny. The K2FeO4has very fine particle substructure (Figure 1b) with its diameter less than 1 μm. Tiny particles of K2FeO4are prone to aggregate and stack into blocks.
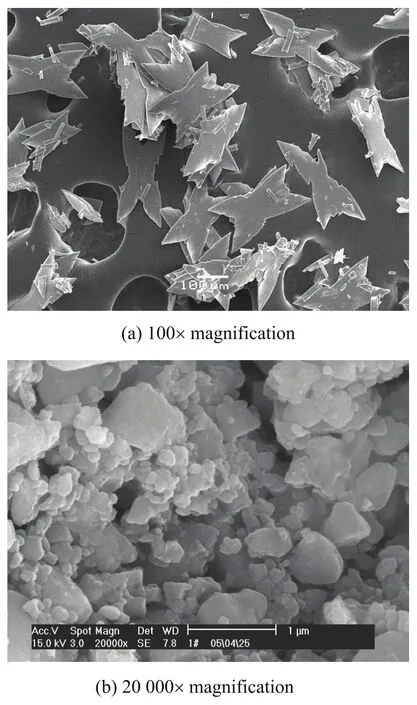
Figure 1 SEM images of synthesized K2FeO4sample
3.3.2 Oxidative/adsorptive desulfurization experiments
Potassium ferrate (VI) is a powerful oxidizing agent throughout the entire pH range with a reduction potential varying from +2.2 V to +0.7 V in acidic and basic solutions, respectively. Potassium ferrate(VI) in conjunction with an appropriate acidic catalyst in nonaqueous media can efficiently oxidize a number of organic compounds in theory. Using phosphomolybdic acid as the catalyst in conjunction with potassium ferrate in the oxidative desulfurization process is a new attempt. Given the slight solubility of phosphomolybdic acid, we applied the water solution of phosphomolybdic acid as the catalyst. A possible mechanism is shown in Figure 2.
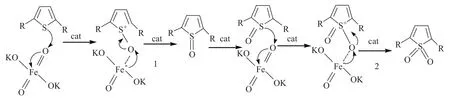
Figure 2 Mechanism of thiophene oxidation by K2FeO4
3.3.2.1 Effect of oxidation temperature on desulfurization
The effect of temperature on the residual sulfur content in simulated oil sample was investigated. As indicated in Figure 3, the residual sulfur content decreased quickly at first with an increasing temperature, but when the reaction temperature increased beyond 30 ℃, the residual sulfur content then increased with the increase in reaction temperature. This phenomenon might be attributed to the opposing effects of temperature. On one hand the high temperature could accelerate the reaction rate, and on the other hand, K2FeO4would be more unstable at increased temperature[13]. Fe (VI) ions would be reduced rapidly and exothermally to form Fe(III) ions and oxygen in strong acidic medium[9]to lose the strong oxidation effect (4K2FeO4+10H2O→4Fe(OH)3+3O2↑+8KOH). In our study, the system was slightly acidic, so the reduction of Fe (VI) ions was not obvious. But at higher temperature, the reduction of Fe (VI) ions was accelerated with a rising temperature.
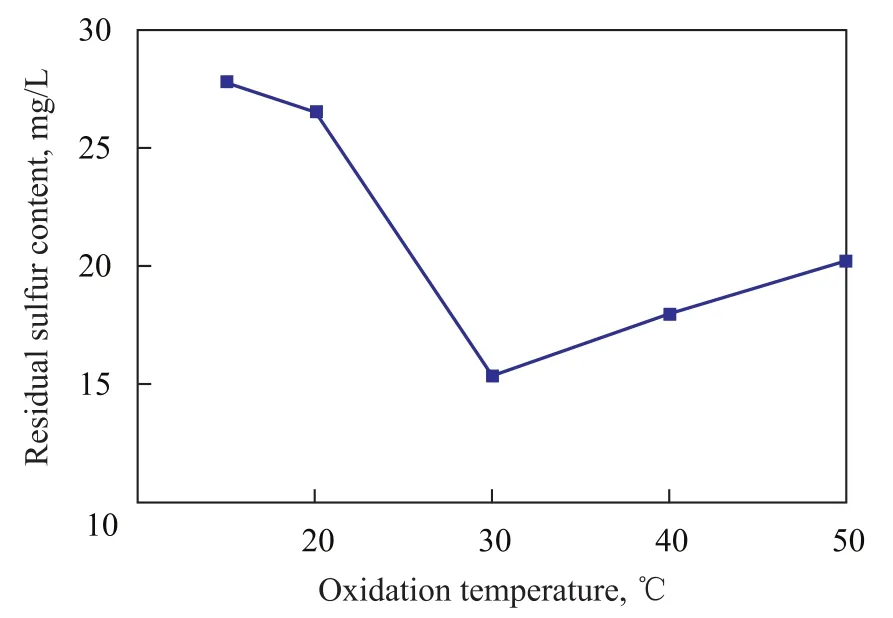
Figure 3 Effect of oxidation temperature on the residual sulfur content in simulated oil
3.3.2.2 Effect of oxidation time on desulfurization
Figure 4 shows the effect of reaction time on residual sulfur content in simulated oil. The results indicate that the residual sulfur content decreased with an increasing oxidation time. When the reaction time was beyond 60 min, the reaction was close to equilibrium and the residual sulfur content almost did not change.
3.3.2.3 Effect of oxidant amount on desulfurization
The effect of oxidant amount on residual sulfur content in simulated oil is shown in Figure 5. The residual sulfur content decreased with an increasing oxidant amount. This phenomenon occurred because the contact opportunity between oxidant and thiophene increased with an increasing oxidant amount, and thiophene could be oxidized to sulfoxide or sulfone which is easily removed by adsorption. When the K2FeO4/thiophene mole ratio was higher than 5.7, the residual sulfur content in simulated oil almost did not change, and a further increase in the oxidant amount was not necessary.
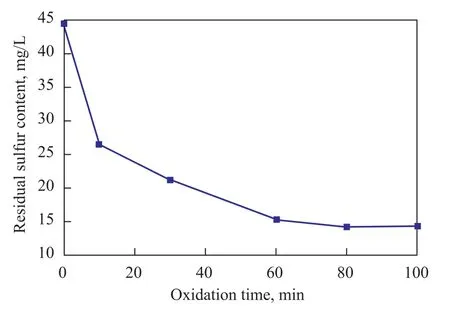
Figure 4 Effect of reaction time on the residual content of sulfur in simulated oil
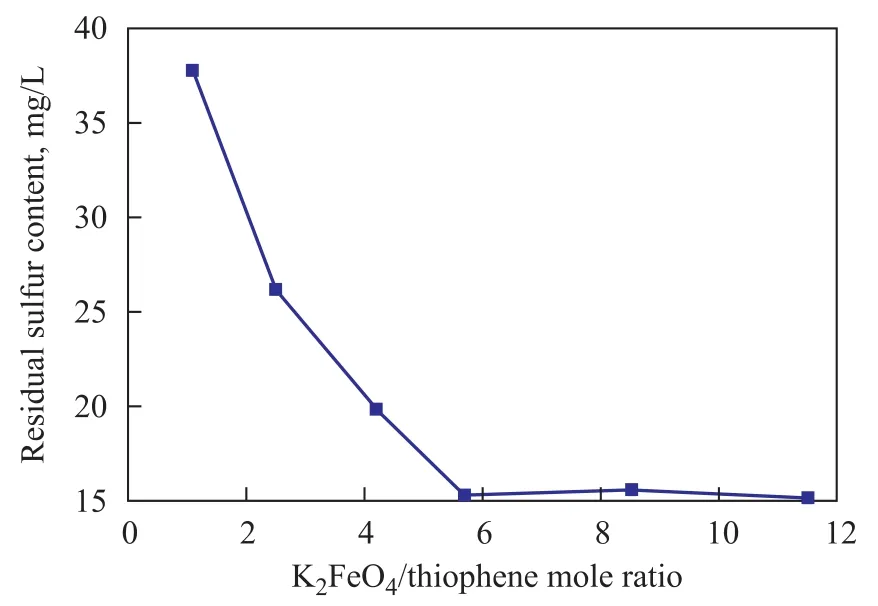
Figure 5 Effect of the amount of oxidant (K2FeO4) on the residual content of sulfur in simulated oil
3.3.2.4 Effect of catalyst amount on desulfurization
Figure 6 shows the effect of catalyst amount on the residual sulfur content in simulated oil. The test results indicated that the residual sulfur content in simulated oil decreased with an increasing catalyst amount at first. However, when the catalyst amount increased further at a phosphomolybdic acid to K2FeO4mole ratio that wasbeyond 0.0482, the residual sulfur content increased inversely with the increase in catalyst amount. It was supposed that the Fe (VI) ions were reduced and decomposed to Fe (III) ions when the acidic catalyst—phosphomolybdic acid increased in large amount.
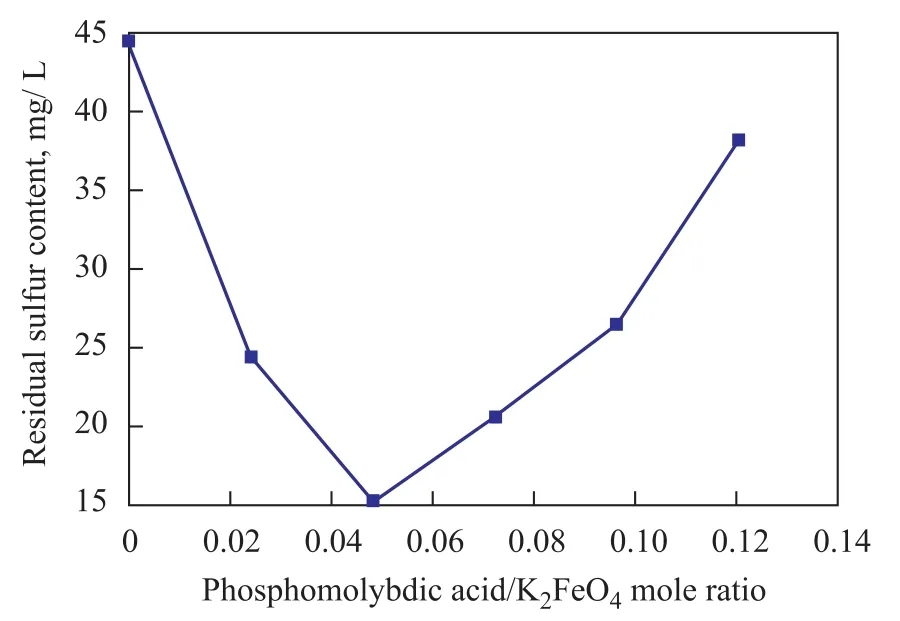
Figure 6 Effect of the amount of catalyst on the residual content of sulfur in simulated oil
4 Conclusions
The new method for deep desulfurization of simulated oil is based on the oxidative-adsorptive desulfurization process using K2FeO4as the oxidant in the presence of phosphomolybdic acid. The suitable oxidation-adsorption desulfurization conditions are specified as follows: a simulated oil sample of 5 mL, a K2FeO4/thiophene mole ratio of 5.7, a phosphomolybdic acid/K2FeO4mole ratio of 0.048 2, an oxidation temperature of 30 ℃, and a reaction time of 60 min. The reaction products were adsorbed by activated carbon, and analysis of relevant products showed that the sulfur content in simulated oil decreased from 200 mg/L to 15.3 mg/L, with the desulfurization rate reaching 92.3%.
Acknowledgements:This work was financially supported by the Science and Technology Program of the Department of Education, Heilongjiang Province (11531012).
[1] Gao Hongshuai, Luo Mingfang, Xing Jianmin, et al. Desulfurization of fuel by extraction with pyridinium-based ionic liquids[J]. Ind Eng Chem Res, 2008, 47(21): 8384-8388
[2] Wang Lei, Shen Benxian. Desulfurization of fluid catalytic cracking diesel oil by extraction with furfuryl alcohol[J]. Journal of East China University of Science and Technology (Natural Science Edition), 2005, 31(5): 563-567 (in Chinese)
[3] Lee J, Beum H T, Ko C H, et al. Adsorptive removal of dimethyl disulfide in olefin rich C4with ion-exchanged zeolites[J]. Ind Eng Chem Res, 2011, 50(11): 6382-6390
[4] Hernandez S P, Fino D, Russo N. High performance sorbents for diesel oil desulfurization[J]. Chem Eng Sci, 2010, 65: 603-609
[5] Kumar S, Srivastava V C, Badoni R P. Oxidative desulfurization by chromium promoted sulfated zirconia[J]. Fuel Process Technol, 2012, 93(1): 18-25
[6] Zhu Wenshuai, Zhu Guopeng, Li Huaming, et al. Oxidative desulfurization of fuel catalyzed by metal-based surfactanttype ionic liquids[J]. J Mol Catal A: Chem, 2011, 347(1): 8-14
[7] Ge Jianhua, Zhou Yuming, Yang Yong, et al. Catalytic oxidative desulfurization of gasoline using ionic liquid emulsion system[J]. Ind Eng Chem Res, 2011, 50(24): 13686-13692
[8] Mei Hai, Mei B W, Yen T F. A new method for obtaining ultra-low sulfur diesel fuel via ultrasound assisted oxidative desulfurization[J]. Fuel, 2003, 82: 406-414
[9] Wood R H. The heat, free energy, and entropy of the ferrate (VI) ion[J]. J Amer Chem Soc, 1958, 80(9): 2038-2041
[10] Delaude L, Laszlo P, Lehance P. Oxidation of organic substrates with potassium ferrate (VI) in the presence of K10 montmorillonite[J]. Tetrahedron Letter, 1995, 36(46): 8505-8508
[11] Song H, Wang B H, Zhang J J, et al. A method to increase the oxidative activity of potassium ferrate: China, CN 1730399[P], 2007 (in Chinese)
[12] Song H, Zhang J, Zhang Z Q. Synthesis of benzaldehyde by green oxidization from benzyl alcohol[J]. Journal of Daqing Petroleum Institute (China), 2004, 28(4): 49-51 (in Chinese)
[13] Song Hua, Wang Yuanyuan. Stability of potassium ferrate in neutral and acidic mediums[J]. Chemistry (China), 2008, 71(9): 696-670 (in Chinese)
Recieved date: 2012-05-23; Accepted date: 2012-09-04.
Ms. Liu Yanxiu, Telephone: +86-459-6504035; E-mail: liuyanxiu1126@163.com.
- 中國煉油與石油化工的其它文章
- Degradation of Nitrobenzene-Containing Wastewater with O3and H2O2by High Gravity Technology
- A Novel Modeling, Simulation and Optimization Approach of Crude Oil Cold Stripping Process
- Feasibility of Intermediate Fluid Vaporizer with Spiral Wound Tubes
- Selecting Suitable Heat Source in Refinery for Multi-effect Distillation Based on Grey System Theory
- Preparation of Alumina Binder-Added PtSnNa/AlSBA-15 Catalyst for Propane Dehydrogenation
- Solid Acid Used as Highly Efficient Catalyst for Esterification of Free Fatty Acids with Alcohols

