Blood group type antigens in pancreatic intraductal papillary mucinous neoplasms
Adriana Handra-Luca
Bobigny, France and Baltimore, USA
Blood group type antigens in pancreatic intraductal papillary mucinous neoplasms
Adriana Handra-Luca
Bobigny, France and Baltimore, USA
BACKGROUND:There are few data on blood group (BG) types and types of pancreatic cancers. The aims of this study were to study BG types and BG-antigens in pancreatic intraductal papillary mucinous neoplasms (IPMNs).
METHODS:BG type and tumor BG-antigen (glycoprotein) expression (studied by immunohistochemistry on tissue microarrays) were analyzed with regard to characteristics of 101 surgically resected pancreatic IPMNs.
RESULTS:Non-O BG type predicted invasive carcinoma independently from high serum CA19-9 and male gender. BG type A was observed more frequently in women than in men. Chronic pancreatitis was more frequently seen in patients with BG type B or AB. Aberrant tumor expression (with regard to BG type) of loss of A antigen expression type occurred in 15.0% of IPMNs and of loss of B antigen expression type in 62.5% of IPMNs. Intraneoplasm BG-antigen expression was not related to dysplasia grade or invasion.
CONCLUSION:The results of the study suggest that in pancreatic IPMN, non-O BG type predicted invasive carcinoma, whereas for intratumor BG-antigen expression no specific patterns were detected with regard to the progression of glandular epithelial dysplasia or invasion. (Hepatobiliary Pancreat Dis Int 2014;13:74-80)
blood group type;
blood group antigen;
immunohistochemistry;
CA19-9;
prognosis;
invasive carcinoma;
pancreas;
intraductal papillary mucinous neoplasm
Introduction
Blood group(BG) type is considered to be significantly associated with the risk of pancreatic cancer, particularly of pancreatic adenocarcinoma. Study participants with BG type O have a lower risk of pancreatic cancer than those with BG type A, AB or B.[1, 2]More recently, BG type A has been reported to be associated with pancreatic adenocarcinoma risk along with other patients' characteristics such as HBsAgpositive/anti-HBc-positive status, anti-HBs-positive/anti-HBc-positive status, diabetes mellitus, cigarette smoking, alcohol drinking, and a family history of other cancer.[3]Greer et al[4]reported a higher frequency of BG type A in pancreatic cancer patients compared to regional blood donors and, a lower frequency of BG type O relatively to blood donors, patients with pancreatic adenocarcinoma having less frequently BG type O as compared with those with other cancers.[5]
Intraductal papillary mucinous neoplasms (IPMNs) are part of the epithelial pancreatic tumor category, and are defined as macroscopically cystic or mass-forming epithelial neoplasms with ductal differentiation, derived primarily from the pancreatic duct system.[6]Risk factors for IPMNs overlap in part with those for pancreatic adenocarcinomas, a history of diabetes, especially with insulin use, chronic pancreatitis, and a family history of pancreatic ductal adenocarcinoma being relevant to the development of IPMNs.[7]These tumors are known to express several ductal cell keratins and glycoproteins such as CA19-9, CEA, TAG72 or mucin glycoproteins.[6]To our knowledge, glycoprotein expression of BG type A and B and BG type distribution in human IPMNs have not been reported since this tumor group, characterized by the entire spectrum of epithelial dysplasia and invasive carcinoma, has been identified and defined.[6]
The aims of this study were to determine the relationships between BG A and B antigens in IPMN patients, both when located on erythrocytes (defining the general background as BG type) and when located on epithelial cells, neoplastic or not (defining a localpancreatic background) and clinicomorphological characteristics in pancreatic IPMNs.
Methods
Patients
Patients with pancreatic IPMN diagnosed between 1998 and 2006 at Johns Hopkins Medical Institutions who had been treated by surgical resection were analyzed in terms of clinical (gender, age, BG type, type and date of surgical resection, serum CA19-9 levels, date of death or of the last visit) and morphological data (tumor size, degree of dysplasia, lymph node metastases, vascular and perineural invasion, presence of mucinous morphology, presence of chronic pancreatitis or presence of pancreatic intraepithelial neoplasia-PanIN-lesions) available. An approval was obtained from the institutional review board. IPMNs were classified according to the WHO classification into invasive IPMNs and non-invasive IPMNs, the latter being further classified according to the highest degree of dysplasia present into low and/or intermediate grade and, into high grade IPMN.[6]
According to current guidelines, histological chronic pancreatitis was de fi ned as pancreatic fi broin fl ammatory changes with unevenly distributed inter- and intralobular fi brosis (leaving the lobular pattern of the organ focally preserved) along with duct changes (obstruction, dilatation or distortion, lined by dystrophic, metaplastic or reactive epithelium) and with varied numbers of lymphocytes, plasma cells and macrophages (either in local accumulations or scattered diffusely throughout the fi brous tissue).[6, 8]Chronic pancreatitis lesions were assessed accordingly on the whole tissue section slides. Since there is no widely accepted standardized system of chronic pancreatitis classification and, that based on the degree and extent of lesions is considered not reproducible, the severity of chronic pancreatitis was not evaluated.[9]PanIN precursor pancreatic ductal lesions have been assessed according to current guidelines, being defined as papillary or flat epithelial ductal neoplasms (non-invasive, confined to the duct), less of 5 mm in size.[6]According to the degree of cytological and architectural atypical, 3 grades of PanIN have been assessed: PanIN1, PanIN2 and PanIN3. Only PanINs present on the tissue microarrays (TMA) slides tissue sections have been studied.
Immunohistochemistry
Immunohistochemistry was performed on slide sections obtained from TMA constructed as previously reported.[10-12]The BG A antigen/glycoprotein, monoclonal mouse (MA1-19693; dilution 1:200, Thermo Scientific, Pierce Protein Research, Rochford, IL, USA) and the BG B antigen/glycoprotein, monoclonal mouse (MA1-19691; dilution 1:100, Pierce Protein Research) have been used. The primary antibody was applied for 60 minutes, the secondary for 30 minutes (Dako EnVision+anti-mouse/HRP polymer, Carpinteria, CA, USA). The slides were treated for 3 minutes with DAB, 4 minutes in hematoxylin. Immunohistochemistry slides (Olympus BX51, 30× objective, Center Valley, PA, USA) were analyzed without knowledge of clinical or morphological data. Expression of A or B antigens was assessed in the non-invasive IPMN in the different dysplasia lesions (low or intermediate grade dysplasia and high grade dysplasia) and in invasive IPMNs (IPMN associated with adenocarcinoma). Cytoplasmic and/or membrane stainings in these lesions were evaluated as the percentage of stained cells with regard to the total number of neoplastic cells.[12]Membrane and/or cytoplasmic expression was also assessed in PanIN lesions available on the TMAs as well as in nontumoral pancreatic acini and ducts, and the percentage of stained cells was noted. The highest percentage noted for each structure (acini, ducts) or lesion type (low or intermediate dysplasia, high grade dysplasia, adenocarcinoma, PanIN1, PanIN2, PanIN3) was retained. Subsequently, the expression pattern was classified as negative or positive (corresponding to the biological classification of expression patterns), and was used as such for the statistical analyses.
Statistical analysis
Patients' characteristics and tumor features including the immunohistochemical ones were compared by using Fisher's exact test or the Chi-square test. For all tests and models, the variables have been considered as binary. Multivariate logistic regression models have been constructed for predicting the presence of invasive adenocarcinoma in IPMN (invasive IPMN, IPMN associated with adenocarcinoma) and the presence of high grade dysplasia or invasive IPMN. The relationship to postsurgical survival was assessed by the Kaplan-Meier method and compared by the log-rank test. Variables associated with a statistical significance of P<0.05 on univariate survival analysis were studied in Cox multivariate survival models. All studied variables have been analyzed for redundancy before constructing the multivariable models. Statistical tests and graphics were performed with the Medcalc (v11.1.1, Belgium) software. A P<0.05 was considered statistically significant.[11, 13]
Results
Characteristics of non-invasive and invasive IPMN
The main clinicopathological characteristics of the studied IPMNs are shown in Table 1. Non-invasive IPMN occurred in 53 patients (low or intermediate grade dysplasia 33 patients, and high grade dysplasia 20 patients) and invasive IPMN in 48 patients. Women showed more frequently BG type A than men (28/43, 65% vs 15/43, 35%, P=0.01). Histological chronic pancreatitis was seen more frequently in BG type B or AB patients than in BG type A or O patients (6/7, 85% vs 12/37, 32%, P=0.01). PanIN1 lesions were observed in 7 patients (6 patients with BG type O and 1 with BG type A), and PanIN2 in 3 patients (2 patients with BG type O and 1 with BG type A).
An elevated serum CA19-9 (>36 U/mL) was observed more frequently in invasive IPMNs than in non-invasive IPMNs (17/26, 65% vs 5/26, 19%, P<0.01). In the multivariate logistic regression model that could be constructed (after checking variables for redundancy), a high serum CA19-9 predicted presence of invasive IPMN, along with the presence of non-O BG type and male gender, independently of these two clinical characteristics. Although the P value for non-O BG type in predicting invasive IPMN was less statistically relevant when comparing invasive versus high grade dysplasia IPMNs as compared to the invasive versus non-invasive IPMNs, the odds ratio for non-O BG type in predicting invasive IPMN increased from 8.05 (in the model considering invasive versus non-invasive IPMNs) to 12.32 (in the model invasive versus high grade dysplasia IPMNs), while the odds ratios for male gender and high serum CA19-9 decreased from 15.21 to 12.70 and, from 28.20 to 10.27, respectively (Table 2).
A shorter overall survival was related to the presence of elevated serum CA19-9 and lymph node metastases as well as lack of chronic pancreatitis (P<0.01, P<0.01 and P=0.02, respectively) (Figs. 1 and 2). Postsurgical overall survival was significantly shorter in patients with invasive IPMNs than in those with non-invasive IPMN (median survival of 23 versus 81 months, P<0.01); the rate of death was significantly higher in patients with invasive IPMNs (30/48, 62.5% vs 13/53, 24.5%, P<0.01). Among the different characteristics, high serum CA19-9 only maintained statistically significant for a shorter overall survival in the Cox multivariate model that could be constructed after checking for redundancy (Table 3).
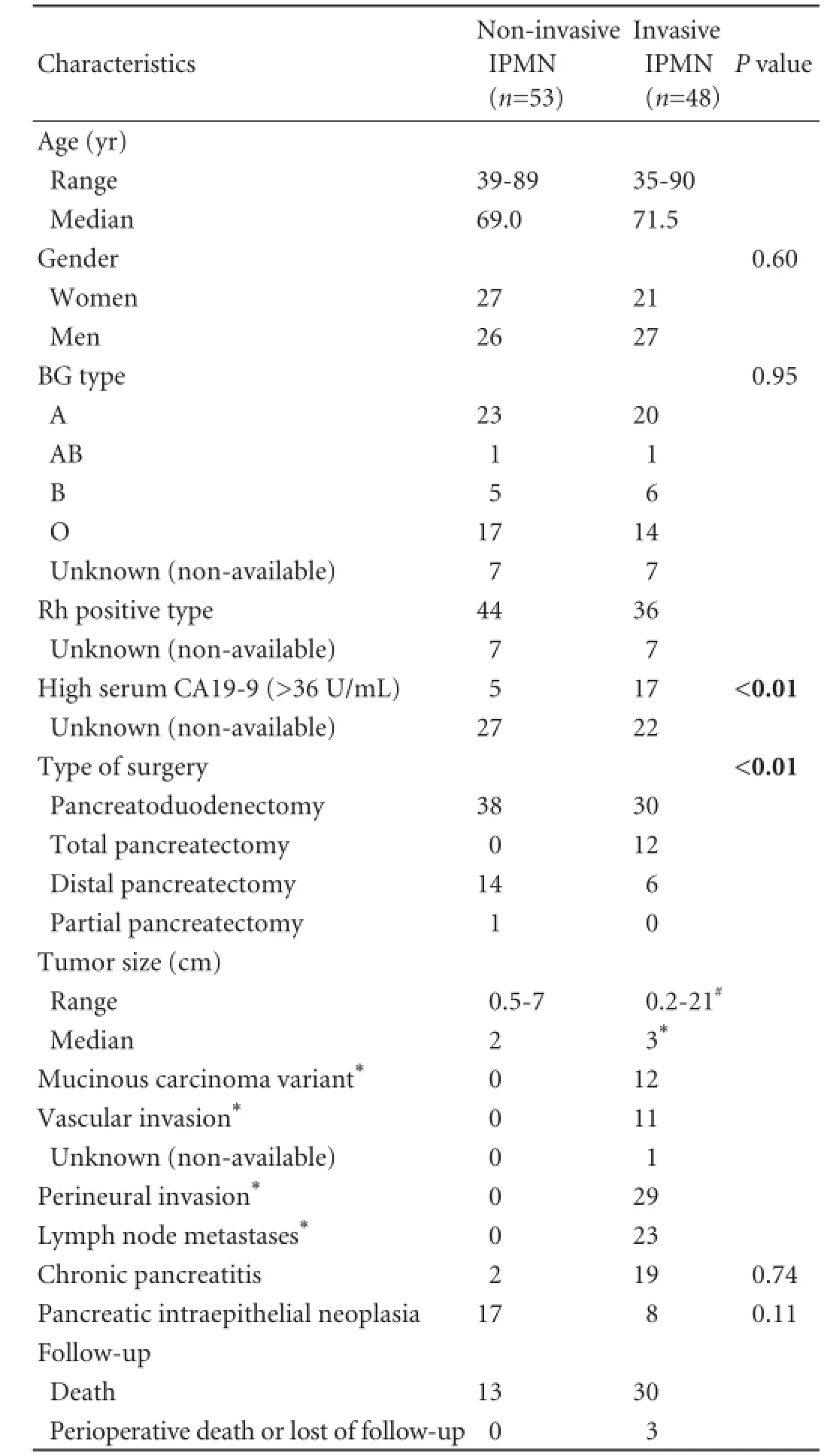
Table 1.Baseline characteristics of the patients and of the pancreatic IPMNs
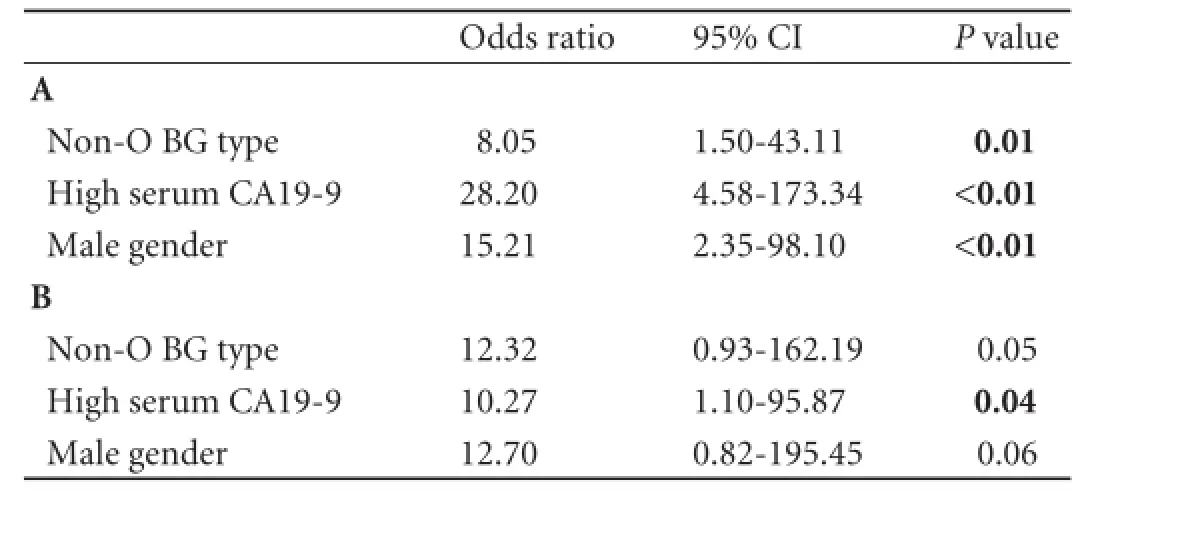
Table 2.Results of multivariate logistic regression models for predicting presence of invasive pancreatic IPMNs when considering the entire series of patients (A) and when considering only the high grade dysplasia and invasive IPMNs (B)
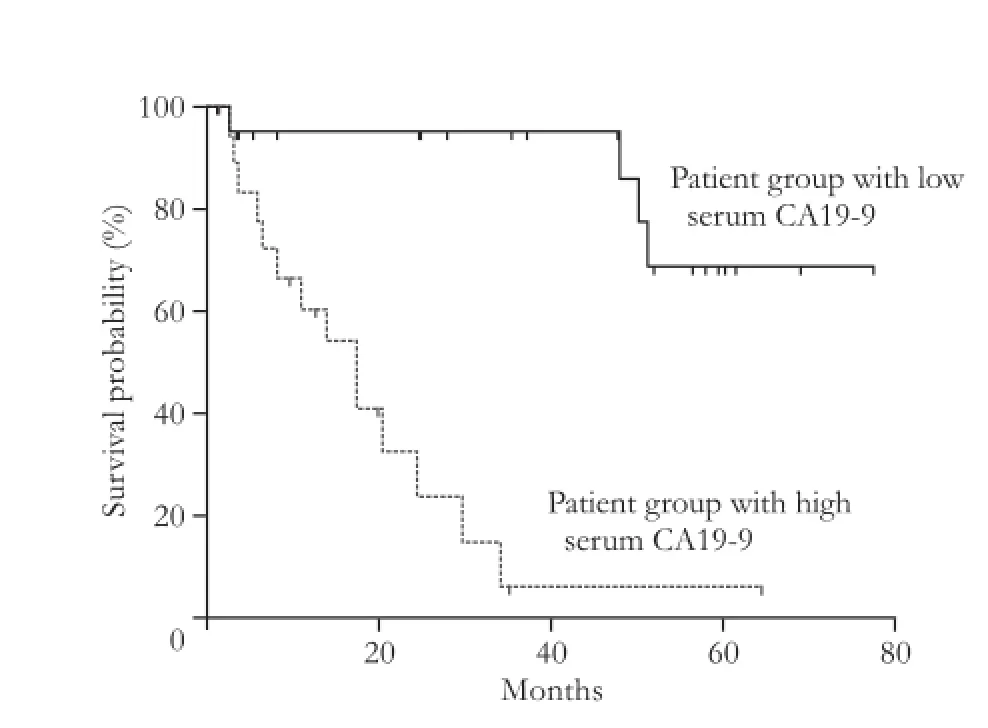
Fig. 1.Postsurgical overall survival curves for IPMN patients according to the serum CA19-9 levels, a high serum CA19-9 being related to a shorter survival.
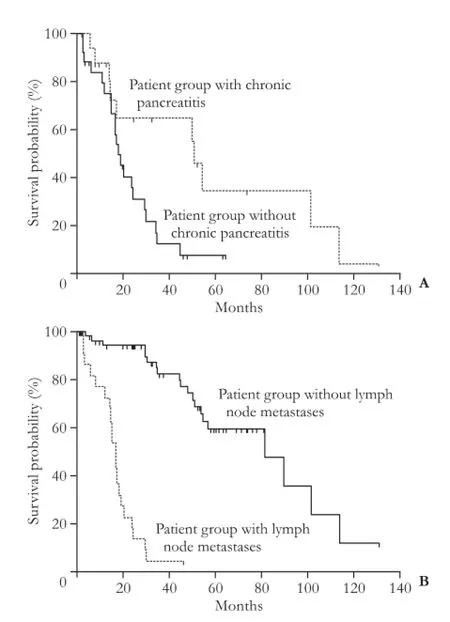
Fig. 2.Postsurgical overall survival curves for IPMN patients according to presence of histological chronic pancreatitis (A) and presence of lymph node metastases (B). Lack of chronic pancreatitis and presence of lymph node metastases related to a worse outcome.
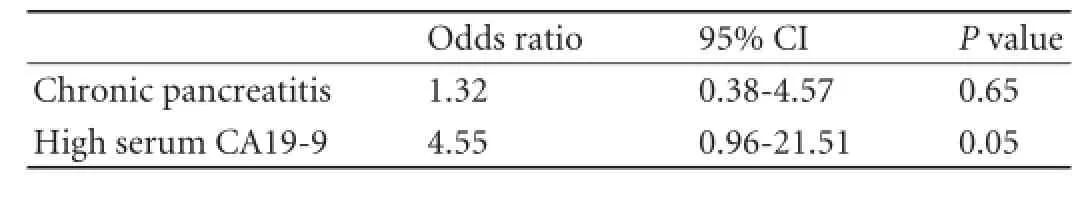
Table 3.Multivariate Cox models for postsurgical overall survival in IPMNs
BG antigen tumor expression in IPMN
The data on IPMN BG antigen expression are shown in Table 4. The expression of each antigen/ glycoprotein correlated strongly to the respective BG type (Fisher P<0.01 for both antigens). The A BG-antigen was expressed in 45% of the IPMNs whereas the B BG-antigen in 4% of the IPMNs. Microscopic aspects of positive normal pancreatic tissue, pancreatic adenocarcinoma and low or intermediate grade IPMN for both antigens are shown in Fig. 3. The percentage of positive cells varied from 0 to 97% for each antigen. Compared to duct expression, the lack of A BG-antigen expression in IPMNs (regardless the dysplasia grade, presence of invasive IPMN or patients' BG type) was directly related to the lack of expression in pancreatic ducts (12/14, 85% vs 12/19, 63%, P=0.02). The immunophenotype for A or B BG-antigen was not different in low or intermediate grade dysplasia IPMNs or in high grade dysplasia IPMNs, when it was associated with more severe lesions (high grade dysplasia or invasive adenocarcinoma and invasive adenocarcinoma, respectively) as compared to when unassociated with more severe lesions. An aberrant expression pattern consisting of loss of A BG-antigen expression (patients with BG type A or AB) occurred in 15.0% of IPMNs. Loss of B antigen expression occurred in 62.5% of IPMNs (patients with BG type B or AB). Intraneoplasm expression of A or B BG-antigen did not vary with dysplasia grade or with presence of invasive IPMN (IPMN associated with adenocarcinoma). For A BG-antigen expression, the dysplasia progression lesions (different types/degrees of dysplasia for a sameIPMN) were positive in 11/22 IPMNs and the dysplasiaadenocarcinoma progression lesions (different types/ degrees of dysplasia and adenocarcinoma for a same IPMN) in 2/4 IPMNs. One tumor showed the intratumor variation of A BG-antigen expression (loss of expression from high grade dysplasia to adenocarcinoma). For B BG-antigen expression, the dysplasia progression lesions were positive in 1/25 IPMNs and the dysplasiaadenocarcinoma progression lesions in none of 7 IPMNs. PanIN lesions showed a similar immunophenotype as the coexisting IPMN (Table 4): A BG-antigen was expressed in 1/2 PanINs2 and in 0/5 PanINs1, whereas B BG-antigen was not expressed in the available 5 PanINs1 and 2 PanINs2.
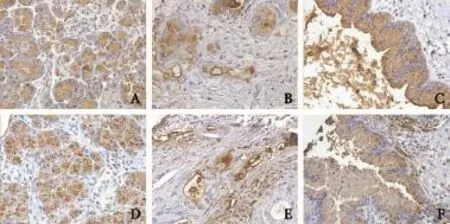
Fig. 3.A and B BG antigen expression in IPMNs. The upper line comprises microscopic figures from A antigen positive cases (normal pancreas, adenocarcinoma IPMN, and low and intermediate grade dysplasia IPMN:A,BandC, respectively) and the bottom line from B antigen positive cases (normal pancreas, adenocarcinoma IPMN, and low and intermediate grade dysplasia IPMN:D,EandF, respectively). Both A and B antigens were expressed in a cytoplasmic or membrane pattern in tumor cells and in normal acinar cells (original magnification ×40).
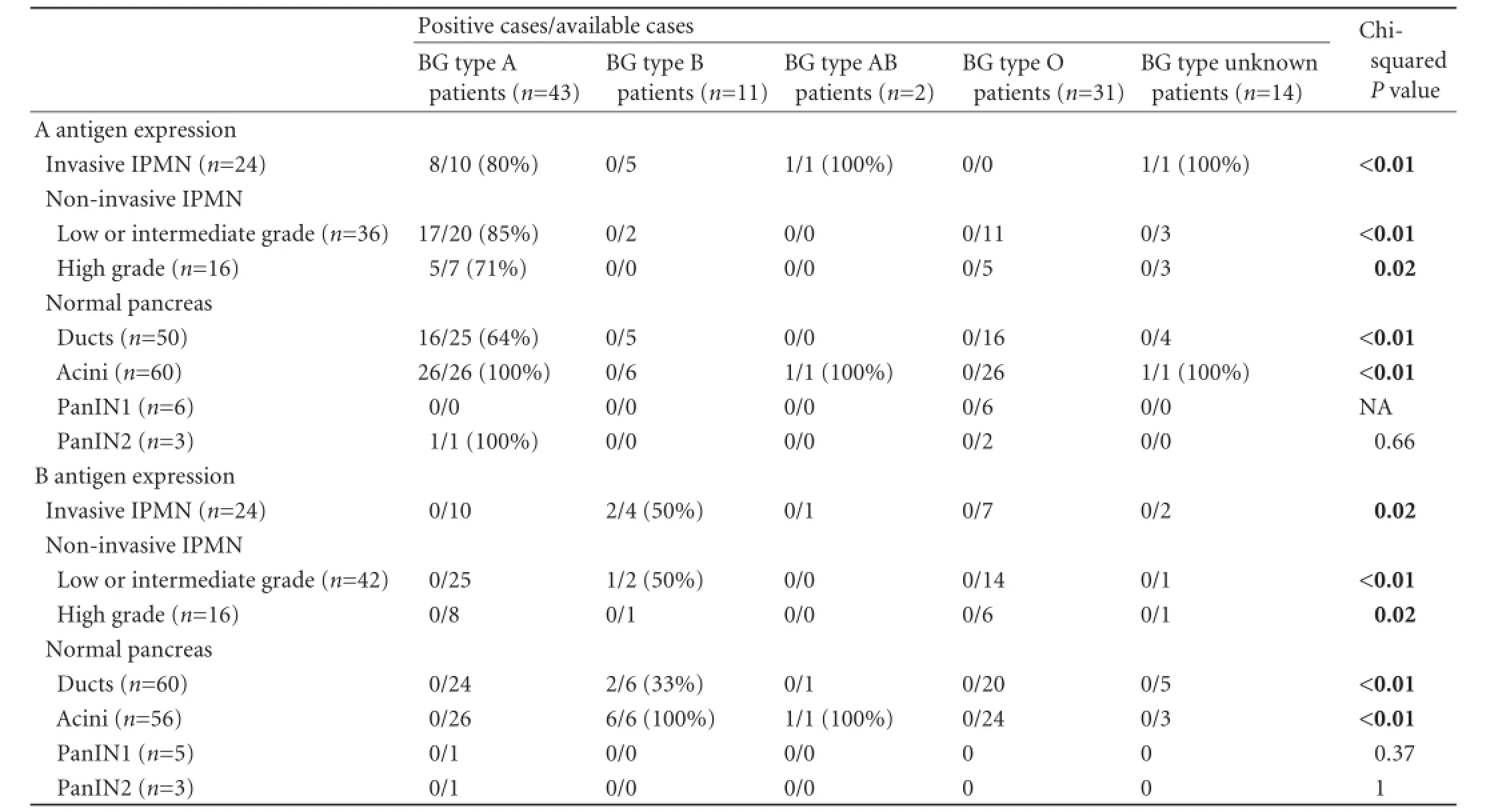
Table 4.Immunohistochemical expression of A and B BG antigens in IPMN according to the patients BG type
Discussion
The results of this study suggest that among patients with pancreatic IPMNs, patients with non-O BG type have a higher risk for invasive IPMN independently of high serum CA19-9 and male gender, which was in agreement with the increased risk of pancreatic adenocarcinoma observed in patients of non-O BG type.[1, 2]Although the statistical relevance of non-O BG type in predicting invasive IPMN was less significant than that of male gender and high serum CA19-9, non-O BG type predicted invasive carcinoma not only in comparison of non-invasive with invasive IPMNs but also in comparison of only high grade dysplasia IPMN with invasive IPMNs. The extent to which tumor BG antigen expression changes, when passing to an invasive adenocarcinoma stage, might be relevant for this relationship, remains difficult to establish. However, results of our study on pancreatic ductal invasive adenocarcinomas[12]suggested interference for lack or loss of tumor A antigen with invasive tumor stage characteristics such as vascular invasion and metastatic disease.
Interestingly, a second clinical variable, male gender, also predicted the presence of invasive IPMN, which parallels the data on pancreatic cysts.[14]The third clinical variable we found predictive of invasive IPMN was high serum CA19-9, which is in agreement with the already reported data.[15-21]Cholestasis as well asseveral benign hepatobiliary and pancreatic conditions are found to influence the concentrations of serum tumor markers,[22-24]Therefore CA19-9 data should be taken cautiously. Interestingly, a high concentration of CA19-9 also predicted invasion in comparison of high grade dysplasia IPMN with invasive IPMN, suggesting a role in the invasive tumor phenotype. Further studies are required to determine the precise significance of the predictive factors of invasive IPMN we have found, in the context of already reported clinical or serum markers such as history of pancreatitis, diabetes, symptoms, jaundice, loss of weight, increase of age, or total level of serum bilirubin.[17, 21 ,22, 25-28]
The results of this study point out two other clinical and pathological correlations, previously not reported to our knowledge. First, there was a relationship between female gender and BG type A in patients with IPMNs, these tumors being more frequent in women with BG type A. Whether this is a reflection of a higher frequency of BG type A in IPMN patients than in the general population[29]remains hypothetical. Second, there was a higher frequency of histological pancreatitis in patients, carriers of B antigen on red blood cells, who are patients with BG type B or AB. Although BG antigens may alter inflammatory response,[2, 5]thus influencing the risk of pancreatic cancer, data on BG type and inflammation are few. Greer et al[30]studied this relationship and found that patients of non-O BG type were less likely to have chronic pancreatitis. In the present series of IPMNs, chronic pancreatitis was found to be related to a better outcome possibly due to earlier symptoms.
We also studied BG glycoprotein expression in tumor specimen from human IPMNs characterized by abundant mucosecretion. Aberrant patterns of BG antigen expression which occurred in this series of IPMNs comprising the entire morphological spectrum from dysplasia to invasive adenocarcinoma were only of loss of expression type, and related more frequently to B antigen expression. For both A and B antigens, the intraneoplasm expression patterns were not related to the dysplasia degree or to the presence of invasive IPMN. Further studies are required to determine the relevance of BG antigens for the progression of epithelial glandular dysplasia, one of the limitations of the present study being the limited number of premalignant IPMNs available for microscopic analysis. Regardless of patients' BG type, lack of A antigen expression in IPMNs was related to ductal lack of expression, suggesting that changes in BG type A antigen expression might precede changes of dysplasia.
We found previously that non-AB BG type predicts death in patients with pancreatic ductal adenocarcinomas,[12]whereas in this series of pancreatic IPMNs, non-O BG type predicted the presence of invasive IPMN. Interestingly, in human epithelial pancreatic ductal adenocarcinoma cells, A BG glycoprotein was related to low invasiveness tumor features such as lack of vascular invasion or few lymph node metastases, whereas B glycoprotein was related to non-mucinous histological type.[12]In IPMNs, BG glycoprotein expression did not show specific expression patterns with progression of dysplasia. However, the biological or clinical relevance of BG type antigens in red blood cells or pancreatic neoplastic cells remains incompletely elucidated, and further investigation is warranted.
Acknowledgments:The author acknowledges Dr M Goggins (Director, Pancreatic Cancer Early Detection Research Laboratory, Johns Hopkins Medical Institutions, Baltimore, MD, USA). The author thanks C Lubek for assistance in technical procedures and SM Hong for advice as well as Prof. B Terris. I thank Dr M Tanaka, Dr M Tachezy, Dr S Navarro and Dr J Greer as well as K Kealoha, Dr VH Tran Thi, Dr M Khallouf i, Dr C Gonzalez and Pr M Reid. I thank P Cusenier with reference search, N Cole, K Blair and LR Butler as well as J Baltaze, N Kemache, L Yahaya, C Adam, S Morard and M Bechani for administrative help.
Contributors:HLA proposed the study, designed the study, wrote the first draft, and analyzed the data. HLA is the guarantor.
Funding:None.
Ethical approval:An approval was obtained from the institutional review board.
Competing interest:No benefits in any form have been received from a commercial party related directly or indirectly to the subject of this article.
1 Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet 2009;41:986- 990.
2 Wolpin BM, Chan AT, Hartge P, Chanock SJ, Kraft P, Hunter DJ, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101:424-431.
3 Wang DS, Chen DL, Ren C, Wang ZQ, Qiu MZ, Luo HY, et al. ABO blood group, hepatitis B viral infection and risk of pancreatic cancer. Int J Cancer 2012;131:461-468.
4 Greer JB, Yazer MH, Raval JS, Barmada MM, Brand RE, Whitcomb DC. Significant association between ABO blood group and pancreatic cancer. World J Gastroenterol 2010;16: 5588-5591.
5 Iodice S, Maisonneuve P, Botteri E, Sandri MT, Lowenfels AB. ABO blood group and cancer. Eur J Cancer 2010;46:3345- 3350.
6 Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of digestive tumors, 4th ed. Lyon: IARC Press; 2010.
7 Capurso G, Boccia S, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, et al. Risk factors for intraductal papillary mucinous neoplasm (IPMN) of the pancreas: a multicentre case-control study. Am J Gastroenterol 2013;108:1003-1009.
8 Kl?ppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol 2007;20:S113-131.
9 Odze RD, Goldblum JR. Surgical Pathology of the GI Tract, Liver, Biliary Tract and Pancreas: Expert Consult, 2nd ed. Philadelphia PA: Saunders Elsevier; 2009.
10 Hong SM, Kelly D, Griffith M, Omura N, Li A, Li CP, et al. Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod Pathol 2008;21: 1499-1507.
11 Handra-Luca A, Hong SM, Walter K, Wolfgang C, Hruban R, Goggins M. Tumour epithelial vimentin expression and outcome of pancreatic ductal adenocarcinomas. Br J Cancer 2011;104:1296-1302.
12 Handra-Luca A. A and B blood group antigens in human pancreatic ductal adenocarcinomas. Oncology 2013;84:141- 149.
13 Hosmer DW, Lemeshow S. Applied Logistic Regression, 2nd ed. New York: Wiley; 2000.
14 Lee CJ, Scheiman J, Anderson MA, Hines OJ, Reber HA, Farrell J, et al. Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg 2008;12:234-242.
15 Fritz S, Hackert T, Hinz U, Hartwig W, Büchler MW, Werner J. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg 2011;98:104-110.
16 Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg 2012;397:93-102.
17 Hirono S, Tani M, Kawai M, Okada K, Miyazawa M, Shimizu A, et al. The carcinoembryonic antigen level in pancreatic juice and mural nodule size are predictors of malignancy for branch duct type intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2012;255:517-522.
18 Lou WH, Wang DS, Ji Y, Xu XF, Kuang TT, Ni XL, et al. Clinical features and prognosis of intraductal papillary mucinous neoplasms of pancreas: analysis of 38 cases. Zhonghua Yi Xue Za Zhi 2006;86:947-950.
19 Maire F, Voitot H, Aubert A, Palazzo L, O'Toole D, Couvelard A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am J Gastroenterol 2008;103:2871-2877.
20 Mimura T, Masuda A, Matsumoto I, Shiomi H, Yoshida S, Sugimoto M, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol 2010;44:e224-229.
21 Shin SH, Han DJ, Park KT, Kim YH, Park JB, Kim SC. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg 2010;34:776-783.
22 Basso D, Fabris C, Plebani M, Del Favero G, Muraca M, Vilei MT, et al. Alterations in bilirubin metabolism during extra- and intrahepatic cholestasis. Clin Investig 1992;70:49-54.
23 Dogan üB, Gümürdülü Y, G?lge N, Kara B. Relationship of CA 19-9 with choledocholithiasis and cholangitis. Turk J Gastroenterol 2011;22:171-177.
24 Ong SL, Sachdeva A, Garcea G, Gravante G, Metcalfe MS, Lloyd DM, et al. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci 2008;53:3213-3217.
25 Fujii T, Ishikawa T, Kanazumi N, Sugimoto H, Nomoto S, Inoue S, et al. Analysis of clinicopathological features and predictors of malignancy in intraductal papillary mucinous neoplasms of the pancreas. Hepatogastroenterology 2007;54: 272-277.
26 Ingkakul T, Sadakari Y, Ienaga J, Satoh N, Takahata S, Tanaka M. Predictors of the presence of concomitant invasive ductal carcinoma in intraductal papillary mucinous neoplasm of the pancreas. Ann Surg 2010;251:70-75.
27 Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillarymucinous tumours of the pancreas. Br J Surg 2003;90:1244- 1249.
28 Nara S, Onaya H, Hiraoka N, Shimada K, Sano T, Sakamoto Y, et al. Preoperative evaluation of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas 2009;38:8-16.
29 Stanford School of Medicine, Blood Center. Blood types in the US. 2008 Available from: http://bloodcenter.stanford.edu/ about_blood/blood_types.html. Retrieved February 2013.
30 Greer JB, LaRusch J, Brand RE, O'Connell MR, Yadav D, Whitcomb DC; NAPS2 Study Group. ABO blood group and chronic pancreatitis risk in the NAPS2 cohort. Pancreas 2011; 40:1188-1194.
Received February 11, 2013
Accepted after revision June 5, 2013
Author Affiliations: Departments of Pathology, Sydney Kimmel Comprehensive Cancer Center, Johns Hopkins Medical Institutions, Baltimore, MD, USA; and APHP Universite Paris Nord Sorbonne Cite-Sce Anatomie Pathologique, GHU Avicenne, 125 rue Stalingrad, Bobigny 93000, France (Handra-Luca A)
Adriana Handra-Luca, MD, PhD, APHP Universite Paris Nord Sorbonne Cite-Sce Anatomie Pathologique, GHU Avicenne, 125 rue Stalingrad, Bobigny 93000, France (Tel: 0033-1-48955555ext2047; Fax: 0033-1-48955602; Email: adriana.handra-luca@avc.aphp.fr)
? 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60010-2
 Hepatobiliary & Pancreatic Diseases International2014年1期
Hepatobiliary & Pancreatic Diseases International2014年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Samaritan donor interchange in living donor liver transplantation
- lntrahepatic Glissonian approach and outflow vascular occlusion during partial hepatectomy
- Complex hepatic outflow reconstruction in domino liver transplantation
- Novel en-bloc resection of locally advanced hilar cholangiocarcinoma: the Rex recess approach
- KAI1 inhibits lymphangiogenesis and lymphatic metastasis of pancreatic cancer in vivo
- Effect of CD74 on the prognosis of patients with resectable pancreatic cancer
