Immunization with Cop-1 promotes neuroprotection and neurogenesis after ischemic stroke
Immunization with Cop-1 promotes neuroprotection and neurogenesis after ischemic stroke
Cerebrovascular diseases are considered to be amongst the most serious public health issues, since they are the third leading cause of death (WHO, 2014) and the most common cause of disability worldwide. Its monetary signif cance is evidenced by the economic burden imposed on health care systems, given that the cost of medical care for a patient that has suf ered a stroke is around $25,741 US dollars every 5 years (Luengo-Fernandez et al., 2012). A stroke occurs as a result of a disturbance or interruption of cerebral blood f ow that signif cantly reduces the supply of oxygen and glucose to the neural tissue. Consequently, several cell death mechanisms (secondary lesion mechanisms) such as necrosis, excitotoxicity, free radical production and inf ammation are triggered (Castillo, 2000). Over the last decades, a variety of therapies with thrombolytic, neuroprotective, and restorative properties have been investigated. However, the results of these studies appear to be limited. That is the case of the tissue plasminogen activator (tPA) – the f rst line of treatment for decades – which is associated with low rates of recanalization and high rates of morbidity. Also, endovascular intervention, particularly mechanical thrombectomy, has been proposed as a promising therapeutic adjunct to tPA for the treatment of stroke; however, until recently, the ef cacy of this therapeutic approach has been controversial (Ding, 2015). Innovative theurapeutic options are currently being developed in order to restore af ected neuronal circuits following a cerebral ischemic event. Some of these innovative therapeutic approaches are based on stem cell transplantation and/or induction of neurogenesis.
After stroke; astrocytes, microglia, and endothelial cells induce an early response in gene expression through the activation of nuclear factor-κB (NF-κB). This event promotes a pro-inf ammatory environment characterized by the expression of interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α) and several chemokines (Fumagalli et al., 2015). These proteins activate adhesion molecules and produce a subsequent inf ltration of inf ammatory cells, especially T lymphocytes specif c to neural constituents. Recruitment and activation of immune cells increase the presence of lytic enzymes and neurotoxic mediators (e.g., free radicals) which in turn, cause secondary damage to the neural tissue.
The immune system plays an essential role in the pathophysiology of some neurodegenerative diseases. It has previously been associated with disease exacerbation (Castillo, 2000). Nevertheless, recent work suggests that inf ammatory cells and even autoimmune T lymphocytes could have the ability to promote neuroprotection (Schwartz and Shechter, 2010). These f ndings provide the basis to conceive a new therapeutic paradigm: Protective autoimmunity (PA), a physiological phenomenon that develops after central nervous system (CNS) damage (Hauben et al., 2000). Paradoxically, the benef cial ef ect of this immune response is exerted by autoreactive T cells directed against neural contituents (Schwartz and Shechter, 2010). In this light, PA might have benef cial ef ects over the secondary mechanisms of stroke and cerebrovascular diseases, nonetheless in order to exert these, it must be modulated. Evidence suggests that PA could be modulated by active inmunization with neural-derived peptides (NDP) in favor of protecting neural tissue after CNS damage (Cruz et al., 2015). Copolymer-1 (Cop-1; Copaxone, glatiramer acetate) is a synthetic polypeptide consisting of four amino acids: L-alanine, L-glutamic acid, L-lysine, and L-tyrosine in a f xed molar ratio of 6.0:1.9:4.7:1.0 and a molecular weight ranging from 4.7 to 11 kDa. Cop-1 has demonstrated to positively modulate PA and induce a strong ef ect over the immune response by binding to the MHC class II molecules on the surface of antigen-presenting cells, without being processed. Vaccination with Cop-1 stimulates T cells, which are activated by determinants common to Cop-1 and myelin basic protein (MBP); suggesting that it has a strong cross-reaction with MBP peptides. Cop-1 increases Th2/3 cytokine secretion patterns, regulatory T cells (Aharoni et al., 2003), IL-4, IL-10, and transforming growth factor-β (TGF-β), a cytokine type that by itself possesses immunomodulatory properties and inhibitis the production of inf ammatory cytokines such as INF-γ, TNF-α and IL-12. Moreover, Cop-1 immunization has the ability to exert neuroregenerative properties. It has proven to increase the production of brain-derived neurotrophic factor (BDNF), insulin-like growth factor 1 (IGF-1) and neurotrophin 3 and 4 (NT-3 and 4) in models of experimental autoimmune encephalomyelitis (EAE) and schizophrenia (Aharoni et al., 2003; Kipnis et al., 2004). Together, these f ndings suggest that modulation of PA–through Cop-1 inmunization– could promote a neuroprotective and neurorestorative environment. Therefore, our group decided to investigate the neuroprotective and neuroregenerative ef ects of this strategy in a focal cerebral ischemia/reperfusion model.
In order to evaluate the neuroprotective ef ect of Cop-1, a model of transient middle cerebral artery occlusion (tMCAo) was developed in our laboratory. Animals were injected with 200 μg of Cop-1 dissolved in saline solution and emulsif ed in an equal volume of complete Freund’s adjuvant. Immunization was applied subcutaneously at the interscapular space immediately after reperfusion (acute phase). In a f rst study, Cop-1 immunized animals presented a signif cant neurological recovery when compared to controls 7 days after ischemia (Ibarra et al., 2007). Additionally, histopathological f ndings had a signif cant correlation with neurological recovery: rats receiving Cop-1 immunization presented a smaller infarct volume after stroke. Such reduction in infarct volume could be related to increased neuroprotection, resulting in less tissue necrosis or inhibition of growth of the ischemic core (Figure 1A). According to recent evidence, these results suggest that Cop-1 specif c immune modulation could be the primary source of neuroprotection after ischemia.
The neurological recovery observed in treated animals led us to investigate the neurorestorative ef ects of Cop-1 over focal cerebral ischemia. During a second experiment (Cruz et al., 2015), immunization with Cop-1 promoted neurological recovery in treated rats (Figure 1B). In addition, a signif cant reduction of infarct volume in Cop-1 immunized animals was observed once again.
In the same work, biochemical studies revealed that treatment with Cop-1 increases the concentration of NT-3 but not BDNF in vivo. It is known that Cop-1 immunization increases the production of BDNF in vitro. However, our f ndings in vivo, showed that Cop-1 augments NT-3, but not BDNF. Additionally, we counted the number of newly formed neurons since existing evidence conf rms that the presence of neurotrophic factors enhances neurogenesis. As a result, we found that Cop-1 increases neurogenesis in the cerebral cortex (CC), the subventricular zone (SVZ) surrounding the ventricles, and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus (Figure 2A). The presence of neural progenitor cells (NPCs) and the increased number of new neurons after immunization could explain, at least in part, the neurological recovery observed in treated animals. Hence, allowing to consider that these newly formed neurons could be able to establish neural connections.
The choroid plexus (CP) plays an important role in modulating the immune response, especif cally PA; thus, it could modulate neurogenesis (Baruch and Schwartz, 2013). Therefore, in order to establish whether Cop-1 is able to modify the microenvironment of the CP, we analyzed the expression of growth factors and citokines 15 days after tMCAo and Cop-1 immunization. Gene expression analysis revealed a considerably higher expression of genes associated with neurogenesis such as NT-3, IGF-1 and also anti-inf ammatory genes like IL-10 (unpublished data).
The promising results provided by Cop-1 led us to examine its ef ect in a model that comprises memory and learning (unpublished data). Aging brings forth an impairment in memory and learning that is clearly associated with a functional def cit of the hippocampal system. This def cit is accompanied by alterations in growth factors (especially BDNF), neurogenesis, and in the hippocampal synapse. We tested whether Cop-1 had a positive ef ect on memory and learning restoration in aged rats. To achieve our goal, 6 month old rats were immunized with Cop-1. Two months later, the spacial memoryand learning of the rats were evaluated, revealing that treatment with Cop-1 improved both variables (unpublished data) (Figure 2B). Interestingly, Cop-1 immunized rats presented increased levels of BDNF (unpublished data). These results indicate that Cop-1 could be an alternative therapy for cognitive impairment and that the ef ect of Cop-1 on BDNF production varies with experimental models.
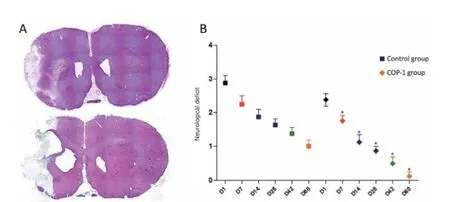
Figure 1 Benef cial ef ects of copolymer-1 (Cop-1).
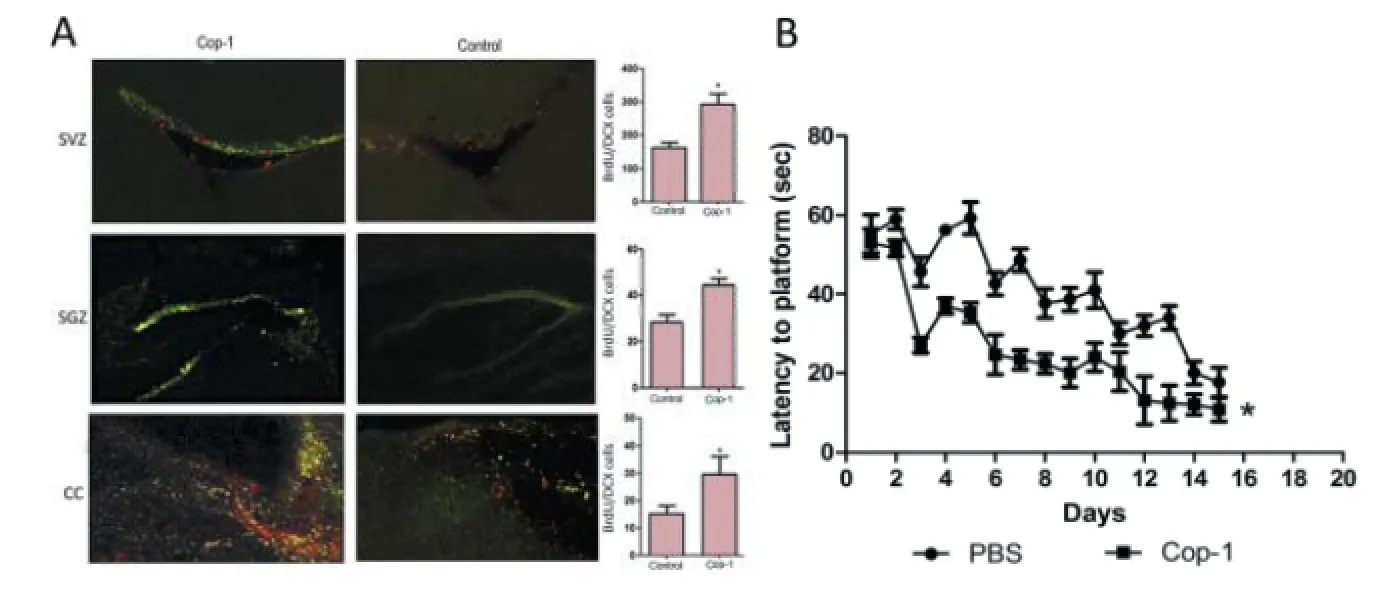
Figure 2 Ef ect of copolymer-1 (Cop-1) on neurogenesis and upon memory and learning.
Immunization with NDP (modulation of PA) could be a succesful therapeutic strategy to control and diminish the progression of secondary degeneration observed after CNS injury. In the case of neurodegenerative diseases caused by functional alterations associated with age, treatment with Cop-1 could be a preventive alternative. Vaccination with synthetic peptides capable of cross-reacting with self-antigens has proven to be benef cial in several neurodegenerative diseases such as multiple sclerosis, damaged optic nerve, Parkinson′s disease (PD), amyotrophic lateral sclerosis (ALS) and others (Ibarra et al., 2007). Cop-1 is an immunomodulatory drug already approved by the Food and Drug Administration (FDA) for the treatment of MS, implying that the safety of the drug has been validated. As a minimally invasive approach for treating neurological diseases involving an inf ammatory response, Cop-1 has the ability to modulate inf ammation and increase local neurotrophic factor production. Preliminary data has led us to believe that active immunization with Cop-1 enhances functional recovery by inducing neuroprotection and neurorestoration. These benef cial ef ects are achieved by avoiding autoimmune disease, local toxicity and by increasing the levels of neurotrophic factors.
In spite of the promising results, a range of new experiments should be designed before Cop-1 therapy can be translated to humans. For instance, the therapeutic window for obtaining the benef cial ef ects of Cop-1, the use of suitable adjuvants for humans, the administration of Cop-1 without any adjuvant, the optimal number of immunizations and the adecuate amount of Cop-1 are some of the topics that must be explored.
As an FDA-approved treatment, Cop-1 could easily be developed for treatment of clinical cerebrovascular diseases or cognitive disorders, with the objective of decreasing mortality and improving the patients’ quality of life. Therapy with Cop-1 represents a promising approach that should be explored in order to optimize the therapeutic strategy for neurodegenerative diseases in the clinical f eld.
We thank Jorge Aguilar Cevallos for his assistance as style reviser and for her comments, which greatly improved this paper.
Yolanda Cruz, Paola Suárez-Meade, Antonio Ibarra*
Facultad de Ciencias de la Salud, Universidad Anáhuac México Norte, Av. Universidad Anáhuac No. 46, Col. Lomas Anáhuac, C.P.52786,
Huixquilucan Edo. de México, México (Cruz Y, Suárez-Meade P, Ibarra A) Proyecto CAMINA A.C., Tlalpan No. 4430 Col. Toriello Guerra, C.P. 14050, México City, México (Ibarra A)
*Correspondence to: Antonio Ibarra, Ph.D., iantonio65@yahoo.com; jose.ibarra@anahuac.mx.
Accepted: 2015-08-03
orcid: 0000-0003-2489-4689 (Antonio Ibarra)
Aharoni R, Kayhan B, Eliam R, Sela M, Arnon R (2003) Glatiramer acetate-specif c T cells in the brain express T helper 2/3 cytokines and brain-derived neurotreophic factor in situ. Proc Natl Acad Sci U S A 24: 14157-14162.
Baruch K, Schwartz M (2013) CNS-specif c T cells shape brain function via the choroid plexus. Brain Behav Immun 34:11-16.
Castillo J (2000) Physiopathology of cerebral ischemia. Rev Neurol 30: 459-464.
Cruz Y, Lorea J, Mestre H, Kim-Lee JH, Herrera J, Mellado R, Gálvez V, Cuellar L, Musri C, Ibarra A (2015) Copolymer-1 promotes neurogenesis and improves functional recovery after acute ischemic stroke in rats. PLoS One 10:1-15.
Ding D (2015) Endovascular mechanical thrombectomy for acute eschemic stroke: a new standard of care. J Stroke 17:123-126.
Fumagalli S, Perego C, Pischiutta F, Zanier ER, Simoni MG (2015) The ischemic environment drives microglia and macrophage function. Front Neurol 6:81.
Hauben E, Nevo U, Yoles E, Moalem G, Agranov E, Mor F, Akselrod S, Neeman M, Cohen IR, Schwartz M (2000) Autoimmune T cells as potential neuroprotective therapy for spinal cord injury. Lancet 355:286-287.
Ibarra A, Avenda?o H, Cruz Y (2007) Copolymer-1 (Cop-1) improves neurological recovery after middle cerebral artery occlusion in rats. Neurosci Lett 425:110-113.
Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M (2004) T cell def ciency leads to cognitive disfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A 101:8180-8185.
Luengo-Fernandez R, Gray AM, Rothwell PM (2012) A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 43:3343-3351.
Schwartz M, Shechter R (2010) Protective autoimmunity functions by intracranial immunosurveillance to support the mind: The missing link between health and disease. Mol Psychiatry 15:342-354.
World health statistics 2014. Geneva: World Health Organization; 2014 (http://apps.who. int/iris/ bitstream/10665/112738/1/9789240692671_eng.pdf).
10.4103/1673-5374.165288 http://www.nrronline.org/
Cruz Y, Suárez-Meade P, Ibarra A (2015) Immunization with Cop-1 promotes neuroprotection and neurogenesis after ischemic stroke. Neural Regen Res 10(11):1733-1734.
 中國(guó)神經(jīng)再生研究(英文版)2015年11期
中國(guó)神經(jīng)再生研究(英文版)2015年11期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
