Combination of fasudil and celecoxib promotes the recovery of injured spinal cord in rats better than celecoxib or fasudil alone
Xiao-lin Hou, Yan Chen, Hua Yin, Wei-gang Duan,
1 Key Laboratory of Molecular Biology for Sinomedicine, Yunnan Key Laboratory for Enthnomedicine, Kunming, Yunnan Province, China
2 Initiative Team of Microenvironment, Yunnan University of Traditional Chinese Medicine, Kunming, Yunnan Province, China
Combination of fasudil and celecoxib promotes the recovery of injured spinal cord in rats better than celecoxib or fasudil alone
Xiao-lin Hou1, Yan Chen1, Hua Yin1, Wei-gang Duan1,2,*
1 Key Laboratory of Molecular Biology for Sinomedicine, Yunnan Key Laboratory for Enthnomedicine, Kunming, Yunnan Province, China
2 Initiative Team of Microenvironment, Yunnan University of Traditional Chinese Medicine, Kunming, Yunnan Province, China
Resistance mechanisms of rho-associated kinase (ROCK) inhibitors are associated with the enhanced expression of cyclooxygenase-2 (COX-2). The therapeutic ef ects of ROCK on nervous system diseases might be enhanced by COX-2 inhibitors. This study investigated the synergistic ef ect of the combined use of the ROCK inhibitor fasudil and a COX-2 inhibitor celecoxib on spinal cord injury in a rat model established by transecting the right half of the spinal cord at T11. Rat models were orally administrated with celecoxib (20 mg/kg) and/or intramuscularly with fasudil (10 mg/kg) for 2 weeks. Results demonstrated that the combined use of celecoxib and fasudil signif cantly decreased COX-2 and Rho kinase II expression surrounding the lesion site in rats with spinal cord injury, improved the pathomorphology of the injured spinal cord, and promoted the recovery of motor function. Moreover, the ef ects of the drug combination were better than celecoxib or fasudil alone. This study demonstrated that the combined use of fasudil and celecoxib synergistically enhanced the functional recovery of injured spinal cord in rats.
nerve regeneration; Rho kinase; fasudil; cyclooxygenase-2; celecoxib; spinal cord injury; NSFC grant; neural regeneration
Funding: The study was supported by the National Natural Science Foundation of China, No. 81060109, and a grant from the Yunnan Provincial Department of Science & Technology in China, No. 2008CD150.
Hou XL, Chen Y, Yin H, Duan WG (2015) Combination of fasudil and celecoxib promotes the recovery of injured spinal cord in rats better than celecoxib or fasudil alone. Neural Regen Res 10(11):1836-1840.
Introduction
The most important step in the recovery of central nervous system injury is to promote neurite growth and to re-establish the physical neural network. In recent years, rho-associated kinase (ROCK) has been recognized as a promising new target for central nervous system injury (Cui et al., 2013), as well as being involved in neural development and neural precursor cell migration (Mueller et al., 2005; Leong et al., 2011; Phillips et al., 2012; Chen et al., 2013; Cui et al., 2013). ROCK is an important regulator of cytoskeleton control, cell adhesion, and gene expression (Leung et al., 1996; Mueller et al., 2005; Groysman et al., 2008; Leong et al., 2011; Feng et al., 2012). There are two isoforms of ROCK, which are encoded by two dif erent genes named ROCK I (ROK α or p160 ROCK) and ROCK II (ROK α) (Mueller et al., 2005). ROCK II is preferentially expressed in the nervous system and plays a pivotal role in neurite outgrowth. After binding to its rho-binding domain and unmasking its catalytic domain, active rho proteins (mainly rho A-GTP), which are activated by membrane signals such as adrenergic receptor activation and prostaglandin E receptor activation (Katoh et al., 1998; Sakurada et al., 2003; Broggini et al., 2010), activate ROCK II. These events lead to substrate phosphorylation (mainly myosin light chain) and trigger cytoskeleton contraction (Mueller et al., 2005) that eventually causes neurite collapse and damage to neural functions (Mueller et al., 2005).
It is believed that neurite outgrowth is the physical foundation for neural network construction and remodeling. Many reports have conf rmed that ROCK II over-expression and/or over-activation inhibited neurite outgrowth induced by nerve growth factor and other stimuli (Lambrechts et al., 2006; Racchetti et al., 2010). In contrast, the low expression of ROCK II and/or ROCK II inhibition triggered or promoted neurite outgrowth (Katoh et al., 1998; Chan et al., 2008). ROCK up-regulation and/or activation have been observed in inf ammation and in many neural disorders, and in animal models ROCK inhibition promoted neural function recovery after nerve damage or brain ischemia/reperfusion injuries (Fournier et al., 2003; Hiraga et al., 2006; Ding et al., 2010).
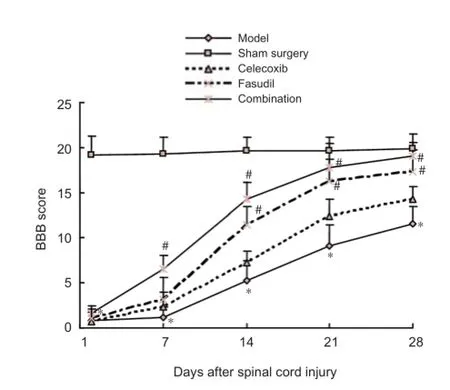
Figure 1 Ef ects of fasudil and celecoxib on locomotor behavior in rats with spinal cord injury.

Figure 2 Ef ects of fasudil and celecoxib on pathological changes in rat spinal cords at 4 weeks after spinal cord injury (hematoxylin-eosin staining).
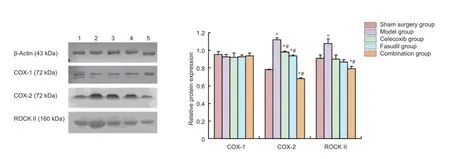
Figure 3 Ef ects of fasudil and celecoxib on COX-2, ROCK II and COX-1 expression in injured spinal tissues of rats at 4 weeks after surgery.
Our previous study in PC12 cells found that ROCK inhibitor Y27632 decreased noradrenalin synthesis and release whereas nerve growth factor increased these processes (Duanet al., 2009). Furthermore, a resistant effect of ROCK inhibitors on neurite outgrowth was observed recently when neural PC12 Adh cells and Neuro-2a cells were exposed to ROCK inhibitors for 3 or more days (Que et al., 2011; Duan et al., 2012). In addition, our previous study indicated the mechanism of resistance was associated with the up-regulation of inf ammatory pathways, especially the cyclooxygenase-2 (COX-2) pathway (Duan et al., 2012; Yin et al., 2015), because in cell cultures, the resistant ef ect was partly improved by a COX-2 selective inhibitor, NS-398 (Duan et al., 2012). This previous evidence suggests that the ef ect of ROCK inhibitors in treating neurological disorders might be enhanced by COX-2 inhibitors. Therefore, this study investigated the synergistic ef ect of a ROCK inhibitor fasudil and a COX-2 inhibitor celecoxib to enhance neural function recovery in spinal cord injury of rats.
Materials and Methods
Spinal cord hemi-transection
Forty adult, clean, female, Sprague-Dawley rats aged 3 months and weighing 280–330 g, were obtained from Jianyang Shuoda Science and Technology Ltd., China (Certification No. SCXK (Chuan) 2008-24). Rats were housed in temperature-controlled (22°C) and humidity-controlled (45–55%) conditions, under natural light. This project was approved by the Experimental Animal Committee of Yunnan University of Traditional Chinese Medicine in China. Forty rats were randomized to f ve groups as follows: sham surgery, model, celecoxib, fasudil and combination groups, with eight rats in each group. All rats were anesthetized with pentobarbital sodium 40 mg/kg. The crest of T11was excised with a pair of nipper pliers, and a hole with a diameter of 2 mm was drilled with the cusp of scissors at the center where the crest was located. Then, a syringe needle was gently placed into the spinal cord via the hole and the right part of the spinal cord was cut by moving the needle to the right in rats. In the sham surgery group, the needle was placed into spinal cord without movement.
Drug administration
When the operation was f nished, rats in the sham surgery and model groups were treated normally. Rats in the celecoxib group were intragastrically administrated with a suspension of celecoxib (20 mg/kg; Pf zer Inc., Dalian, China), and a suspension of celecoxib containing 0.5% sodium carboxymethylcellulose was made from the capsules. Rats in the fasudil group were intramuscularly administrated with fasudil hydrochloride injection (10 mg/kg; Tianjin Chase Sun Pharmaceutical Co., Ltd., Tianjin, China) via the dorsal muscle. Rats in the combination group were administrated with both a suspension of celecoxib (20 mg/kg) and fasudil hydrochloride (10 mg/kg). The fasudil and celecoxib doses were based on doses administered to adults and these were adjusted in a pre-study. Administration was once every day for 2 weeks. Subsequently, all rats were treated normally for another 2 weeks, and then sacrif ced either for histological examination or for western blot assay.
Behavioral analysis
All rats were subjected to behavioral examination preoperatively, and at 1, 7, 14, 21, and 28 days after surgery. The Basso-Beattie-Bresnahan (BBB) locomotor rating scale (Basso et al., 1995) was used to analyze individual components of limb movement, weight support, plantar and dorsal stepping, forelimb-hindlimb coordination, paw rotation, toe clearance, trunk stability, and tail placement. Scores from 0 to 21 were given based on these observations. The BBB scores of normal rats were 21.
Histological examination
Four rats in each group at 4 weeks after surgery were perfused with 4% paraformaldehyde (pH 7.2) via the left ventricle after euthanasia. The vertebral column including the injury site and the surrounding area (2 mm) was harvested, and immersed in 4% paraformaldehyde until a routine histological operation was conducted. Paraf n sections of the spinal cord through the lesion were cut parasagittally or paracoronally (10 μm). Transverse sections were collected from the spinal cord rostral and caudal to the injury site, and coronal sections were also collected from the spinal cord proster and posterior to the injury site. The sections were stained with a hematoxylin-eosin staining kit. Images were obtained with a light microscope (Nikon, Tokyo, Japan).
Western blot assay
The remaining four rats in each group at 4 weeks after surgery were sacrif ced and their spinal cords were carefully removed. Spinal cord at 2.5 mm from the lesion site was discarded. The remaining spinal cord of 5 mm was homogenized with PBS in ice-cold water. The homogenate was centrifuged at 6,000 × g, 4°C for 10 minutes and the supernatant was collected as a protein sample for western blot assay as previously described (Fukuda et al., 2005) with some modif cations. Brief y, protein (50 μg, 5 μg/μL × 10 μL) of dif erent samples was used for sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein was transferred to a nitrocellulose f lter at 10 V for 60 minutes in a Semi-Dry Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA, USA). The nitrocellulose f lter was blocked with 3% bovine serum albumin at room temperature for 2 hours, incubated in a solution containing rabbit anti-rat ROCK II monoclonal antibody (1:400; Merck Millipore Ltd., Darmstadt, Germany), rabbit anti-rat COX-1 monoclonal antibody (1:400; Boster Company, Wuhan, Hubei Province, China), rabbit anti-rat COX-2 monoclonal antibody (1:400; Boster Company) and rabbit anti-rat β-actin monoclonal antibody (1:400; Boster Company) at 4°C overnight. The f lter was rinsed three times with Tris-HCl buffer with Tween-20 solution (20 mM Tris-HCl, pH 7.5, 0.05% Tween-20) each for 10 minutes, and incubated in a solution containing goat anti-rabbit antibody linked with horseradish peroxidase (1:800; Boster Company) for an additional 2 hours. The nitrocellulose f lter was rinsed with Tris-HCl buf er and Tween-20 solution for 10 minutes three times and developed on X-ray f lms by enhanced chemiluminescence detection kits (Pierce Biotechnology Inc.,Rockford, IL, USA). The X-ray f lms were scanned by a gel imager (Bio-Rad, Hercules, CA, USA). The results were expressed as the optical density ratio to β-actin.
Statistical analysis
Data are expressed as the mean ± SD. One-way analysis of variance followed by the least signif cant dif erence post hoc test was performed to compare the dif erence between groups. A value of P < 0.05 was considered statistically signif cant. The statistical analysis was conducted with SPSS for Windows 16.0 (SPSS, Chicago, IL, USA).
Results
Combined administration of fasudil and celecoxib improved locomotor behavior of rats with spinal cord injury
The body weight of rats after surgery increased slowly. Rat locomotor activities in the celecoxib, fasudil and combination groups were similar to that of the model group immediately after injury. However, the recovery of rats in the celecoxib, fasudil or combination groups was increased compared to controls (P < 0.05). The recovery of rat locomotor activity was enhanced in the combination group compared with the model, celecoxib and fasudil groups (Figure 1).
Ef ects of fasudil and celecoxib on pathological changes in rats with spinal cord injury
Hematoxylin-eosin staining in transverse sections showed that no micropathological injury was visible in the sham surgery group rats. However, abundant cell death was observed in the damaged spinal tissue in rats from the model group. The damaged spinal tissue was recovered to a certain extent in the celecoxib, fasudil, and combination groups. The recovered tissues in rats of the fasudil and combination groups were similar to those in the sham surgery group, but with the presence of slight cellular edema and inf ammation (Figure 2).
Ef ects of fasudil and celecoxib on protein expression of COX-2, ROCK II, and COX-1 in injured spinal cord tissues of rats
The results of the western blot assay showed that at 28 days after spinal cord injury, the expression of COX-2 and ROCK II in injured spinal tissues was up-regulated in the model group, followed by the celecoxib group, fasudil group and combination group (Figure 3). The expression prof ling of COX-2 in injured spinal tissues was similar to that of ROCK II. COX-1 is a constitutively expressed molecule and its expression in injured spinal tissues was maintained at a stable level in all the dif erent groups.
Discussion
That ROCK inhibitors are promising agents for neurological disorders, and have been widely accepted by pharmacologists (Mueller et al., 2005; Chen et al., 2013). However, their therapeutic ef ect was further enhanced in a spinal cord injury model (Hiraga et al., 2006) and in our previous studies (Que et al., 2011; Duan et al., 2012).
In the present study, fasudil was intramuscularly administrated and celecoxib was orally administrated. The only ROCK inhibitor licensed for clinical use is fasudil and this is administered via injection (fasudil is not stable enough for oral administration). Most COX-2 inhibitors are prepared orally. Ideally, the delivery route of both drugs should have been the same. However, as the main aim of our study was to indicate the clinical application of the drugs, we consider the ef ects of dif erent delivery routes to be negligible. The different ways of drug delivery could, however, introduce some interference in the therapeutic ef ect. However, we consider these ef ects to be negligible as the celecoxib group and fasudil group are control groups for the combined group. The benef cial synergistic ef ects can be found by comparing the combined group with the fasudil group and celecoxib group.
Other studies demonstrated that spinal cord injury causes inf ammatory reactions that prevent spinal cord recovery and the up-regulation of COX-2 and ROCK II (Sch?fers et al., 2004; Conrad et al., 2005). These evidences were conf rmed by the present study. Based on these f ndings, it could be deduced that either COX-2 or ROCK inhibition might promote the recovery of spinal cord injury, and this was conf rmed by Schafers’s group (Sch?fers et al., 2004) and Conrad’s group (Conrad et al., 2005), respectively. Furthermore, Schwab et al. (2004) suggested that COX inhibitors might be useful for the treatment of spinal cord injury, and are candidates for a safe, synergistic, adjuvant treatment option in combination with cell-specif c approaches of rho inactivation. This would minimize the pool of rhoA+(whose ef ector protein is ROCK) cells at the lesion site following spinal cord injury (Schwab et al., 2004). However, the synergestic ef ect of a COX inhibitor and ROCK inhibitor in improving spinal cord injury has not been conf rmed directly. In the present study, the combined application of a ROCK inhibitor (fasudil) and a COX-2 inhibitor (celecoxib) conf rmed the synergestic ef ect, because behavioral scores of the combination group were higher than those of the other groups except the sham surgery group. The results of histological examination also supported the synergestic ef ect. The ef ect of the ROCK inhibitor in enhancing spinal cord injury (Hiraga et al., 2006) was conf rmed in the present study. However, slightly dif erent from a previous report (Schwab et al., 2004), there was a trend in the benef cial ef ect of celecoxib, but it was not statistically signif cant.
Our previous study (Duan et al., 2012) suggested that: (1) inf ammatory reactions might cause the over-expression of COX-2 and other bio-macromolecules; (2) COX (especially COX-2) would catalyze arachidonic acid from membrane phospholipids to prostaglandins (PGs), including thromboxane A2, PGF2α, and PGE2; (3) these PGs might dif use out of the cell to act on neural cells and glial cells; (4) PGs activated their membrane receptors whose ef ector protein was ROCK II; (5) receptor activation would result in the contraction of the cytoskeleton; and (6) that eventually, cytoskeleton contraction would cause neurite collapse. In the present animal model, two factors, inf ammation and theprolonged use of a ROCK inhibitor, might have contributed to COX-2 over-expression (Sch?fers et al., 2004; Duan et al., 2012; Yin et al., 2015). However, it was dif cult for us to distinguish between these mechanisms in the animal model. Nevertheless, a benef cial synergistic ef ect was observed after the combined use of ROCK inhibitor fasudil and COX-2 inhibitor celecoxib.
In summary, the combined use of ROCK inhibitor fasudil and celecoxib synergistically enhanced the functional recovery in spinal cord injury in rats.
Author contributions: WGD conceived and designed the study as well as analyzed and interpreted the data. YC carried out histological examination. HY performed western blot assay. WGD wrote the paper. All authors performed animal experiments and approved the f nal version of the paper.
Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open f eld testing in rats. J Neurotrauma 12:1-21.
Broggini T, Nitsch R, Savaskan NE (2010) Plasticity-related gene 5 (PRG5) induces f lopodia and neurite growth and impedes lysophosphatidic acid- and nogo-A-mediated axonal retraction. Mol Biol Cell 21:521-537.
Chan CC, Roberts CR, Steeves JD, Tetzlaff W (2008) Aggrecan components dif erentially modulate nerve growth factor-responsive and neurotrophin-3-responsive dorsal root ganglion neurite growth. J Neurosci Res 86:581-592.
Chen K, Zhang W, Chen J, Li S, Guo G (2013) Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regen Res 8:3027-3035.
Chen KE, Zhang WB, Chen J, Li SM, Guo GQ (2013) Rho-associated protein kinase modulates neurite extension by regulating microtubule remodeling and vinculin distribution. Neural Regen Res 8:3027-3035.
Conrad S, Schluesener HJ, Trautmann K, Joannin N, Meyermann R, Schwab JM (2005) Prolonged lesional expression of RhoA and RhoB following spinal cord injury. J Comp Neurol 487:166-175.
Cui QH, Zhang YB, Chen H, Li JM (2013) Rho kinase: a new target for treatment of cerebral ischemia/reperfusion injury. Neural Regen Res 8:1180-1189.
Ding J, Li QY, Yu JZ, Wang X, Sun CH, Lu CZ, Xiao BG (2010) Fasudil, a Rho kinase inhibitor, drives mobilization of adult neural stem cells after hypoxia/reoxygenation injury in mice. Mol Cell Neurosci 43:201-208.
Duan WG, Que L, Lv XM, Li QF, Yin H, Zhang LY (2012) Tolerance of neurite outgrowth to Rho kinase inhibitors decreased by cyclooxygenase-2 inhibitor. Neural Regen Res 7:2705-2712.
Duan WG, Shang J, Jiang ZZ, Yao JC, Yun Y, Yan M, Shu B, Lin Q, Yu ZP, Zhang LY (2009) Rho kinase inhibitor Y-27632 down-regulates norepinephrine synthesis and release in PC12 cells. Basic Clin Pharmacol Toxicol 104:434-440.
Feng JF, Liu J, Zhang XZ, Zhang L, Jiang JY, Nolta J, Zhao M (2012) Guided migration of neural stem cells derived from human embryonic stem cells by an electric f eld. Stem Cells 30:349-355.
Fournier AE, Takizawa BT, Strittmatter SM (2003) Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23:1416-1423.
Fukuda T, Takekoshi K, Nanmoku T, Ishii K, Isobe K, Kawakami Y (2005) Inhibition of the RhoA/Rho kinase system attenuates catecholamine biosynthesis in PC 12 rat pheochromocytoma cells. Biochim Biophys Acta 1726:28-33.
Groysman M, Shoval I, Kalcheim C (2008) A negative modulatory role for rho and rho-associated kinase signaling in delamination of neural crest cells. Neural Dev 3:27.
Hiraga A, Kuwabara S, Doya H, Kanai K, Fujitani M, Taniguchi J, Arai K, Mori M, Hattori T, Yamashita T (2006) Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J Peripher Nerv Syst 11:217-224.
Katoh H, Aoki J, Ichikawa A, Negishi M (1998) p160 RhoA-binding kinase ROKalpha induces neurite retraction. J Biol Chem 273:2489-2492.
Lambrechts A, Jonckheere V, Peleman C, Polet D, De Vos W, Vandekerckhove J, Ampe C (2006) Prof lin-I-ligand interactions inf uence various aspects of neuronal dif erentiation. J Cell Sci 119:1570-1578.
Leong SY, Faux CH, Turbic A, Dixon KJ, Turnley AM (2011) The Rho kinase pathway regulates mouse adult neural precursor cell migration. Stem Cells 29:332-343.
Leung T, Chen XQ, Manser E, Lim L (1996) The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16:5313-5327.
Mueller BK, Mack H, Teusch N (2005) Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 4:387-398.
Phillips HM, Papoutsi T, Soenen H, Ybot-Gonzalez P, Henderson DJ, Chaudhry B (2012) Neural crest cell survival is dependent on Rho kinase and is required for development of the mid face in mouse embryos. PLoS One 7:e37685.
Que L, Duan WG, Zhang LY, Jiang ZZ (2011) Differences of neurite outgrowth induced by rho kinase inhibitors between in PC12 cell line and PC12 Adh cell line. Yunnan Daxue Xuebao: Ziran Kexue Ban 33:370-372.
Racchetti G, Lorusso A, Schulte C, Gavello D, Carabelli V, D’Alessandro R, Meldolesi J (2010) Rapid neurite outgrowth in neurosecretory cells and neurons is sustained by the exocytosis of a cytoplasmic organelle, the enlargeosome. J Cell Sci 123:165-170.
Sakurada S, Takuwa N, Sugimoto N, Wang Y, Seto M, Sasaki Y, Takuwa Y (2003) Ca2+-dependent activation of Rho and Rho kinase in membrane depolarization-induced and receptor stimulation-induced vascular smooth muscle contraction. Circ Res 93:548-556.
Sch?fers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C (2004) Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol 185:160-168.
Schwab JM, Conrad S, Elbert T, Trautmann K, Meyermann R, Schluesener HJ (2004) Lesional RhoA+cell numbers are suppressed by anti-inf ammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia 47:377-386.
Yin H, Hou X, Tao T, Lv X, Zhang L, Duan W (2015) Neurite outgrowth resistance to rho kinase inhibitors in PC12 Adh cell. Cell Biol Int doi: 10.1002/cbin.10423.
Copyedited by Croxford L, Pack M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
*Correspondence to: Wei-gang Duan, Ph.D., deardwg@126.com.
orcid: 0000-0002-9006-861X (Wei-gang Duan)
10.4103/1673-5374.170314 http://www.nrronline.org/
Accepted: 2015-06-16
- 中國神經(jīng)再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces

