Autologous mesenchymal stem cells applied on the pressure ulcers had produced a surprising outcome in a severe case of neuromyelitis optica
Adriana Octaviana Dulamea, Mirela-Patricia Sirbu-Boeti Coralia Bleotu, Denisa Dragu, Lucia Moldovan, Ioana Lupescu Giancarlo Comi
1 U.M.F. Carol Davila, 8 Bulevardul Eroii Sanitari, Bucharest, Sector 2, Romania
2 Department of Neurology, Fundeni Clinical Institute, 258 Soseaua Fundeni, Bucharest, Sector 5, Romania
3 National Virology Institute Stefan S. Nicolau, 285 Mihai Bravu Avenue, Bucharest, Sector 3, PO 77, PO Box 201, Romania
4 National Institute of Research and Development for Biological Sciences, 296 Splaiul Independen?ei, Bucharest, Sector 6, PO Box 17-16, Romania
5 Universita Vita-Salute San Raff aele, 58 Via Olgettina, Milan, Italy
Autologous mesenchymal stem cells applied on the pressure ulcers had produced a surprising outcome in a severe case of neuromyelitis optica
Adriana Octaviana Dulamea1,2,#,*, Mirela-Patricia Sirbu-Boeti1,2,#, Coralia Bleotu3, Denisa Dragu3, Lucia Moldovan4, Ioana Lupescu1,2, Giancarlo Comi5
1 U.M.F. Carol Davila, 8 Bulevardul Eroii Sanitari, Bucharest, Sector 2, Romania
2 Department of Neurology, Fundeni Clinical Institute, 258 Soseaua Fundeni, Bucharest, Sector 5, Romania
3 National Virology Institute Stefan S. Nicolau, 285 Mihai Bravu Avenue, Bucharest, Sector 3, PO 77, PO Box 201, Romania
4 National Institute of Research and Development for Biological Sciences, 296 Splaiul Independen?ei, Bucharest, Sector 6, PO Box 17-16, Romania
5 Universita Vita-Salute San Raff aele, 58 Via Olgettina, Milan, Italy
Recent studies provided evidence that mesenchymal stem cells (MSCs) have regenerative potential in cutaneous repair and profound immunomodulatory properties making them a candidate for therapy of neuroimmunologic diseases. Neuromyelitis optica (NMO) is an autoimmune, demyelinating central nervous system disorder characterized by a longitudinally extensive spinal cord lesion. A 46-year-old male diagnosed with NMO had relapses with paraplegia despite treatment and developed two stage IV pressure ulcers (PUs) on his legs. The patient consented for local application of autologous MSCs on PUs. MSCs isolated from the patient’s bone marrow aspirate were multiplied in vitro during three passages and embedded in a tridimensional collagen-rich matrix which was applied on the PUs. Eight days after MSCs application the patient showed a progressive healing of PUs and improvement of disability. Two months later the patient was able to walk 20 m with bilateral assistance and one year later he started to walk without assistance. For 76 months the patient had no relapse and no adverse event was reported. The original method of local application of autologous BM-MSCs contributed to healing of PUs. For 6 years the patient was free of relapses and showed an improvement of disability. The association of cutaneous repair, sustained remission of NMO and improvement of disability might be explained by a promotion/optimization of recovery mechanisms in the central nervous system even if alternative hypothesis should be considered. Further studies are needed to assess the safety and effi cacy of mesenchymal stem cells in NMO treatment.
neuromyelitis optica; Devic’s syndrome; mesenchymal stem cells; multiple sclerosis; pressure ulcers
Funding: This work was supported by the Romanian Ministry of Education and Research (Research project: Alternative therapies for major tissue defects 42136/01.10.2008).
Dulamea AO, Sirbu-Boeti MP, Bleotu C, Dragu D, Moldovan L, Lupescu I, Comi G (2015) Autologous mesenchymal stem cells applied on the pressure ulcers had produced a surprising outcome in a severe case of neuromyelitis optica. Neural Regen Res 10(11):1841-1845.
Introduction
Mesenchymal stem cells (MSCs) are a subset of adult progenitor cells isolated from hematogenous bone marrow and tissues derived from mesoderm (Dominici et al., 2006). Experimental studies provided evidence about the eff ect of bone marrow mesenchymal stem cells (BMMSCs) on epidermal regeneration (McFarlin et al., 2006; Dash et al., 2009; Wu et al., 2010). Evidence from preclinical studies suggested the capacity of MSCs to inhibit pathogenic immune responses and release neuroprotective and pro-oligodendrogenic molecules favouring tissue repair (Di Nicola et al., 2002; Zhang et al., 2005, 2006; Gerdoni et al., 2007). Small clinical studies showed safety and tolerability of MSCs and stabilization or mild improvement in patients with multiple sclerosis (Karussis et al., 2010; Yamout et al., 2010; Bonab et al., 2012; Lalu et al., 2012; Llufriu et al., 2014). Neuromyelitis optica (NMO, Devic’s syndrome) is an infl ammatory, demyelinating central nervous system disorder characterized by concurrence of optic neuritis and myelitis in a relapsing course. A humoral autoimmune mechanism is involved, targeting aquaporin 4 channels on astrocytes resulting in demyelination and axonal injury (Wingerchuk and Weinshenker, 2008). We report the suprising outcome of local application, on pressure ulcers, of autologous BMMSCs in a case of severe NMO.
Case Report
In March 2003, a 46-year-old male without previous medical and family history presented paraesthesia and pain in both legs followed by progressive paraparesis (left leg 2/5 BMRC, right leg 3/5 BMRC). Spinal cord magnetic resonance imaging (MRI) revealed a lesion with edematous component (T2 and FLAIR hyperintensity and T1 hy-pointensity) at T2–4level, producing an increase in volume of the spinal cord and a reduction of the peri-spinal spaces, with central, ring-shape contrast enhancement corresponding to a demyelinating lesion. Brain MRI showed three small demyelinating lesions, with hyperintensity in T2 sequences, localized in the subcortical white matter of the left frontal lobe and right parietal lobe (Figure 1). The patient tested negative for Borrelia Burgdorferi, human immunodeficiency virus, hepatitis B and C viruses. Serum angiotensin converting enzyme, antinuclear antibodies, antineutrophil cytoplasmic antibodies, anti Sm antibodies, and anti-double stranded DNA antibodies were negative. The patient refused lumbar puncture. A diagnosis of transverse myelitis was formulated. Until April 2008 the patient had three relapses: on September 2006 with paraplegia, on May 2007 with diminished visual acuity of the right eye, paraparesis (2/5 BMRC) and in November 2007 with paraplegia. After recovery from the last relapse the patient’s Expanded Disability Status Scale (EDSS) score was 6.5. Visual evoked potentials (VEPs) revealed right optic neuritis. The relapses have been treated with methylprednisolone 1 g intravenously once daily for 5 days and as chronic treatment: methylprednisolone oral, followed by pulse therapy with intravenous cyclophosphamide and then oral azathioprine associated to methylprednisolone. In April 2008, the patient presented a new relapse with decreased visual acuity of the right eye and paraplegia. At the admission into the Department of Neurology of Fundeni Clinical Institute, the neurologic examination showed in the right eye disc pallor and visual accuity (corrected) 20/40 and in the left eye visual accuity corrected 20/20, paraplegia, bilateral pyramidal syndrome, superfi cial, vibratory and position sense hypoesthesia in right leg, anesthesia of left leg, urinary retention, with frequent urinary infections, sexual dysfunction, fatigue, anxiety, helpless bedridden patient, with no self-care (EDSS 9, Hauser Ambulation Index AI 9) and three PUs: two stage IV PUs on the lateral left malleolus (8/7 cm) and on lateral side of thigh (3 cm) respectively and a stage III PU on right calcaneum. Brain and spinal cord MRI showed hypotrophy of the spinal cord at T2–4level, the three subcortical lesions were still present and no new demyelinating lesions appeared (Figure 2A–C). VEPs showed a P100 latency of 115 ms and amplitude N7-P100 of 4 μV in the right eye and a P100 latency of 112 ms and an amplitude N7-P100 of 12 μV in the left eye. Cerebrospinal fl uid analysis showed 30 mg/dl protein, 52 mg/dl glucose, no oligoclonal bands and no cells. Immunofl uorescence on monkey tissue (Laboraf Diagnostica e Ricerca San Raff aele Milano, Italy) disclosed the presence of NMO IgG anti-aquaporin-4 antibodies in serum sample. NMO was diagnosed based on clinical signs and course with relapses, the presence of a spinal cord MRI lesion on three vertebral segments and NMO-IgG seropositive status (Wingerchuk and Weinshenker, 2008).
Methods
In order to treat the PUs, the patient was referred to the Department of General Surgery and Liver Transplantation. Cultures of wound secretions came positive for Proteus and Stafi lococcus aureus. Systemic antibiotic therapy and local treatment with Sorbalgon, Atrauman Ag, and TenderWet were conducted to clean the wounds. The patient was offered a plastic surgery consultation and the possibility to choose between skin graft and local application of autologous MSCs for the treatment of PUs. He opted for the last choice.
The approval by the Ethics Committee of Fundeni Clinical Institute was obtained for application of biological dressing with autologous BMMSCs. The patient signed the written informed consent for the treatment of PUs with autologous BMMSCs. The patient was investigated to exclude any ongoing malignant disease with the standard check-up procedure used for transplanted patients. The serum tests checked for viral and oncologic status were negative.
The bone marrow aspirate was obtained from the right posterior superior iliac spina for BMMSCs isolation. Mononuclear cells were separated by centrifugation over a Biocoll gradient (Biochrom, Germany) and suspended in alpha-MEM containing 10% autologous serum and penicillin-streptomycin mixture (Biochrom, Germany), followed by plating at an initial seeding density of 1 × 106/cm2. After 3 days, the nonadherent cells were removed and monolayers of adherent cells were cultured until confl uence. Cells were trypsinized with 0.25% trypsin and subcultured at densities of 5,000–6,000 cells/cm2. After the fi rst passage, the culture of MSCs became homogenous and demonstrated a high proliferation potential. The cell surface marker identifi cation was performed by Epics XL FACS analyzer (Beckman Coulter) using fl uorescein isothiocyanate (FITC) or phycoerythin (PE)-labeled monoclonal antibodies. Phenotypic characterization confi rmed MSCs: CD105+, CD90+, CD34–, CD45–, CD3–, CD14–(Figure 3). A normal karyotype of MSCs was confi rmed. The biological dressing was made of 8 × 106BM-MSCs seeded into a 30 cm2collagen-agarose sponge placed on a decellularized human arterial fragment using a previous protocol (Sirbu-Boeti et al., 2009).
Stage IV PUs were completely covered with the biological dressing.
Results and Discussion
The evolution of the wounds was followed daily for the fi rst 2 weeks and then every 2 days for other 2 weeks. Two months later the left lateral femoral PU was completely epithelized. The healing of the left malleolar PU was completed 5 months later with normal function of ankle joint. The right calcanean PU healed with dry dressing (Figure 4).
Concomitent with pressure ulcers’ healing the authors noticed a progressive improvement in the neurological status of the patient. Since this change was not anticipated before the procedure, topical application of MSCs being made with the intention to treat the pressure ulcers, the authors had not planned to assess the immunological status of the patient before and after the procedure.
However the neurostatus was daily recorded by the neurologist and noticed in a dairy by the patient’s spouse and later by the patient himself. First a recovery of sensitivity at the treated leg occurred. Two weeks later the movements of the fi ngers of both legs improved. One month later the patient recovered his sensitivity for both legs, was able to move in bed by himself and to sit, the paraparesis was 3/5 BMRC for the left and right legs, but spasticity of both legs occurred. EDSS improved from 9.0 to 8.0, AI 9. At 2 months, patient’s exam-ination revealed: in the right eye disc pallor and visual acuity 20/40, in the left leg BMRC 3 for hip flexors, knee flexors and extensors, plantar fl exion and extension, in the right leg BMRC 3 for hip fl exors, BMRC 2 for knee fl exors and extensors, BMRC 2 for plantar fl exion and extension, spasticity of both legs, moderate limb ataxia of both legs, moderate decrease of superfi cial and mild decrease of vibration sense of left leg, urinary retention, fatigue and the patient was able to walk 5 meters with constant bilateral assistance (EDSS 7.0, AI 7). At four months neurologic examination showed: in the right eye disc pallor and visual acuity 20/40, superfi -cial hypoesthesia on left leg, paraparesis (left leg BMRC 3/5, right leg BMRC 4/5), spasticity on fl exors of the left inferior limb, no ataxia, 40 meters ambulation with constant bilateral assistance (EDSS 6.5, AI 6). Eight months later the patient was ambulatory with unilateral assistance (cane) for 150 m without rest (EDSS 6.0, AI 4). In May 2009, at 11 months, brain and spinal cord MRI showed some new T2 lesions in the frontal regions and unchanged aspect at thoracic spinal cord (Figure 2D–F). VEPs and optic nerve MRI were not performed. At 12 months the patient was ambulatory without aid or rest for more than 300 m, EDSS 4.5, AI 3. At 32 months the patient was able to ride bicycle and to shovel snow (EDSS 3.5, AI 2). At 76 months after MSCs therapy the patient was free of relapse, with EDSS 3.5, AI 2, needs only 4 mg methylprednisolone every other day and had negative malignancy screening. Figure 5 and Supplementary Video online illustrate the neurological evolution of the patient.
The successful healing of both PUs was expected. Other studies reported rapid wound healing with reduced scarring after administration of mesenchymal stem cells. They showed that MSCs stimulate angiogenesis and induce fi broblast migration due to a paracrine eff ect (Rodriguez-Menocal et al., 2015). The innovation consisted in the application of BM-MSCs embedded in a collagen-agarose sponge that has the advantage of alleviating the cell damage which is associated with injection barotrauma and avoiding the side eff ects secondary to other administration routes (e.g., intravenous, intra-arterial).
However the spectacular and long-term improvement of disability cannot be explained only by healing of PUs since the patient was paraplegic long before the development of PUs.
Several studies showed that mesenchymal stem cells modulate the innate and adaptive immunity mostly through the secretion of paracrine factors (“bystander eff ect”) (Krampera et al., 2006; Karussis et al., 2008; Uccelli et al., 2011; Keating, 2012).
In our case, a possible explanation of the improvement of disability secondary to the local application of BMMSCs on PUs might be that local infl ammation facilitated the migration of BMMSCs into the peripheral lymph nodes producing a peripheral immunomodulation since the recovery lasted. An alternative explanation for the partial remission could be the absence of further relapses. However the interpretation of the outcome must be made with caution since, appart from the neurological evolution of the patient, we do not have other arguments to sustain that the long term remission was a consequence of the local application of mesenchymal stem cells.
Conclusions
This is the first report of topical application of a biological dressing embedded with autologous in vitro expanded BMMSCs in an NMO patient with stage IV PUs that accelerated the healing of PUs and was associated with a spectacular and long term improvement of patient’s disability (from EDSS 9 to EDSS 3.5). This curious association could be explained by a promotion / optimization of recovery mechanisms in the central nervous system even if alternative hypothesis should be considered. Further studies are needed in order to assess the safety and effi cacy of mesenchymal stem cells in NMO treatment.
Acknowledgments: We thank Mr. Stefan Constantinescu and Mrs. Melania Dulamea for preparing the movie and fi gures of the paper and Mrs. Monica Marinescu for the proofreading of the English version of the paper.
Confl icts of interest: None declared.
Supplementary data: Supplementary information is available at http://www.nrronline.org.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, Nikbin B, Lotfi J, Khorramnia S, Motamed MR, Togha M, Harirchian MH, Moghadam NB, Alikhani K, Yadegari S, Jafarian S, Gheini MR (2012) Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther 7:407-414.
Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC (2009) Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 12:359-366.
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecifi c mitogenic stimuli. Blood 99:3838-3843.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defi ning multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315-317.
Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, Pedemonte E, Mantegazza R, Frassoni F, Mancardi G, Pedotti R, Uccelli A (2007) Mesenchymal stem cells eff ectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol 61:219-227.
Karussis D, Kassis I, Kurkalli BG, Slavin S (2008) Immunomodulation and neuroprotection with mesenchymal bone marrow stem cells (MSCs): a proposed treatment for multiple sclerosis and other neuroimmunological/neurodegenerative diseases. J Neurol Sci 265:131-135.
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S (2010) Safety and immunological eff ects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol 67:1187-1194.
Keating A (2012) Mesenchymal stromal cells: new directions. Cell Stem Cell 10:709-716.
Krampera M, Pasini A, Pizzolo G, Cosmi L, Romagnani S, Annunziato F (2006) Regenerative and immunomodulatory potential of mesenchymal stem cells. Curr Opin Pharmacol 6:435-441.
Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ (2012) Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One 7:e47559.
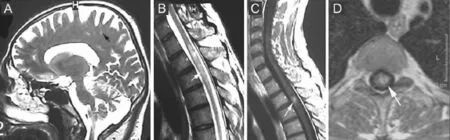
Figure 1 Magnetic resonance imaging (MRI) evaluation in 2003.
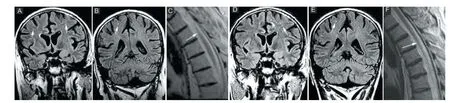
Figure 2 Magnetic resonance imaging (MRI) evaluation in 2008 (A–C) and 2009 (D–F).
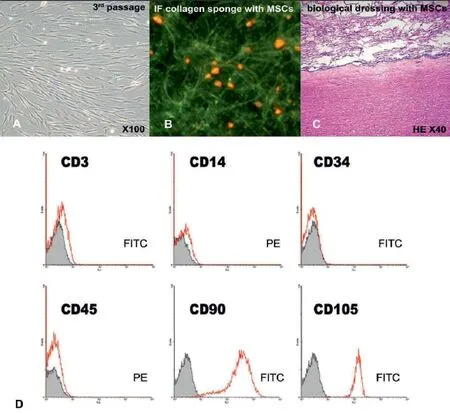
Figure 3 Microscopy and phenotypic characterization of the bone marrow mesenchymal stem cells culture.
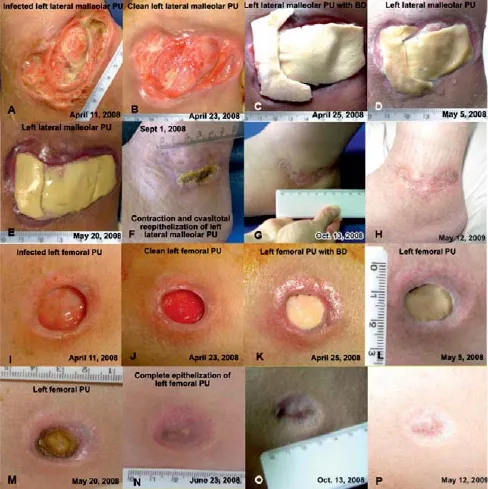
Figure 4 Macroscopic aspect of pressure ulcers (PUs) of the patient.
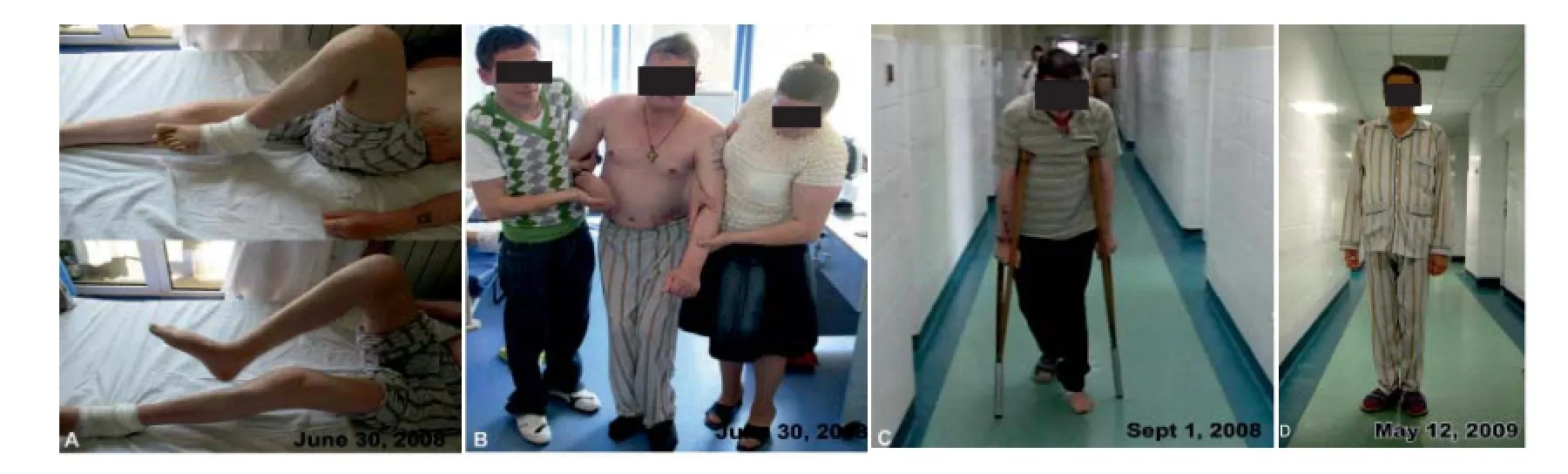
Figure 5 Neurological evolution of the patient after local biological dressing with mesenchymal stem cells (MSCs) culture.
Llufriu S, Sepulveda M, Blanco Y, Marin P, Moreno B, Berenguer J, Gabilondo I, Martinez-Heras E, Sola-Valls N, Arnaiz JA, Andreu EJ, Fernandez B, Bullich S, Sanchez-Dalmau B, Graus F, Villoslada P, Saiz A (2014) Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS One 9:e113936.
McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, Bansal M, Li Y, Chopp M, Dulchavsky SA, Gautam SC (2006) Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen 14:471-478.
Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E (2015) Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther 6:24.
Sirbu-Boeti MP, Chivu M, Paslaru LL, Efrimescu C, Herlea V, Pecheanu C, Moldovan L, Dragomir L, Bleotu C, Ciucur E, Vidulescu C, Vasilescu M, Boicea A, Manoiu S, Ionescu MI, Popescu I (2009) Transplantation of mesenchymal stem cells cultured on biomatrix support induces repairing of digestive tract defects, in animal model. Chirurgia 104:55-65.
Uccelli A, Laroni A, Freedman MS (2011) Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol 10:649-656.
Wingerchuk DM, Weinshenker BG (2008) Neuromyelitis optica. Curr Treat Options Neurol 10:55-66.
Wu Y, Zhao RC, Tredget EE (2010) Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells 28:905-915.
Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A (2010) Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 227:185-189.
Zhang J, Li Y, Lu M, Cui Y, Chen J, Noffsinger L, Elias SB, Chopp M (2006) Bone marrow stromal cells reduce axonal loss in experimental autoimmune encephalomyelitis mice. J Neurosci Res 84:587-595.
Zhang J, Li Y, Chen J, Cui Y, Lu M, Elias SB, Mitchell JB, Hammill L, Vanguri P, Chopp M (2005) Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol 195:16-26.
*Correspondence to: Adriana Octaviana Dulamea, M.D., Ph.D., adrianadulamea@gmail.com.
# These authors contributed equally to this work.
orcid: 0000-0002-5056-0191 (Adriana Octaviana Dulamea)
10.4103/1673-5374.165325 http://www.nrronline.org/
Accepted: 2015-08-26
 中國(guó)神經(jīng)再生研究(英文版)2015年11期
中國(guó)神經(jīng)再生研究(英文版)2015年11期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces
