Does the intrathecal propofol have a neuroprotective ef ect on spinal cord ischemia?
Murat Sahin, Huriye Gullu, Kemal Peker, Ilyas Sayar, Orhan Binici, Huseyin Yildiz
1 Department of Anesthesiology and Reanimation, Faculty of Medicine, University of Amasya, Amasya, Turkey
2 Department of Anesthesiology and Reanimation, Mengucek Gazi Research and Training Hospital, Erzincan, Turkey
3 Department of General Surgery, Faculty of Medicine, University of Erzincan, Erzincan, Turkey
4 Department of Pathology, Faculty of Medicine, University of Erzincan, Erzincan, Turkey
5 Department of Anesthesiology and Reanimation, Faculty of Medicine, University of Sutcu Imam, Kahramanmaras,Turkey
Does the intrathecal propofol have a neuroprotective ef ect on spinal cord ischemia?
Murat Sahin1,*, Huriye Gullu2, Kemal Peker3, Ilyas Sayar4, Orhan Binici2, Huseyin Yildiz5
1 Department of Anesthesiology and Reanimation, Faculty of Medicine, University of Amasya, Amasya, Turkey
2 Department of Anesthesiology and Reanimation, Mengucek Gazi Research and Training Hospital, Erzincan, Turkey
3 Department of General Surgery, Faculty of Medicine, University of Erzincan, Erzincan, Turkey
4 Department of Pathology, Faculty of Medicine, University of Erzincan, Erzincan, Turkey
5 Department of Anesthesiology and Reanimation, Faculty of Medicine, University of Sutcu Imam, Kahramanmaras,Turkey
The neuroprotective ef ects of propofol have been conf rmed. However, it remains unclear whether intrathecal administration of propofol exhibits neuroprotective ef ects on spinal cord ischemia. At 1 hour prior to spinal cord ischemia, propofol (100 and 300 μg) was intrathecally administered in rats with spinal cord ischemia. Propofol pre-treatment greatly improved rat pathological changes and neurological function def cits at 24 hours after spinal cord ischemia. These results suggest that intrathecal administration of propofol exhibits neuroprotective ef ects on spinal cord structural and functional damage caused by ischemia.
nerve regeneration; propofol; pre-treatment; spinal cord; ischemia; neuroprotection; paraplegia; neural regeneration
Sahin M, Gullu H, Peker K, Sayar I, Binici O, Yildiz H (2015) Does the intrathecal propofol have a neuroprotective ef ect on spinal cord ischemia? Neural Regen Res 10(11):1825-1829.
Introduction
Paraplegia, an occasional but serious complication seen after surgical repair of thoracoabdominal aortic aneurysms, has been conf rmed to be attributed to ischemia of the spinal cord caused by interruption of blood flow during aortic cross-clamping (Hsieh et al., 2005). The reported incidence is between 4% and 33% (Ilhan et al., 1999, 2004). A variety of factors including microcirculatory disturbance, inf ammatory factors, cellular necrosis and apoptosis, or biochemical auto-destruction (calcium ion overloading, free radicals, stimulatory amino-acids, etc.) have been proposed to explain the occurrence of paraplogia, the exact mechanisms remain largely unknown (Sahin et al., 2014). The pathophysiological processes responsible for the development of ischemic/ hypoxic injury of the spinal cord are obscure (Kale et al., 2011; Sahin et al., 2014). Lumbar drains, intercostal artery re-implantation, left heart bypass and hypothermic circulatory arrest can be protective against the development of paraparesia or paraplegia following aortic surgery (Tetik et al., 2000; Umehara et al., 2010; Saito et al., 2011; Smith et al., 2011), but their complex and invasive nature is inevitably associated with additional complications, limiting their widespread prophylactic utility (Saito et al., 2011). Pharmaceutical agent(s) with potent protective ef ects are currently unavailable, but some compounds have been shown to be useful in reducing the inf ammatory and metabolic injury resulting from the destructive ef ect of ischemia/reperfusion on the spinal cord (Smith et al., 2011). Therefore, neurological def cits arising from ischemia/reperfusion injury may potentially be corrected using pharmacological treatments (Saaf et al, 2011). However, in spite of multimodal ef orts aiming at reducing the incidence of spinal cord ischemia, a signif cant elimination of the risk has not been possible until now. Human studies on the reduction of spinal cord ischemia risk after thoracoabdominal aortic aneurysm repair focused mainly on additional invasive procedures, rather than using a preventive drug treatment (Tetik et al., 2000; Gravereaux et al., 2001; Chiesa et al., 2005). However, ischemia risk has not been eliminated so far using these methods. In addition, such methods (e.g., drainage of cerebrospinal f uid, intravenous steroid treatment, additional measures in highrisk patients, thoracic endograft repair) have their own risks (Gravereaux et al., 2001).
Propofol (2,6-diisopropylphenol) is a widely used short-acting intravenous anesthetic agent. In vitro studies suggest that ef ects of propofol are associated with the inhibition of N-methyl-D-aspartate (NMDA) receptor (Xu, 2004). Several reports have also suggested an inhibitor ef ect of propofol on gamma-amino-butyric acid (GABA) receptors (Nadeson et al., 1997; Nishiyama et al., 2004; Wang et al., 2004; Geo et al., 2005; Vasileiou et al., 2009). This agent also has analgesic properties (Xu et al., 2004; Ji et al., 2013). When administered at non-sedative doses, it exerts anxiolytic ef ects and has strong antioxidant properties. There is a study suggesting a neuro-protective ef ect of propofol (Vasileiou et al., 2009).
Intrathecal drug administration is mainly used for the purpose of analgesia and anesthesia as an alternative routeusually when suf cient ef cacy cannot be achieved with high oral dose or parenteral administration with acceptable side ef ects (Smith et al., 2008). A number of laboratory and clinical studies are currently focusing on drug administration into cerebrospinal f uid thus bypassing blood-brain barrier. Cerebrospinal administration has many theoretical advantages when compared to intravascular route. Intrathecal administration bypasses cerebrospinal f uid barrier thus providing high drug levels in cerebrospinal f uid rapidly. Since the drug is directly present in cerebrospinal fluid, lower doses can be used, resulting in potentially reduced systemic toxicity. In addition, drugs have longer half-lives in the cerebrospinal f uid due to very low level of protein binding and enzymatic activity when compared to plasma (Misra et al., 2003).
Considering the absence of literature data on the protective ef ect of propofol on spinal ischemia/reperfusion injury, we aimed to examine its potential protective role in ischemic spinal cord injury when administered as an intrathecal pre-treatment.
Materials and Methods
Ethics statement
Approval was obtained for protocols used in this study from the Animal Care Committee of Kahramanmaras Sutcu Imam University, Kahramanmaras, Turkey (Permission No. 2013/03-6). All ef orts were made to minimize animal discomfort and reduce the number of animals used.
Animals
Fifty-four adult male Wistar rats, weighing 400–425 g, were selected and provided by Animal Research Center, Ataturk University, Erzurum, Turkey. Our study protocol was based on a previous animal study method reported by Sahin et al. (2014) and Hsieh et al. (2005). All rats were maintained under the same physiological and biological properties (22 ± 2°C and 12-hour dark/light cycle) and fed with standard rat food and water ad libitum.
Intrathecal catheterization
Three days before spinal cord ischemia, intrathecal catheterization for drug delivery was performed. Rats were placed in a plastic container and anesthetized with 1.5–2% isof uran. After shaving the head and posterior neck, the head was fixed anteriorly to allow maintenance of isof urane anesthesia with facial mask. A skin incision on the posterior nuchal area was made and occipital muscles were separated from the base of the skull. A polyethylene catheter (PE-10, BD Intramedic?Polyethylene Tubing, Becton, Dickinson and Company, New Jersey, USA) was advanced until the lumbar expansion area along the cysternal membrane and externalized at the back of the head. Rats showing motor impairment during the procedure were excluded from the study and immediately euthanized.
Ischemia/reperfusion procedure
Anesthesia maintenance in rats anesthetized within the plastic boxes was done using 1.5–2% isof urane administered via face masks (Anesthesia WorkStation AWS, Hallowel EMC, Pittsf eld, MA, USA). Heating pads were placed under the rats to maintain a normal body temperature. The tail artery was cannulated with a 22G catheter for intra-arterial heparin infusion. The left carotid artery proximal artery pressure (PAP) was measured after cannulation with a 20G catheter. For spinal cord ischemia, the left femoral artery was exposed, and a 2F Fogarty catheter (Edwards Lifesciences, Irvine, CA, USA) was advanced through the thoracic aorta. The tip of the catheter was localized at the junction of the left subclavian artery, which corresponds to a catheter length of 10.8–11.4 cm as reported in other studies (Yaksh et al., 1976; Taira et al, 1996). Immediately after the placement of the arterial catheter, 200 U (0.2 mL) of heparin (Nevparin, Mustafa Nevzat Ilac, Istanbul, Turkey) was injected through the tail artery. The balloon was f lled with 0.05 mL of physiological saline for 11 minutes to induce spinal cord ischemia. The diastolic arterial pressure (DAP) was measured at the tail artery to assess the ef ectiveness of occlusion. PAP was measured from the left carotid artery and maintained around 40 mmHg. DAP, PAP and body temperature were monitored before and during ischemia/reperfusion using the Philips Intellivalue MP30 (Philips, USA). After ischemia was obtained, the balloon was def ated. After completing all procedures, catheters were removed and wounds were closed. Protamine sulphate (Protamin HCl, Onko&Kocsel, Istanbul, Turkey) 4 mg was injected subcutaneously to counteract the anti-coagulant ef ect of heparin.
Drug administration
In the f rst part of the study, rats were randomized into four experimental groups (n = 6) to assess the ef ect of intrathecal propofol on neurological signs and histopathological changes: control group, propofol 100 and 300 μg groups, and the sham group.
One hour before the induction of ischemia, 10 μL of physiological saline was injected in the control group, while rats in the propofol 100 μg group received propofol 100 μg, rats in the propofol 300 μg group received propofol 300 μg in 10 μL of physiological saline solution (Nishiyama et al., 2004). A 50 μL of Hamilton syringe was used for drug administration. In the sham group, a Fogarty catheter was placed but the balloon was not inf ated and PAP was decreased to 40 mmHg for 11 minutes. No medication was given to the sham group.
Neurological investigation
After spinal ischemia, rats were transferred to their cages for recovery and their neurological functions were assessed in the f rst 24-hour postoperative period. For the assessment of motor functions, hind limb placing/stepping ref ex was recorded. Ambulation in the hind limbs was graded as follows (Hsieh et al., 2005): 0, normal (symmetrical and coordinated hind limb movements); 1, the first toe is immobile while walking, but ataxia is present; 2, knuckle walking; 3, absence of knuckling, but some mobility exists in the hind limbs; 4,no movement in the hind limbs. The placing/stepping ref ex was assessed by dragging the dorsum of the hindpaw along the edge of a surface. Normally, this requires a coordinated pulling-up and placing response, and was graded as follows (Hsieh et al, 2005): 0, Normal; 1, weak; 2, no stepping.
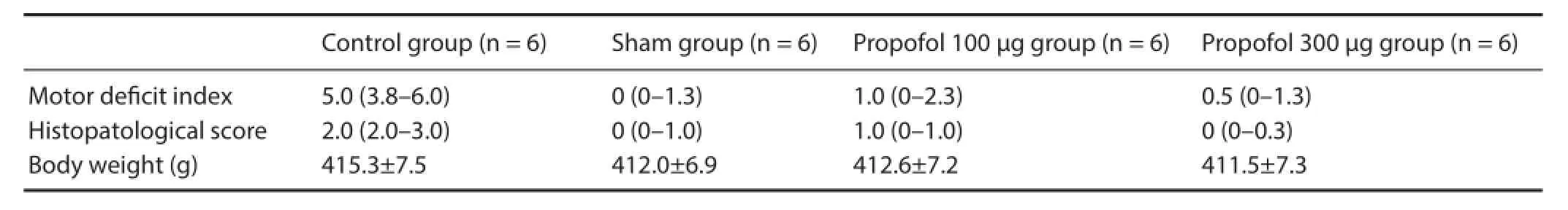
Table 1 Motor def cit index, histopathological score and body weight in rats with spinal cord injury at 24 hours after surgery
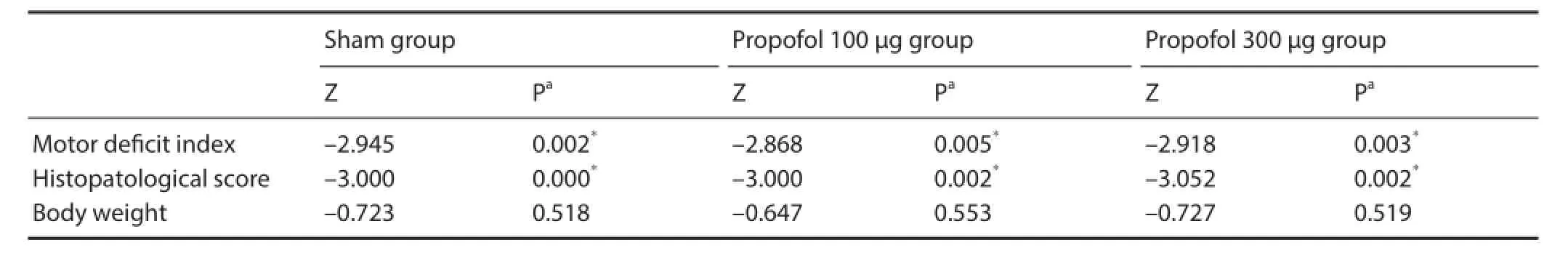
Table 2 Statistical results of comparisons with the control group
A motor def cit index (MDI) score was calculated for each rat as the sum of both scores with a maximum of 6 (score of 4 for ambulation and 2 for the lacing/stepping ref ex). MDI was calculated by an observer blinded to the treatments used.
Tissue samples and histopathological assessment
After observation of the motor behavior, rats were anesthetized by intraperitoneal ketamine injection (10 mg/kg), which was followed by the transcardiac perfusion of 100 mL of heparinized physiological saline. Immediately after this, 150 mL of 4% paraformaldehyde with phosphate buffer was given. Then, the lumbar expansion of the spinal cord at L3or L4was removed and kept in the same f xative at 4°C overnight. The samples were embedded in paraf n and 5 μm thick transverse cross sections were prepared and stained with hematoxylin and eosin. All slides were evaluated using a light microscope (Olympus, Tokyo, Japan). The samples were assessed by a pathology specialist blinded to treatment groups. For all rats, acute grey matter injury was calculated on the basis of the proportion of death or abnormal cells in the ventral horn as follows: 0, No neuronal injury or death; 1, mild injury (< 10%); 2, moderately severe injury (10–50%); and 3, severe injury (> 50%). For each rat, the score corresponds to the f ndings in the right and left hemicords in three consecutive sections.
Survival evaluation
In the second part of the study, the survival rate during the 28 day follow-up period was assessed in the remaining 30 rats, which were randomized into three groups with 10 rats in each group: physiological saline, propofol 100 μg, and propofol 300 μg groups. Interventions to these groups were the same as the f rst part of the study.
Statistical analysis
Statistical analysis was performed using SPSS software (version 17.0; SPSS, Chicago, IL, USA). Dif erences in motor and histopathological scores (MDI and histopathologic score expressed as a median (Q1–Q3)) were evaluated using the non-parametric Mann-Whitney U test (Monte Carlo signif cance test). Fisher’s exact probability test was used for the comparisons in survival analyses. P values less than 0.05 were considered statistically signif cant.
Results
Neurological function
There was no signif cant dif erence in body weight among the groups. The rats were weighted at the beginning of the study. There were significant differences in MDI and histopathology scores between the groups at 24 hours after spinal cord ischemia (Tables 1, 2). After the 11thminute of the aortic occlusion by proximal controlled hypotension (40 mmHg), normal motor functions were observed in the sham group, while acute f accid paraplegia developed in the control group. At 24 hours after reperfusion, acute f accid paraplegia and spastic paraplegia occurred in the control group. At 24 hours after spinal cord ischemia, one of the six rats in the propofol 100 μg group showed a slight decrease in the mobility of the hind limbs (MDI 2 and 3) with no signif cant paraplegia in the remaining f ve rats (MDI 1). In the propofol 300 μg group, no rats had severe paraplegia (MDI 0 and 1).
Histopoatological change in spinal cord tissue
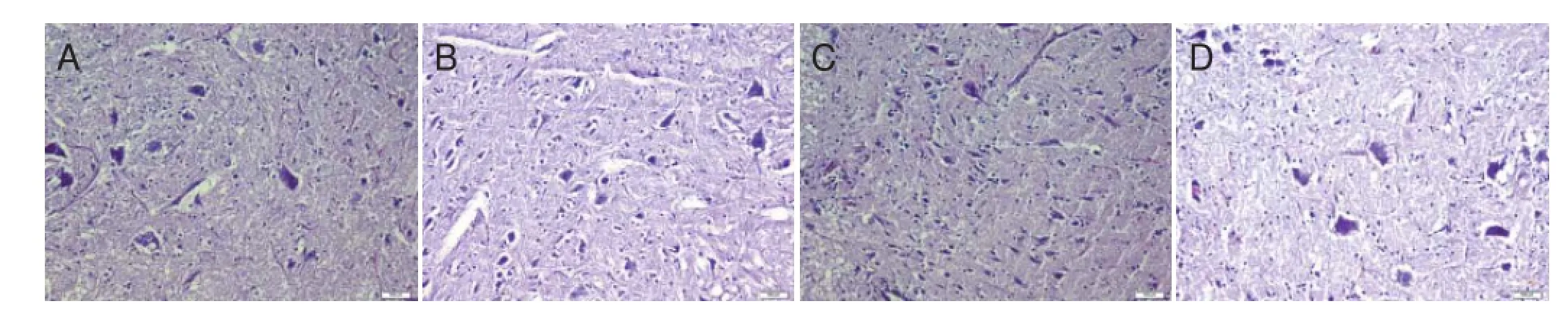
Figure 1 Histopathological changes in the lumbar expansion of rat spinal cord 24 hours after ischemia/reperfusion (× 200, hematoxylin-eosin staining).
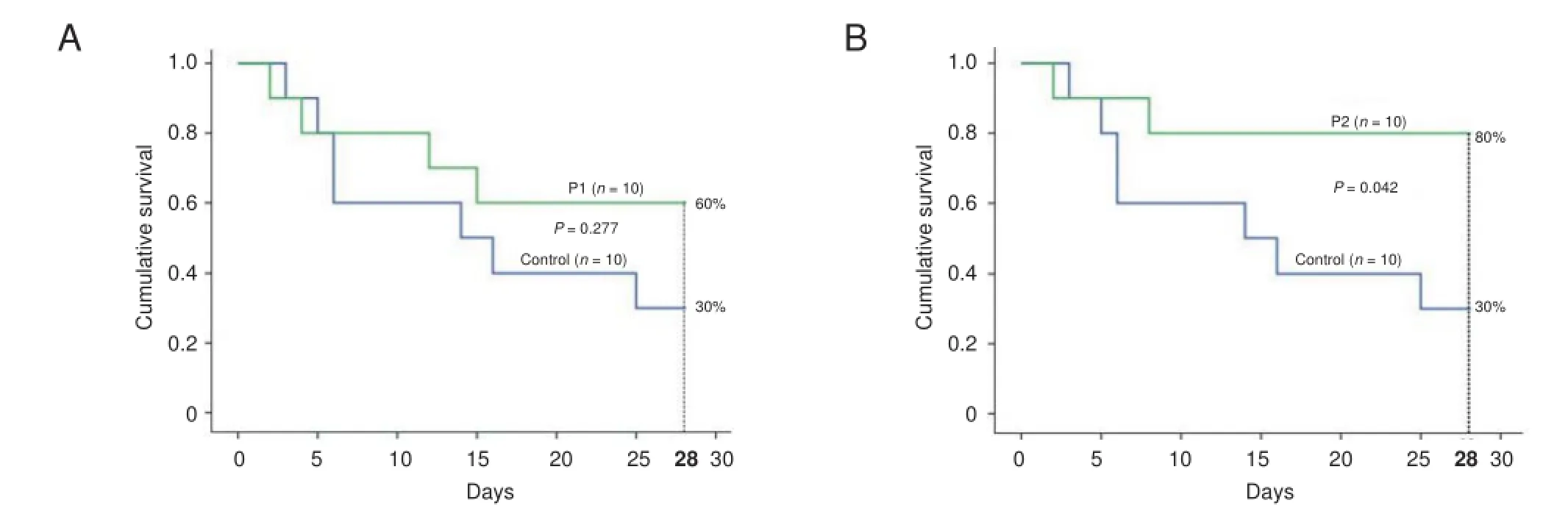
Figure 2 Impact of intrathecal propofol on survival of rats with spinal cord ischemia.
At 24 hours, in histopathological evaluation, two rats in the control group had severe neuronal injury of the lumbar spinal cord, while the remaining four rats had moderately severe neuronal injury (Figure 1A). In the sham group, two rats had mild neuronal injury, whereas four rats had no neuronal injury (Figure 1B). In the propofol 100 μg group, four rats had moderate neuronal injury, and two rats had no injury (Figure 1C). In the propofol 300 μg group, one rat had mild neuronal injury, and the remaining f ve rats had no injury (Figure 1D).
Survival evaluation
The therapeutic ef ects of propofol 100 and 300 μg on rat survival were examined. In the control group (n = 10), only three rats survived beyond 28 days after spinal cord injury. Six rats from propofol 100 μg group and eight rats from propofol 300 μg group survived beyond 28 days, respectively, without loss of good-level motor functions after spinal cord ischemia. These f ndings corresponded to higher survival rates (i.e., 60% and 80%) in propofol 100 μg and propofol 300 μg groups than in the control group (30%) during this time period (Figure 2). Fisher’s exact probability test results showed there was no signif cant dif erence in survival rate between control and propofol 100 μg groups but it existed between control and propofol 300 μg groups.
Discussion
Our results show that pre-treatment with propofol (100 μg or 300 μg, intrathecal) was associated with a signif cant decrease in hind limb motor dysfunction due to ischemic spinal cord injury 24 hours after ischemia/reperfusion in rats. In addition, pre-treatment with propofol 100 or 300 μg was able to prevent histopathological changes 24 hours after ischemia in the spinal cord.
The underlying mechanisms for the neuroprotective ef ect of propofol are not well understood. However, in a rabbit model of experimental spinal cord ischemia, Zeng et al. (2009a) demonstrated a neuroprotective ef ect of propofol infusion at room temperature or at 4°C throughout ischemia. Both approaches were associated with lower malonylaldehyde concentrations in the spinal cord as compared to control and sham groups, while they were associated with higher superoxide dismutase concentrations. Thus, they proposed that propofol may exhibit neuroprotective ef ects on spinal cord injury through regulating malonylaldehyde and superoxide dismutase concentrations. In the study by Zeng et al. (2009a), the infusion was performed via the left femoral artery during cross-clamping. In a rabbit study by Lin et al. (2008), intra-aortic and intravenous propofol infusions were performed during infrarenal occlusion. In rabbits receiving intra-aortic infusion, propofol concentration at the spinal level of L4–6was higher than at the level of T6–8. Intravenous administration did not result in significant increases in both treatment and control groups and at both L4–6and T6–8segments, and no signif cant dif erence in propofol concentration was observed between treatment and control groups.The incidence of paraplegia was lower in the intra-aortic infusion group than in the control and intravenous infusion groups, while there was no signif cant dif erence between the control and intravenous infusion groups.
These authors concluded that intra-aortic propofol infusion resulted in better neurological outcomes than intravenous propofol infusion. Similarly, in a rabbit study by Ke et al. (2005) where spinal cord ischemia was induced by aortic cross-clamping, intravenous infusion of propofol was found to prevent cell apoptosis in the spinal cord and this ef ect was attributed to changes in Bax and Bcl-2 protein levels, which were achieved by its regulation of malonylaldehyde and superoxide dismutase concentrations. In the study by Zeng et al. (2009b), propofol infused through a catheter positioned at the distal part of the aortic clamp inhibited the accumulation of excitatory amino acids in the ischemic spinal cord and thereby provide neuroprotective ef ects.
Although propofol is a widely utilized general anesthetic agent, its mechanism of action has not been well elucidated (Wang et al., 2004; Ji et al., 2013). Several reports have also suggested a certain degree of anti-inf ammatory ef ect of propofol (Ke et al., 2005; Line et al., 2008; Zeng et al., 2009a, b).
To conclude, our results suggest that pre-treatment with intrathecal propofol at 100 μg and 300 μg can prevent against spinal cord ischemia. This approach also benef ts for the prevention of spinal cord ischemia-related complications.
Author contributions: MS conceived the study and wrote the paper. All authors were invovled in the data analysis, synthesis, and interpretation, and approved the final version of this paper. Conf icts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded, stringently reviewed by international expert reviewers.
Chiesa R, Melissano G, Marocco Trischitta MM, Civillini E, Setaccci F (2005) Spinal cord ischemia after elective stent-graft repair of the thoracic aorta. J Vasc Surg 42:11-17.
Ge Z, Zeng Y, Tan Y (2005) Ef ects of intrathecal 6-hydroxydopamine, α1 and α2 adrenergic receptor antagonists on antinociception of propofol in mice. Acta Pharmacol Sin 26:186-191.
Gravereaux EC, Faries PL, Burks JA, Latessa V, Spielvogel D, Hollier LH, Marin ML (2001) Risk of spinal ischemia after endograft repair of thoracic aortic aneurysms. J Vasc Surg 34:997-1003.
Hsieh YC, Liangs WY, Tsai SK, Wong CS (2005) Intratecal Ketorolac pretreatment reduced spinal cord ischemic injury in rats. Anesth Analg 100:1134-1139.
Ilhan A, Yilmaz HR, Armutcu F, Gurel A, Akyol O (2004) The protective ef ect of nebivolol on ischemia/reperfusion injury in rabbit spinal cord. Prog Neuropharmacol Biol Psychiatry 28:1153-1166.
Ilhan A, Koltuksuz U, Ozen S, Uz E, Ciralik H, Akyol O (1999) The effects of caf eic acid phenetyl ester (CAPE) on spinal cord ischemia/ reperfusion injury in rabbits. Eur J Cardiothorac Surg 16:458-463.
Ji W, Cui C, Zhang Z, Liang J (2013) Paradoxic ef ects of propofol on visceral pain induced by various TRPV1 agonists. Exp Ther Med 5:1259-1263.
Kale A, B?rcek A?, Emmez H, Y?ld?r?m Z, Durda? E, Lortlar N, Kurt G, Do?ulu F, K?l?? N (2011) Neuroprotective ef ects of gabapentin on spinal cord ischemia-reperfusion injury in rabbits. J Neurosurg Spine 15:228-237.
Ke QB, Hou J, Chen C, Fang W, Tang HQ, Li QH, Sun DH (2005) Ef ect of propofol on spinal cord apoptosis associated with aortic cross-clamping in rabbits. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 17:426-429.
Lin Y, Liao Z, Zhang J, Zhang L (2008) Comparison of propofol concentration in the spinal cord between intra-aortic and intravenous infusion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 22:431-434.
Misra A, Ganesh S, Shahiwala A, Shah SP (2003) Drug delivery to the central nervous system: a review. J Pharm Pharmaceut Sci 6:252-273.
Nadeson R, Goodchild CS (1997) Antinociceptive properties of propofol: Involvement of spinal cord γ-aminobutyric acid A receptors. J Pharmacol Exp Ther 283:1181-1186.
Nishiyama T, Matsukawa T, Hanaoka K (2004) Intrathecal propofol has analgesic ef ects on inf ammation-induced pain in rats. Can J Anesth 51:899-904.
Sahin M, Sayar I, Peker K, Gullu, H, Yildiz H (2014) Protective ef ect of intrathecal paracetamol on spinal cord injury in rats. Iran Red Crescent Med J 16: e22151.
Saito T, Tsuchida M, Umehara S, Kohno T, Yamamoto H, Hayashi J (2011) Reduction of spinal cord ischemia/reperfusion injury with simvastatin in rats. Anesth Analg 113:565-571.
Shaaf S, Afrooz MR, Hajipour B, Dadashi A, Hosseinian MM, Khodadadi A (2011) Anti-oxidative ef ect of lipoic acid in spinal cord ischemia/reperfusion. Med Print Pract 20:19-22.
Smith HS, Deer TR, Staats PS, Singh V, Sehgal N, Cordner H (2008) Intrathecal drug delivery. Pain Physician 11:89-104.
Smith PD, Puskas F, Fullerton DA, Meng X, Cho D, Clevland JC, Weyant MJ, Reece TB (2011) Attenuation of spinal cord ischemia and reperfusion injury by erythropoietin. J Thorac Cardiovasc Surg 141:256-260.
Taito Y, Matsala M (1996) Ef ect of proximal arterial perfusion pressure on function spinal cord blood f ow and histopathologic changes after increasing intervals of aortic occlusion in the rat. Stroke 27:1850-1858.
Tetik O, Gurbuz A (2000) Spinal cord protection. TGKDCD 8:587-592.
Umehara S, Goyagi T, Nishikawa T, Tobe Y, Masaki Y (2010) Esmolol and landiolol, selective β1-adrenoreceptor antagonists provide neuroptrtection against spinal cord ischemia and reperfusion in rats. Anest Analg 110:1133-1137.
Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, Papadimitriou L (2009) Propofol. A review of its non-anaesthetic ef ects. Eur J Pharmacol 605:1-8.
Wang Q, Cao J, Zeng Y, Dai T (2004) GABAA receptor partially mediated propofol-induced hyperalgesia at superspinal level and analgesia at spinal cord level in rats. Acta Pharmacol Sin 25:1619-1625.
Xu A, Duan S, Zeng Y (2004) Ef ects of intrathecal NMDA and AMPA receptors agonists or antagonists on antinociception of propofol. Acta Pharmacol Sin 25:9-14.
Yaksh TL, Rudy TS (1976) Chronic catheterization of the spinal subarachnoid space. Phisiol Behav 17:1031-1036.
Zeng J, Lin YJ, Wang QY, Fang H, Yao JY (2009a) Protective ef ect of hypothermic propofol on ischemic spinal cords. Sichuan Da Xue Xue Bao Yi Xue Ban 40:593-597.
Zeng J, Yao J, Wang Q, Zhang Y, Weng H (2009b) Ef ect of propofol on spinal excitatory amino acid accumulation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23:723-726.
Copyedited by Vinit S, Zhang K, Li CH, Song LP, Zhao M
*Correspondence to: Murat Sahin, M.D., muratsahin4006@hotmail.com.
orcid: 0000-0003-1180-2204 (Murat Sahin)
10.4103/1673-5374.170312 http://www.nrronline.org/
Accepted: 2015-09-08
- 中國神經(jīng)再生研究(英文版)的其它文章
- Intracellular sorting pathways of the amyloid precursor protein provide novel neuroprotective strategies
- The role of the Rho/ROCK signaling pathway in inhibiting axonal regeneration in the central nervous system
- VEGF in the nervous system: an important target for research in neurodevelopmental and regenerative medicine
- Studying neurological disorders using induced pluripotent stem cells and optogenetics
- Ef cacy of glucagon-like peptide-1 mimetics for neural regeneration
- Compliant semiconductor scaf olds: building blocks for advanced neural interfaces

