Larval and Juvenile Growth Performance of Manila Clam Hybrids of Two Full-Sib Families
HUO Zhongming, YAN Xiwu, *, ZHAO Liqiang, LIANG Jian, YANG Feng,and ZHANG Guofan
?
Larval and Juvenile Growth Performance of Manila Clam Hybrids of Two Full-Sib Families
HUO Zhongming1), YAN Xiwu1), *, ZHAO Liqiang1), LIANG Jian1), YANG Feng1),and ZHANG Guofan2)
1),,116023,2),,,266071,
In order to determine whether growth performance could be improved by hybridizing full-sib families of Manila clam (), crosses between two full-sib families including self and reciprocal crosses were carried out. The effects of heterosis, combining ability and interaction on the growth of shell length were estimated. The results showed that the growth of hybrid larvae was intermediate between parents on days 6 and 9. Heterosis on shell length was observed, which varied at juvenile stage. The cross ofA×♀B (varied between 10.41% and 68.27%) displayed larger heterosis thanB×♀A (varied between 1.89% and 32.33%) did, suggesting thatA×♀B was an ideal hatchery method of improving the growth performance of Manila clam. The variances of general combining ability (GCA), special combining ability (SCA) and interaction (I) were significant in shell length (<0.05), indicating that both additive and non-additive genetic factors were important contributors to the growth of larvae and juveniles. The GCA for shell length ofA×♀B was higher than that ofB×♀A at both larval and juvenile stages. This confirmed that the cross betweenAand ♀Bshowed great growth in shell length. In summary, the growth of Manila clam seeds could be improved by hybridizing selected parents from large numbers of full-sib families.
Manila clam;; growth; hybrids; family
1 Introduction
One method of improving production in aquaculture is to produce a hybrid animal that has a better performance than either of its parents because of heterosis (Newkirk, 1980). The application of hybridization between genetically different populations or inbred lines in bivalve mollusks has been successfully practiced in recent years and is receiving increasing attention in species such as Pacific oyster,(Hedgecock, 1995; Hedge- cock and Davis, 2007), American oyster,(Mallet and Haley, 1983), Pacific abalone,(Deng, 2010), catarina scallop,(Cruz and Ibarra, 1997), and hard clam,(Manzi, 1991)The Manila clam,, is mainly distributed along the coasts of Pacific and Indian oceans and is now cultured for commercial purposes in a number of European, American and Asian countries, especially in China. By 2011, the annual production of Manila clam in China had increased to over 3.2 million tons (DOF, 2011),accounting for 90% of the world (Zhang and Yan, 2006). Despite its economic importance, the industry has not yet benefited from its genetic improvement. A breeding program for Manila clam initiated in 2007 established 45 full-sib families, aiming to select out varieties or lines with rapidly growing shell length. The results indicated that a large portion of the variance of shell length was genetically additive (Huo, 2010).
The significance of the heterotic performance of different populations is strongly affected by the genetic backgrounds of parents. In comparison, hybrid vigor (heterosis), the superiority of hybrids produced by crossing inbred parent lines, has long been recognized in. It was found that the improvement of quantitative traits such as shell length and weight is more efficient than that of other traits (Hedgecock, 1995; Hedgecock and Davis, 2007). This finding indicated that hybridization among full-sib families in large size may produce fast growing lines.
In the present study, two full-sib families with fast growing shell length were selected out for testing our expectation. Our objective was to evaluate heterosis, combining ability and interaction of shell length of Manila clam. Our results will aid to determining whether hybridization among full-sib families is effective for raising fast growing Manila clam seedlings.
2 Materials and Methods
2.1 Base Stock and Experimental Design
The two full-sib families with the fastest growth in shell length were selected from 45 full-sib families for hybridization. Two hundred clams were randomly taken from the two full-sib families (designated as family A and family B) for measurement, and 20 clams each full-sib family for larger size were selected as the base stock. Gametes were obtained with the method of Yan(2012). The clams were induced to spawn by drying for 8h in shade. The water temperature was 23℃when spawning occurred. Each adult was placed in a 2L beaker for spawning. The eggs from 1 female of each family were divided into two equal parts, and fertilized with the sperm from family A and family B. The crosses between two full-sib families including self and reciprocal crosses were shown in Table 1. The experiments were carried out three times using different sets of parental clams and beakers.
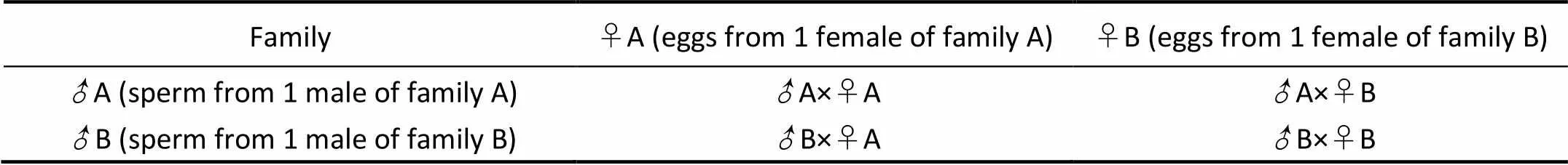
Table 1 Crosses between two full-sib families including self and reciprocal crosses
2.2 Larval and Juvenile Rearing
The fertilized eggs of each group were hatched at an initial density of 40–50 eggsmL?1. After about 24h, the fertilized eggs developed into D-hinge larvae. Larvae and juveniles in all experimental groups were reared under the same environmental condition. Larval rearing and the juvenile and adult nursery were done as described by Zhang and Yan (2006). Initially, the D-hinged larval density in each replicate bucket was 5–6 larvaemL?1. This density was reduced to 1–2 juvenilesmL?1after metamorphosis because of juvenile growth. During larval rearing, the water was completely exchanged every day with sand-filtered seawater, and the temperature was maintained at 25–28℃. Salinity was kept at 28–29. As the larvae grew, the sieves were changed to those with a mesh size of 50–150μm after day 10. The larvae were fed withduring days 1–3 and a mixture ofandspp. (2:1) from day 4 to the juvenile stage. Larvae were fed at a rate of 2000–10000 cells mL?1d?1on days 1–3 and 30000–60000 cells mL?1d?1from day 4 to the juvenile stage (Zhang and Yan, 2006).
After 60d, the juveniles were placed in bags (20cm× 25cm) with a mesh size of 700μm and cultured at the nursery ponds (700m×150m×1.5m). The stocking densities were adjusted every 15d, starting with 100–150 clams bag?1and ending with 30–50 clams bag?1.
Thirty individuals were sampled per replicate and killed with 4% formaldehyde with their shell lengths measured using a microscope (100×) on days 3, 6, 9, and 30 and for juveniles on days 60 and 90.
2.3 Data Analysis
Differences in shell length of the hybrid and self-cross lines were analyzed using one-way ANOVA followed by Tukey multiple comparison implemented in SPSS. The significance level for all analyses was set at<0.05. The formula for calculating general (GCA) and specific (SCA) combining abilities and interaction (I) were obtained according to Griffing’s (1956) Method 1 (complete diallele set), Model 1. Heterosis was calculated using the formula:
where1is the average shell length of a cross, andpis the average shell length of both parental families.
3 Results
3.1 Comparisons of Growth in Shell Length
The growth of shell length is shown in Figs.1, 2 and Table 2. The larval shell lengths of the hybrid lines (A×♀B andB×♀A) were larger than those of both self-cross lines (A×♀A andB×♀B) on day 3 (Fig.2; Table 2). However, the shell lengths of the larvae were between those of the self-cross on days 6 and 9 (Fig.2; Table 2).
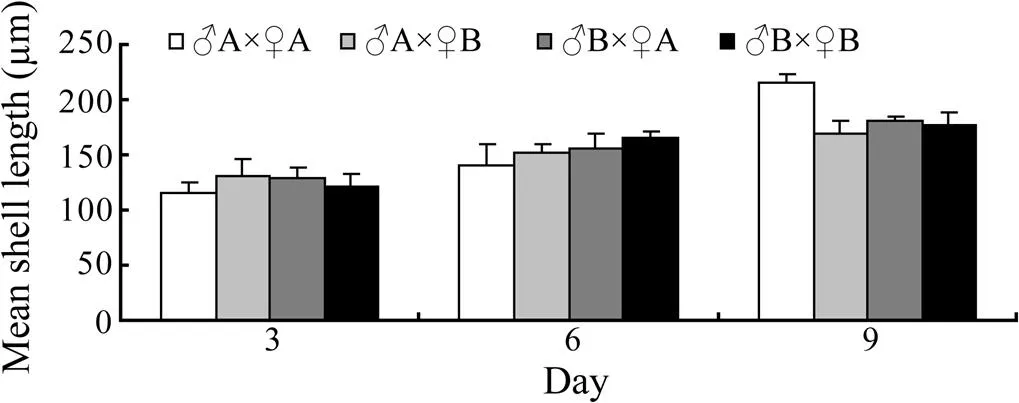
Fig.1 Comparisons of growth in shell length of crosses between two full-sib families of Manila clams at the larval stage.
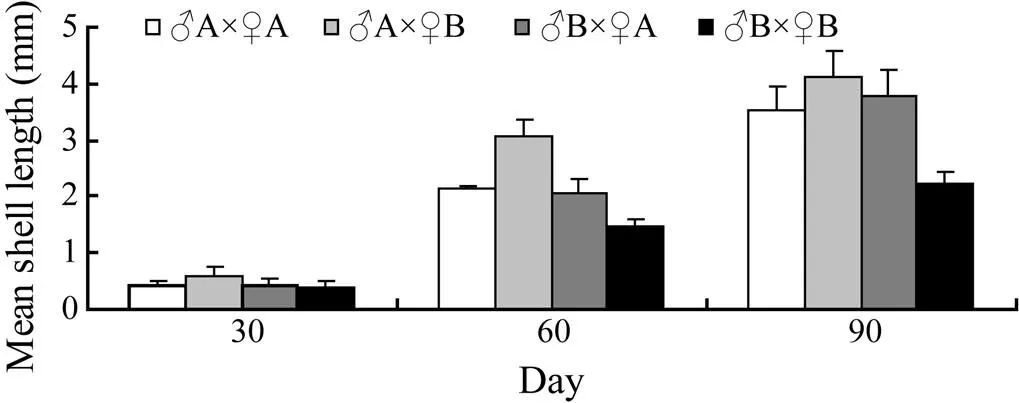
Fig.2 Comparisons of growth in shell length of crosses between two full-sib families including self and reciprocal crosses of Manila clams at the juvenile stage.
At the beginning of day 30, the juveniles of hybrid lineA×♀B grew faster than those of the self-cross lines (A×♀A andB×♀B). The Tukey multiple comparisons showed that the difference was not significant betweenA×♀B andA×♀A, but it was significant betweenA×♀B andB×♀B on day 30. The difference was significant betweenA×♀B andA×♀A orB×♀B on days 60 and 90. The juvenile shell length of hybrid lineB×♀A was between those of self-cross linesA×♀A andB×♀B on day 60; that ofB×♀A was larger than those ofA×♀A andB×♀B but smaller than that ofA×♀B on day 90.
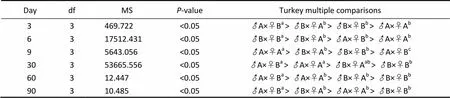
Table 2 Analysis of variance for the shell length of crosses between two full-sib families including self and reciprocal crosses
Notes: Different letters within each row indicate that the means are significantly different (<0.05).
3.2 Heterosis
The heterosis in shell length of the hybrid lines varied between 10.41% and 68.27% forA×♀B and between 1.89% and 32.33% forB×♀A (Figs.3 and 4).
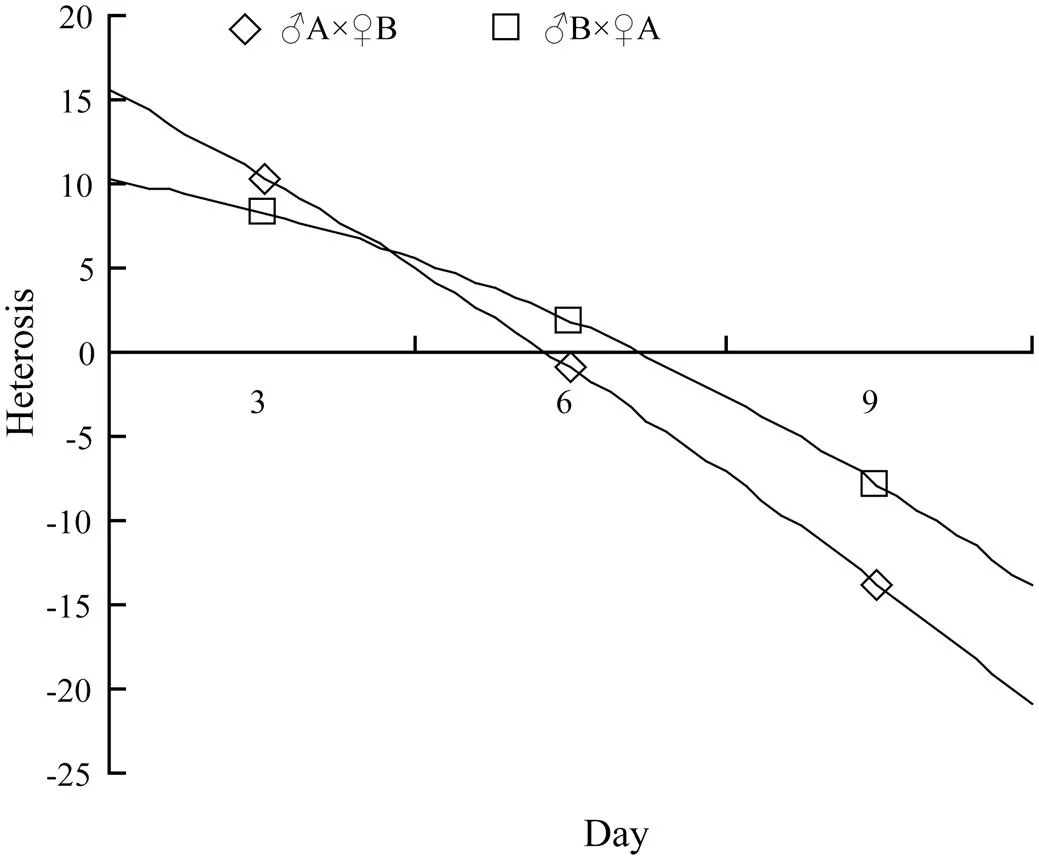
Fig.3 The heterosis values of hybrid lines ♂A×♀B and ♂B×♀A at larval stage.
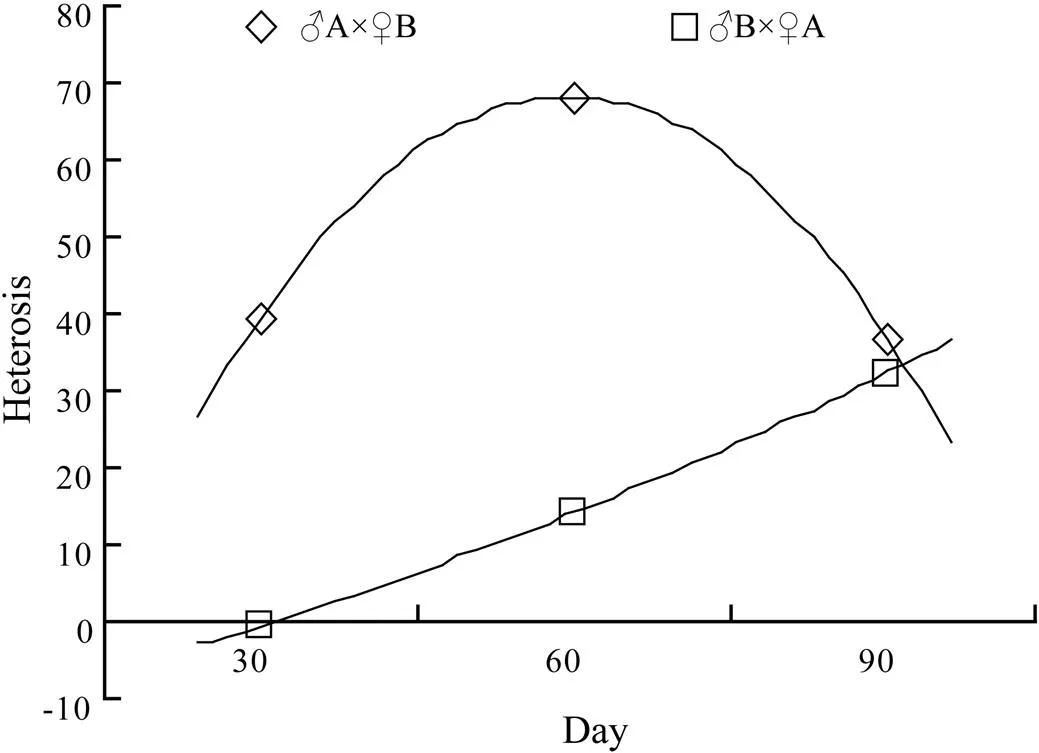
Fig.4 The heterosis values of hybrid lines ♂A×♀B and ♂B×♀A at juvenile stage.
3.3 Combining Ability
The variances ofGCA, SCA and Ion shell length are shown in Table 3. The analysis of the three variances in the shell length was significant (<0.05). The mean squares (MS) were higher in GCA than in SCA from day 6 to day 90.The GCA, SCA and I of shell length for hybridlines are presented in Figs.5a–d. The GCA of hybrid lineA×♀B was higher than that ofB×♀A at both larval and juvenile stages. At the beginning of day 30,the SCA values inthe shell length ofA×♀B cross were statistically higher than the I valuesofB×♀A.
4 Discussion
The larvae of the hybrid lines did not exhibit heterosis in the shell length. This result likely was due to a maternal effect whereby the main nutrition comes from the maternal egg, which could have contributed to shell growth.Thus the growth was significantly larger in self- cross lineB×♀B than in hybrid lineB×♀A andA×♀B on day 6, and it was significantly larger inA×♀A than inA×♀B andB×♀A on day 9. For mollusk species, maternal effects on fitness traits such as growth and survival are common during the larval stage due to variation in egg quality (Deng., 2010). The amount of energy reserves in the form of lipids from eggs has been shown to be a strong determinant of success of early stages of larval development (Cragg and Crisp, 1991). In the Manila clam, for example, Yan. (2012) reported that the growth was significantly affected by maternal origin at the larval stage of Manila clam. In abalone, Deng. (2010) observed the maternal effects on the larval growth and metamorphosis of.Newkirk. (1977) reported a signi- ficant maternal effect on the survival of larvae up to day 6 in crosses of the oyster,. Xu. (2009)suggested that the maternal effects caused by egg quality should decrease with increasing age and may fade after 9 days in the larvae of.
Hybridization are frequently used in plant and animal breeding research to obtain the additive components of genetic variance of quantitative characters in a fixed set of geographical populations or inbred parent lines (Deng., 2010; Mallet and Haley, 1984; Hedgecock and Davis, 2007). Heterosis has been widely observed in oysters (Mallet and Haley, 1984; Newkirk, 1977; Hedge- cock., 1995; Hedgecock and Davis, 2007), scallops (Cruz and Ibarra, 1997; Zheng, 2006; Zhang, 2007), and abalones (Deng, 2010; You, 2009). Taken together, the previous results indicate that hy- bridization among the geographical populations and inbred parent lines could provide important tools for selective breeding programs in shellfish culture.
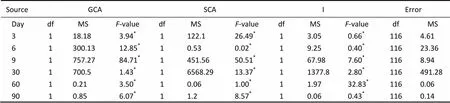
Table 3 Analysis of variance of combining ability on shell length of crosses between two full-sib families including self and reciprocal crosses of Manila clams
Note:*donates significant difference (<0.05).
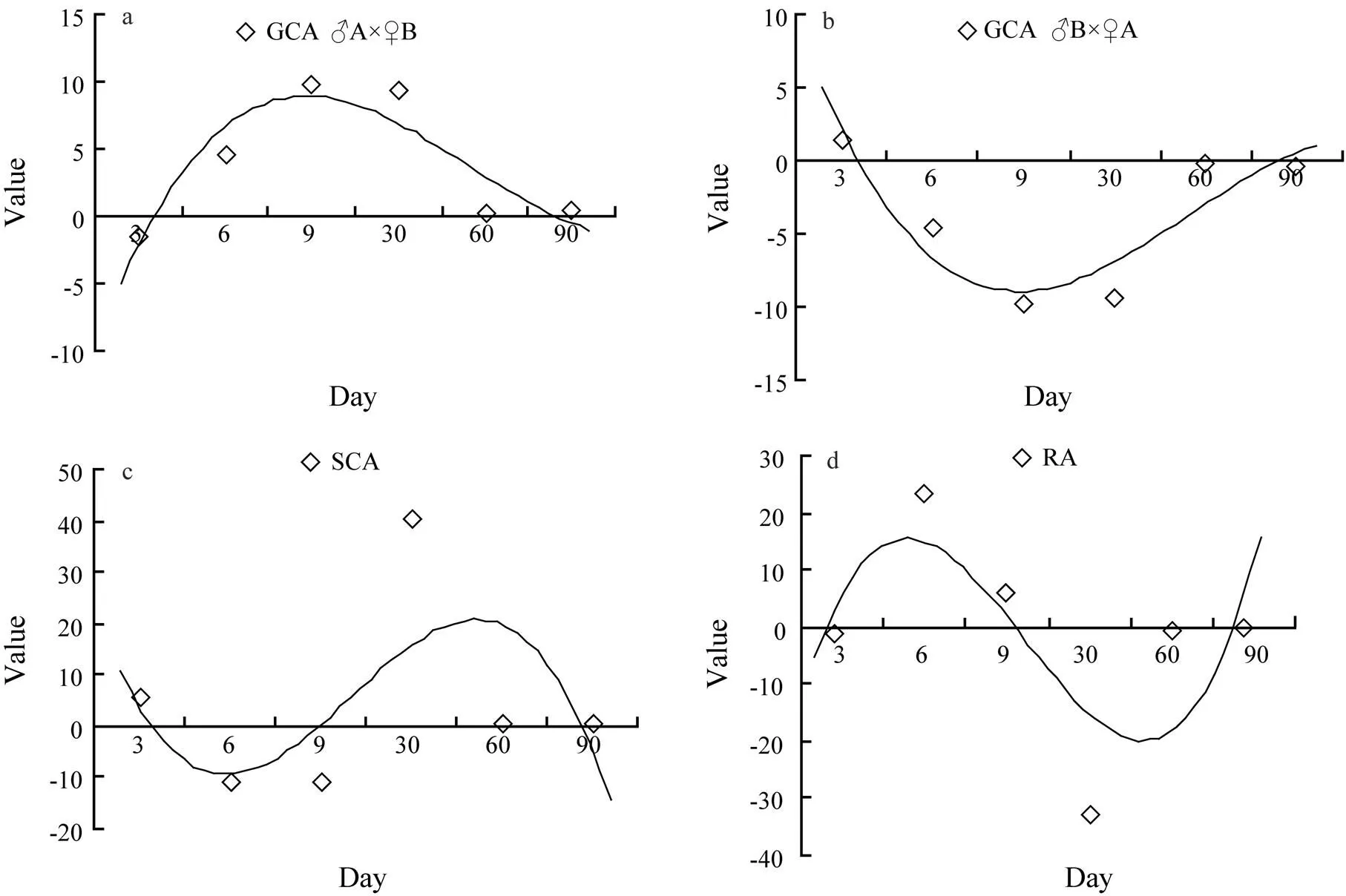
Fig.5 Estimates of GCA (a and b), SCA (c) and I (d) in the shell length of crosses between two full-sib families including self and reciprocal crosses of Manila clams.
Heterosis for growth and survival in bivalve mollusks was demonstrated experimentally in crosses of inbred Pacific oysters,, by Hedgecock(1995) using the dominance, over dominance and epistasis hypotheses. Crosses between inbred parent lines are expected to increase heterozygosity, reduce the effects of inbreeding depression, and enhance fitness, resulting in heterosis (Hedgecock and Davis, 2007; Deng., 2010). Results from the crosses between two full-sib families including self and reciprocal crossesof the Manila clam con- sistently showed heterosis for the shell length at the juvenile stage, and the magnitude of the heterosis varied on different days. The shell length of juvenile from hybrid lineA×♀ B exceeded that of the better self-cross line. TheA×♀B displayed larger heterosis for the shell length thanB×♀A. It is predicted that the cross ofA×♀B would be an ideal hatchery method to improve the growth of Manila clam seed.
The general combining ability (GCA) is a measure of the additive genetic action, while the special combining ability (SCA) and interaction (I) are assumed to be the non-additive genetic action (Deng, 2010; Sprague, 1942). For the present study, we calculated general and specific combining abilities using methods described initially by Griffing (1956). The large GCA variances observed in the present study indicated that additive gene actions were involved in expression of the shell length. However, it is interesting to find that positive and large SCA and I existed, suggesting that non-additive compo- nents of variance were also important contributors to the growth of larvae and juveniles of the Manila clam. This is consistent with the findings of previous studies, which have shown non-additive genetic variation in the growth traits of several species, such as the American oyster,(Mallet, 1984), the catarina scallop,(Cruz, 1997), and the Pacific abalone,(Deng, 2010). However, the mean squares were higher in GCA than in SCA from day 6 to day 90, suggesting that the GCA of shell length was dominant. In the present study,the GCA for the shell length ofA×♀B was higher than that ofB×♀A at both larval and juvenile stages. This confirmed again that the cross betweenAand ♀Bcould produce the best hybridprogeny with fast growth in shell length.
Our purpose was to determine the additive components of genetic variance and to find evidence concerning heterosis for shell length of larvae and juveniles in the Manila clam. The heterosis and combining ability for shell length tested in this study suggested that the growth of Manila seed could be improved by hybridizing selected families on a large scale.
Acknowledgements
We thank the anonymous reviewers for their helpful comments on this work. We thank Dalian Hairi Fisheries Food Limited Corporation for providing technical support. This research was supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-48) and grants from the ‘863’ Project of China (2012AA10AA400).
Cragg, S. M., and Crisp, D. J., 1991. The biology of scallop larvae. In:Shumway, S. E., ed., Elsevier Scientific Publishing Company, Amsterdam, 75- 132.
Cruz, P., and Ibarra, A. M., 1997. Larval growth and survival of two Catarina scallop(, Sowerby, 1835) populations and their reciprocal crosses., 212: 95-110.
Deng, Y. W., Liu, X., Zhang, G. F., and Wu, F. C., 2010. Heterosis and combining ability: A diallel cross of three geo- graphically isolated populations of Pacific abaloneIno.,28: 1195-1199.
Department of Fisheries (DOF),2011.. Agriculture Press, Beijing, 288pp.
Griffing, B., 1956.Concept of general and specific combining ability in relation to diallel crossing systems.,9: 463-493.
Hedgecock, D., and Davis, J. P.,2007. Heterosis for yield and crossbreeding of the Pacific oyster., 272: 17-29.
Hedgecock, D., McGoldrick, D. J., and Bayne, B. L., 1995. Hybrid vigor in Pacific oysters: An experimental approach using crosses among inbred lines., 137: 285-298.
Huo, Z. M., Yan, X. W., Zhao, L. Q., Zhang, Y. H., Yang, F., and Zhang, G. F., 2010. Effects of shell morphological traits on the weight traits of Manila clam ()., 30: 251-256.
Mallet, A. L., and Haley, L. E., 1983. Growth rate and survival in pure population matings and crosses of the oyster, 40: 948-954.
Manzi, J. J., Hadley, N. H., and Dillon, R. T., 1991. Hard clam,, broodstocks: Growth of selected hatchery stocks and their reciprocal crosses.,15: 17-26.
Newkirk, G. F., Waugh, D. L., and Haley, L. E.,1977. Genetics of larval tolerance to reduced salinities in two populations of oysters,., 34: 383-387.
Newkirk, G. F.,1980. Review of the genetics and the potential for selective breeding of commercially important bivalves., 19: 209-288.
Sprague, G. F., and Tatum, L. A., 1942. General and special combining ability in simple crosses of corn.,34: 923-932.
Xu, F., Zhang, G. F., Liu, X., Zhang, S. D., Shi, B., and Guo, X. M., 2009. Laboratory hybridization betweenand., 28: 453-458.
Yan, X. W., Huo, Z. M., Yang, F., and Zhang, G. F., 2012.Heritability of larval and juvenile growth for two stocks of Manila clam., DOI: 10.1111/j.1365-2109.2012.03250.x.
You, W. W., Ke, C. H., Luo, X., and Wang, D. X.,2009. Growth and survival of three small abalonepopulations and their reciprocal crosses.. 40: 1474-1480.
Zhang, G. F., and Yan, X. W., 2006.A new three-phase culture method for Manila clam,, farming in northern China., 258: 452-461.
Zhang, H. B., Liu, X., Zhang, G. F., and Wang, C. D.,2007. Growth and survival of reciprocal crosses between two bay scallops,Say andLamarck., 272: 88-93.
Zheng, H. P., Zhang, G. F., Guo, X. M., and Liu, X., 2006.Heterosis between two stocks of the bay scallop,Lamarck (1819)., 25: 807-812.
(Edited by Qiu Yantao)
10.1007/s11802-015-2354-1
(March 31, 2013; revised September 30, 2013; accepted March 31, 2015)
. Tel: 0086-411-84763026 E-mail: yanxiwu@dlou.edu.cn
ISSN 1672-5182, 2015 14 (3): 564-568
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
 Journal of Ocean University of China2015年3期
Journal of Ocean University of China2015年3期
- Journal of Ocean University of China的其它文章
- Research on China’s Aquaculture Efficiency Evaluation and Influencing Factors with Undesirable Outputs
- Sustainability Evaluation of Different Systems for Sea Cucumber (Apostichopus japonicus) Farming Based on Emergy Theory
- Tolerance, Oxygen Consumption and Ammonia Excretion of Ophiopholis sarsii vadicola in Different Temperatures and Salinities
- Effect of Shrimp (Litopenaeus vannamei) Farming Waste on the Growth, Digestion, Ammonium-Nitrogen Excretion of Sea Cucumber (Stichopus monotuberculatus)
- Proline with or without Hydroxyproline Influences Collagen Concentration and Regulates Prolyl 4-Hydroxylase α (I) Gene Expression in Juvenile Turbot (Scophthalmus maximus L.)
- Cloning, Expression and Activity Analysis of a Novel Fibrinolytic Serine Protease from Arenicola cristata
