Study on the Effect of Catalyst Properties on Residue Hydroconversion
(SINOPEC Research Institute of Petroleum Processing, Beijing 10083)
Study on the Effect of Catalyst Properties on Residue Hydroconversion
Tong Fengya; Yang Qinghe; Li Dadong; Dai Lishun; Deng Zhonghuo
(SINOPEC Research Institute of Petroleum Processing, Beijing 10083)
The effect of catalyst properties on residue oil hydroconversion was studied at moderate operating conditions (at a temperature of 400 ℃, an initial hydrogen pressure of 10 MPa, and a reaction time of 4 h) in a batch mode slurry phase with different catalyst samples. The results showed that the catalyst acidity had a good effect on residue conversion and MCR (micro carbon residue) conversion but brought about higher coke yield. Residue conversion was thermally induced but the catalyst acidity changed its conversion route. A catalyst with higher metal loading, higher hydrogenation activity and appropriate pore size had higher sulfur and metal removal rate, higher MCR conversion and also a lower coke formation. The activity of spent commercial catalyst AS1 and DS1 was slightly lower than the corresponding fresh ones but was still high enough for residue oil hydroconversion. It assumes that the role of the catalyst is to activate hydrogen species toward reaction with an aromatic carbon radical to yield a cyclohexadienyl type intermediate which will turn into liquid and also to absorb the mesophase which can easily aggregate to form coke.
residue oil; hydroconversion; catalyst; slurry phase; mechanism
1 Introduction
The rapid increase in the demand for fossil fuel, the steady decrease in the availability of light crude oils and the potential economics of processing heavy oil have made researchers look for an ef fi cient way of utilizing the inferior heavy crude oils. Residuum, which accounts for almost 60% by volume of the crude oil, is the heaviest fraction and it concentrates about 90% of total sulfur and nitrogen contents and almost all the metals, predominantly nickel and vanadium compounds. If the crude is more inferior, the content of impurities is much higher and the treating process is more dif fi cult. In a word, residue oil is dif fi cult to process but has to be processed.
Till now, all the technologies that are used to process residue oil are based on carbon rejection, hydrogen addition and combination of these two methods. Carbon rejection method includes technologies such as visbreaking, thermal cracking, catalytic cracking and coking. The utilization of carbon rejection technologies can bring in lower investment and operating costs but will reduce the yield of liquid products. Compared to the carbon rejection method, the hydrogen addition technologies have the potential of offering better yields of high-quality motor fuels, and are becoming the focused research fi eld[1]. Nowadays, hydrogen addition technologies can be divided into four types depending on the reactor type used, including the fi xed-bed, the moving-bed, the ebullated-bed and the slurry-bed[2].
Among the four hydrogen addition technologies, the fi xed-bed process is the most experienced one and takes up about 80% of market share. But a severe catalyst deactivation associated with coke and metal deposition (mainly Ni, V, Fe and Ca) usually leads to a short operating time, which is not longer than one and a half year, and furthermore, a small portion of feedstock requires a metal content of less than 100 μg/g and a MCR (micro carbon residue) equating to less than 12%[3]. The slurry-bed technologies have a capability to process almost any kind of feeds without the concerns about catalysts deactivation, because most of catalysts are dispersed and used oncethrough[4]. Besides, plenty of advantages can be brought forth by slurry reactors, for example: slurry catalysts promote quicker reactions by lowering the particle size andconsequently exposing the active sites to reactants, and mass transfer and heat transfer are promoted, so that the highly exothermic reactions can be conducted without the presence of any hot spots[5]. However, most of the existing slurry technologies operate under severe conditions (at a temperature of higher than 420 ℃). At this operating mode, a great amount of solids and gases was produced, and also a high content of contaminants such as sulfur, nickel and vanadium species are present in the valuable liquids[6]. Consequently, a slurry bed- fi xed bed combined process has been proposed. The main function of the slurry bed process is to offer suitable feed for the fixed bed reactor or extend the operating time of the fi xed bed reactor.
As a result, reducing the solid and gas formation is a key point in slurry technology. The formation of solids can be divided into two parts, one is the polymerization of coke precursors, and the other is the precipitation of partially hydrocracked asphalthenes and polycondensed aromatics because of a more paraf fi nic system which is formed due to the small paraf fi n molecules and the hydrogenation of double bonds[7]. The coke precursors are polymerized mainly because the active hydrogen atoms are not suf ficient. The gas is produced mainly because of deep cracking of paraf fi nic chains of asphaltenes and the molecules resulted from the reaction. During the residue hydroprocessing, thermal cracking reaction and hydrogenation reaction are concurrent and take place in parallel[8-9]. It has been revealed that under low severity reaction conditions the reactions occur by catalytic route and the solid and gas formation is low, while under high severity conditions thermal reactions are more dominant and a larger amount of solid and gas is produced[10]. Catalyst properties also have a strong influence on the solid and gas formation. The previous studies have considered more about the operating conditions and paid little attention to the catalyst properties.
The aim of this work was to study the residue hydrocraking over finely supported catalysts of different properties in slurry bed to gain a suitable feed for the fi xed bed process. The experiments were run in a batch mode and the conversion of heteroatoms (mainly S, Ni, and V) and MCR and the coke yield were examined as the difference in catalyst activity.
2 Experimental
2.1 Materials
The feedstock of the experiments was a mixture of commercial VR and HCO provided by the Sinopec Qingdao Branch Company with its typical properties detailed in Table 1. The properties and serial number of catalysts used in the experiments are listed in Table 2. SD1 and SA2 were the commercial spent catalysts originating from catalysts D1 and A2, respectively. The spent catalysts were first extracted by toluene to remove the remaining oil and then the toluene remaining in the catalyst sample was volatilized. All the catalyst samples used in these experiments were crushed and sieved, and a proper particle size fraction, which was smaller than 0.1 mm, was used in tests.

Table 1 Properties of the feedstock oil
2.2 Experimental procedure
The experiments were carried out in a stirred batch autoclave (Parr 4757). A 0.5-L stainless steel autoclave was loaded with 200 g of residue (after being heated to ~100 ℃) and a certain amount of catalyst. A theoretical amount of CS2was sprinkled to the hot residue and immediately stirred. Then the autoclave was loaded and fl ushed with hydrogen to 10 MPa, after this procedure the run was started. When the temperature reached 350 ℃, a duration of 2 hours was ensured for the catalyst sulfurization. It took 4 hours for conducting the reaction at 400 ℃. After the experiments were fi nished, the gas was vented. The liquid and catalystmixture was dissolved in toluene and then fi ltered. Toluene was then removed from the fi ltrate by a rotary evaporator to collect the liquid product. The total liquid product was weighed carefully. The liquid product was analyzed to determine the S, MCR and vanadium contents.
2.3 Measurements
The removal rates in this paper are de fi ned by the general equation shown below:

whereAis one of MCR, S content, vanadium (V) content, or nickel content;AFis the mass fraction ofAin feedstock, %;WFis the mass ofAin the feedstock, g;APis the mass fraction ofAin liquid product, %; andWPis the mass ofAin the liquid product, g.
Residue conversion=(the mass of >500 ℃ fraction in feedstock - the mass of >500 ℃ fraction in liquid product)/the mass of >500 ℃ fraction in feedstock×100%.
Coke yield=(the mass of the feedstock - the mass of gas -the mass of liquid)/the mass of feedstock×100%.
3 Results and Discussion
3.1 Effect of metal loading on residue oil hydroconversion
Metal loading on catalyst has an important effect on the number of hydrogenation active sites. To study metal loading of catalyst, three catalysts (named D0, D1 and D2) with the same support but different metal loadings were used in the residue hydroconversion process in slurry phase. The composition of catalyst samples is presented in Table 2. The catalyst D0 has no metal loading, while the metal loading on D2 was two times that on D1. The experimental results are shown in Figure 1. Figure 1 clearly shows that with an increasing metal loading, the sulfur, nickel, vanadium and MCR conversion also increased, but the residue conversion was not signi fi cantly changed. As the total metal loading increased from 0% (D0) to 17.9% (D2), the coke yield decreased from 6.5% to 1.6%. It was considered that the residuum conversion belonged primarily to thermal reactions and that catalyst could promote the sulfur, nitrogen and metal removal, as well as aromatics saturation[11]. Thermal cracking reaction could convert high molecular weight substances to lower molecular weight substances, and then the lower molecular weight substances would have access to the active sites of the catalyst through the catalyst pores to remove sulfur, nitrogen and metals while the aromatics contained in these lower molecular weight substances could be hydrogenated or could be turned into coke if the active sites were deactivated. Therefore, thermal and catalytic reactions acted sequentially via thermal scission and hydrogenation, and then the two reactions acted concurrently via coke formation and hydrogenation.[8]The last one was a key process to inhibit coke formation and could be successfully controlled by increasing catalyst metal loading. However, the increasing metal loading had a bad effect on the dispersing of metal on the catalyst and also could increase the cost of the process, so the metal loading should be optimized.

Table 2 Properties of the spent commercial catalyst

Figure 1 Effect of metal loading on residue oil hydroconversion
3.2 Effect of different metals on residue hydroconversion
Different metals show hydrogenation activity in a de-creasing order shown below for the hydrodesulfurization reactions: Co-Mo>Ni-Mo>Ni-W>Co-W; for the hydrodenitrogenation reactions: Ni-W=Ni-Mo>Co-Mo>Co-W; and for the aromatics or ole fi ns saturation reactions: Ni-W>Ni-Mo>Co-Mo>Co-W[12]. To study the metal type effect, two catalysts (named A1 and D2) with the same support but different metal types were used in the residue hydroconversion process in slurry phase. D2 was a NiMo catalyst containing 24.6% of metals, while A1 was a CoMo catalyst which contained 17.9% of metals. The experimental results are shown in Figure 2. Figure 2 clearly depicts that the two catalysts had the same residue conversion performance. This occurred mainly because the residue conversion was induced by thermal reaction. In comparison with the catalyst A1, the catalyst D2 had higher nickel, vanadium and MCR conversion rates, and a lower coke yield, which was ascribed to a higher hydrogenation activity of the NiMo catalyst.

Figure 2 Effect of different metal on residue hydroconversion
3.3 Effect of catalyst textural properties on residue hydroconversion
In the heterogeneous catalytic system, the reactants fi rstly arrive at the catalyst surface, and then diffuse through the catalyst pores to reach the active sites where the reactions will take place. As a result, textural properties of a catalyst such as pore volume and pore diameter should be optimized to carry out different reactions. Ancheyta[13]offered the suitable textural properties of the catalysts adapted to the reactants and reactions. For the heavy oil and residual oil hydroprocessing process, the best pore diameter should be larger than 18 nm. To study the effect of textural properties, two catalysts (named A2 and D2) with the same metal loading albeit with different textural properties were used in the residue hydroconversion process in slurry phase. The properties of the catalyst are shown in Table 3. The pore diameter distribution of the two catalysts is shown in Figure 4. The catalyst A2 had a higher BET surface area and a lower total volume. The pore size of the catalyst A2 ranged mainly from 5 nm to 15 nm, while that of the catalyst D2 ranged mainly from 4 nm to 40 nm. The experimental results are described in Figure 5.It can be seen from Figure 4 that the catalyst D2 had a higher residue conversion, a higher metal, sulfur and MCR conversion, and also a higher coke yield. The molecules of reactant in the residue had a large size and this reactant would fi rst enter the pores of the catalyst before the reaction could proceed. The pores of the catalyst A2 were too small to let the large reactants in and were also easily susceptible to plugging on the pore mouth. However, with a high dispersion of active metals on the catalyst inner surface, high surface area and moderate pore volume catalysts were extremely active for HDS. So the catalyst A2 attained a higher sulfur conversion rate.

Table 3 Properties of catalyst D2 and catalyst A2

Figure 3 Particle diameter distribution of catalysts A2 and D2

Figure 4 Effect of catalyst textural properties on residue hydroconversion
3.4 Effect of catalyst acidity on residue hydroconversion
To study the catalyst acidity effect, two catalysts (named H1 and D2) with the same metal loading and type albeit with different acidity were used in the residue hydroconversion process in slurry phase. In the course of preparing the catalyst H1, 25% of USY zeolite were added to the support. The experimental results are shown in Figure 5. It can be seen from Figure 5 that the two catalysts had a similar sulfur and metal conversion, but the catalyst H1 displayed a higher residue and MCR conversion rates and higher coke yield. The residue upgrading process can undergo along with both the free radical chain mechanism via thermal reaction and the carbonium ion mechanism by acid catalyst. The latter has a lower activation energy and can more readily take place, but it is extremely sensitive to nitrogen compounds especially the basic nitrogen compounds. During the experiments, the acidity of the catalyst D2 was too weak to induce the latter reaction while the catalyst H1 could. The suggested mechanism for carbon residue (MCR or CCR) conversion and residue conversion consists of the cracking of aliphatic side chains followed by the saturation of aromatic rings and the opening of hydroaromatic rings[8]. The opening of hydroaromatic rings has a strong relationship with the catalyst acidity[14]. That is why the high acidity catalyst H1 had achieved a higher conversion of residue and MCR. Although the high acidity catalyst could bring about high residue conversion, the coke yield was also high and this catalyst would be easily deactivated by the poisonous substance contained in the residue. So in the residual oil hydroconversion process, catalyst with high acidity is not used till now.

Figure 5 Effect of catalyst acidity on residue oil hydroconversion
3.5 Hydroconversion with spent catalyst
Some technologies for residue hydroconversion in slurry phase are being operated with the once through mode and the catalysts are not recycled[15-16]. It is advisable to use catalysts with low cost. Every year there are plenty of spent catalysts discharged from the commercial plants at refineries and they can possibly be a good option for the slurry phase residue hydroconversion process. It is reported that the spent HDS catalyst from commercial resid hydrotreating plant showed some activity for upgrading residue in slurry phase[17].
To study the possible utilization of spent catalyst, two spent catalysts (named AS1 and DS1) were used in the residue hydroconversion process in slurry phase. The catalyst AS1 was the spent commercial catalyst A1 while the catalyst DS1 was the spent commercial catalyst D1. The catalyst properties are shown in Table 2. The content of V2O5in AS1 was 3.9% and its carbon content was 11.5%, while the relevant indicators of the catalyst DS1 was 27.2% and 6.0%, respectively. The comparison of properties between the fresh and spent catalysts is shown in Figure 6 and Figure 7. The data in these two Figures indicate that the spent catalysts had lower sulfur and metals conversion than the fresh catalysts, but showed higher MCR conversion and coke yield. However, the spent catalysts all had a relatively high activity for residue hydroconversion, which might occur because the catalysts were ground before use. Once the catalyst was ground, the active sites of the catalyst were more readily exposedto the reactants. Figure 6 and Figure 7 also show that the two spent catalysts had different activity.
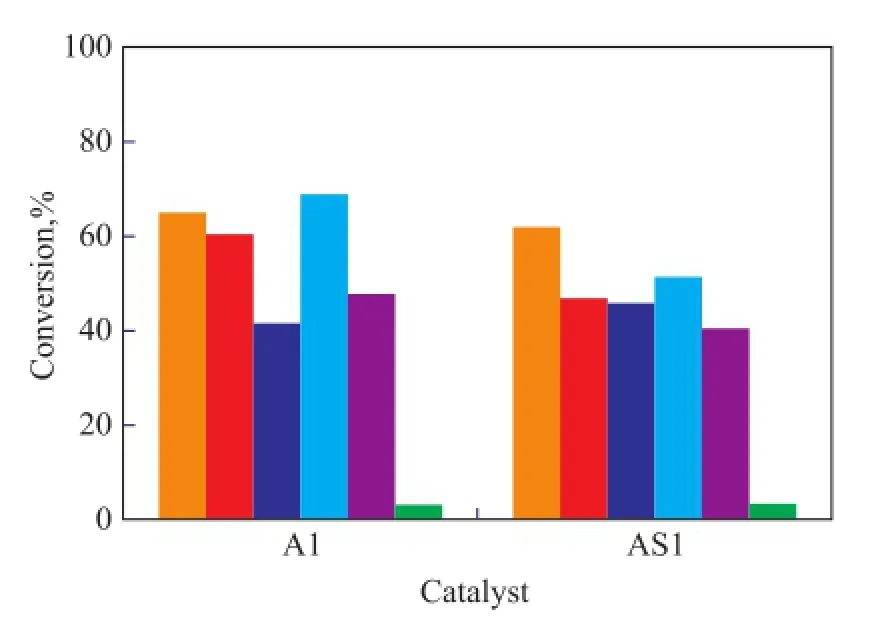
Figure 6 Activity comparison between A1 and AS1
3.6 Catalysis mechanism
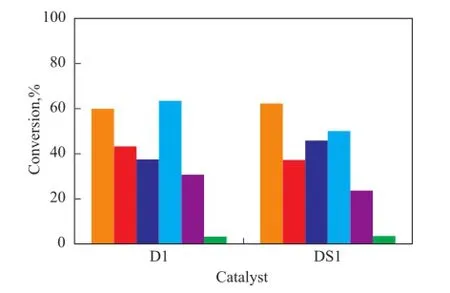
Figure 7 Activity comparison between D1 and DS1
The catalyst plays an important role in residue hydroconversion in slurry phase, but the mechanism of the catalyst is not yet clear. Researchers[18]have proposed a mechanism, which is presented in Figure 8. It assumes that the main role of catalyst is to activate hydrogen toward the reaction with an aromatic carbon radical to give a cyclohexadienyl type intermediate. Once the intermediate is formed, the molecule decomposes through a series of reactions to form the distillate and gases. This is the process that can reduce coke formation otherwise the intermediate will be quickly turned into coke (also named as solids). Besides, the catalyst also has the ability to adsorb the mesophase which can be easily aggregated to form coke.

Figure 8 Proposed catalysis mechanism
4 Conclusions
The effect of catalyst properties on residue hydroconversion was studied under moderate operating conditions (at a temperature of 400 ℃, an initial hydrogen pressure of 10 MPa, and a reaction time of 4 h) in a batch mode slurry phase in the presence of different catalysts. The testresults showed that the catalyst acidity had a good effect on residue conversion and MCR (micro carbon residue) conversion but brought about higher coke yield. The residue conversion was induced by the thermal reaction, but the catalyst acidity changed its conversion route. A catalyst with higher metal loading, higher hydrogenation activity and appropriate pore size had higher sulfur, metals and MCR conversion along with a lower coke formation. The activity of spent commercial catalysts AS1 and DS1 was slightly lower than the corresponding fresh ones but was still high enough for conducting the residue hydroconversion.
It assumes that the role of the catalyst is to activate hydrogen toward reaction with an aromatic carbon radical to give a cyclohexadienyl type intermediate, which will turn into liquid and also to adsorb the mesophase which can be easily aggregated to form coke.
[1] Mosio-Mosiewski J, Morawski I. Study on single-stage hydrocracking of vacuum residue in the suspension of Ni–Mo catalyst [J]. Applied Catalysis A: General, 2005, 283(1): 147-155
[2] Castaneda L C, Mu?oz J A, Ancheyta J. Current situation of emerging technologies for upgrading of heavy oils [J]. Catalysis Today, 2014, 220: 248-273
[3] Rankel L A. Hydrocracking vacuum resid with Ni-W bifunctional slurry catalysts [J]. Fuel Processing Technology, 1994, 37(2): 185-202
[4] Rezaei H, Liu X, Ardakani S J, et al. A study of Cold Lake vacuum residue hydroconversion in batch and semi-batch reactors using unsupported MoS2catalysts [J]. Catalysis Today, 2010, 150(3): 244-254
[5] Angeles M J, Leyva C, Ancheyta J, et al. A review of experimental procedures for heavy oil hydrocracking with dispersed catalyst [J]. Catalysis Today, 2014, 220: 274-294
[6] Ortiz-Moreno H, Ramírez J, Sanchez-Minero F, et al. Hydrocracking of Maya crude oil in a slurry-phase batch reactor. II. Effect of catalyst load [J]. Fuel, 2014, 130: 263-272
[7] Martinez-Grimaldo H, Ortiz-Moreno H, Sanchez-Minero F, et al. Hydrocracking of Maya crude oil in a slurry-phase reactor. I. Effect of reaction temperature [J]. Catalysis Today, 2014, 220: 295-300
[8] Kim J, Longstaff D C, Hanson F V. Catalytic and thermal effects during hydrotreating of bitumen-derived heavy oils [J]. Fuel, 1998, 77(15): 1815-1823
[9] Khorasheh F, Rangwala H A, Gray M R, et al. Interactions between thermal and catalytic reactions in mild hydrocracking of gas oil [J]. Energy Fuels, 1989, 3(6): 716-722
[10] Martínez J, Ancheyta J. Modeling the kinetics of parallel thermal and catalytic hydrotreating of heavy oil [J]. Fuel, 2014, 138: 27-36
[11] Heck R H, Rankel L A, DiGuiseppi F T. Conversion of petroleum resid from Maya crude: Effects of H-donors, hydrogen pressure and catalyst [J]. Fuel Processing Technology, 1992, 30(1): 69-81
[12] Li Dadong. Hydrotreating Technologies and Processes [M]. Beijing: China Petrochemical Press, 2004 (in Chinese)
[13] Ancheyta J, Rana M S, Furimsky E. Hydroprocessing of heavy petroleum feeds: Tutorial [J]. Catalysis Today, 2005, 109(1): 3-15
[14] Leyva C, Ancheyta J, Travert A, et al. Activity and surface properties of NiMo/SiO2–Al2O3catalysts for hydroprocessing of heavy oils [J]. Applied Catalysis A: General, 2012, 425: 1-12
[15] Niemann K, Wenzel F. The Veba Combi-Cracking technology: An update [J]. Fuel Processing Technology, 1993, 35(1): 1-20
[16] Drago G, Guitian J, Krasuk J, et al. The development of HDH process, a refiner’s tool for residual upgrading [J]. American Chemical Society, Division of Petroleum Chemistry, 1990, 4(35): 66
[17] Sakabe T, Yagi T. Crack residua with spent HDS catalyst [J]. Hydrocarbon Processing, 1979, 59(12): 103-107
[18] Kennepohl D, Sanford E. Conversion of Athabasca bitumen with dispersed and supported Mo-based catalysts as a function of dispersed catalyst concentration [J]. Energy & Fuels, 1996, 10(1): 229-234
Received date: 2015-09-17; Accepted date: 2015-11-27.
Dr. Yang Qinghe, Telephone: +86-10-82368123; E-mail: yangqh.ripp@sinopec.com.
- 中國煉油與石油化工的其它文章
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Molecular Simulations of FCC Dry Gas Components Adsorption in Zeolite Y1
- Intrinsic Kinetic Modeling of Thermal Dimerization of C5Fraction
- Synthesis and Properties of Dendritic Long-Chain Esters as Crude Oil Flow Improver Additives
- Method for Control of Particle Size and Morphology of Paraf fi n/Polystyrene-Divinylbenzene Microcapsules
- Study on Absorption and Regeneration Performance of Novel Hybrid Solutions for CO2Capture

