Substitution of egg yolk with different concentrations of soybean lecithin in trisbased extender during bulls' semen preservability
Gamal A El-Sisy, Walid S El-Nattat, Reda I El-Sheshtawy, Amal M Abo El-Maaty
Department of Animal Reproduction and Artificial Insemination, Veterinary Division National Research Centre, Egypt
Substitution of egg yolk with different concentrations of soybean lecithin in trisbased extender during bulls' semen preservability
Gamal A El-Sisy*, Walid S El-Nattat, Reda I El-Sheshtawy, Amal M Abo El-Maaty
Department of Animal Reproduction and Artificial Insemination, Veterinary Division National Research Centre, Egypt
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Bulls
Objective:To investigate the effect of various concentrations of soybean lecithin as an alternative for egg yolk in bull semen extender on post-chilling and post-thaw sperm quality characteristics.Methods:Semen ejaculates were collected from three mature bulls' once/week for 5 weeks. After initial evaluation the approved ejaculates were pooled and extended gradually 1:7 with tris-citrate-fructose egg yolk extender (control) and tris-citrate-fructose (TCF)+different concentration of soybean lecithin (0.5%, 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, 3.5% and 4.0%) to ensure 60 million motile spermatozoa/mL, and then proceeded an adopted international cryopreservation protocol. Frozen straws were thawed at 37 ℃ for 30 s. The parameters studied were sperm motility, viability, abnormality, membrane integrity and normal intact acrosome percentages in chilled and frozen-thawed semen.Results:The substitution of egg yolk into TCF with 1% soya lecithin significantly (P<0.0 001) ameliorated the maintenance of semen characters motility (86.67%±0.80%), life (87.60%±0.88%), and membrane integrity (82.47%±0.94%), meanwhile it had significantly (P<0.0 001) reduced the abnormality (13.20%±0.60%) of spermatozoa to its least value compared to some other concentrations in use. Moreover, the addition of 1.5% of soya lecithin to TCF had maintained the semen characteristics compared to the control tris-citrate-fructose egg yolk. The significantly higher mean values of post-thawing sperm motility% were observed in 1% soybean lecithin (56.00% ±1.00 %) as compared to the control (41.00%±1.00%).Conclusion:1% to 1.5% soya lecithin can effectively alternate egg yolk as cryoprotective additives for cryopreservation extender, without any detrimental effects on post-chilling and post-thaw semen quality in cattle bull.
1. Introduction
Artificial insemination together with perfect progeny testing programs have a fundamental effects on the rate of genetic improvement, enhancing of gene merits and amplifying of chosen reproductive characteristics in farm animals. Cryopreservation is the main essential tool for long-standing storage of semen and control of venereal diseases[1]. However, cryopreservation yields detrimental effects on post-thawing sperm quality and fertilization process[1,2]. Optimization of freezing extender to obtain the best quality of post-thaw semen is crucial. In general, farm animal semen cryopreservation medium includes: A nonpenetrating cryoprotectant (a source of lipoprotein to provide protection against cold shock such as egg yolk, milk, or soybean lecithin), a penetrating cryoprotectant (glycerol, ethylene glycol, or dimethyl sulfoxide, etc.), ionic or nonionic substances to maintain a suitable osmotic pressure and pH, (buffer such as Tris or Test, etc.), energy source substrate (i.e., glucose or fructose), antibiotics (penicillin, streptomycin, etc.) and other additives, such as enzymes and antioxidants[3,4]. Egg yolk is the most widely used cryoprotectant in the composition of cryopreservation extenders of mammalian spermatozoa; yet, efforts have been made to find ways to substitute it due to the possibility of transporting pathogenic microorganisms, production of harmful metabolites and toxins, the lack of standardization, and the presence of steroid hormones and substances that inhibit metabolic exchanges or decrease the motility of sperm, all resulting in reduced semen quality[5-9]. In addition, the bio-security in various countries for semen transport is extremely essential to avoid the hazard of transporting avian influenza through egg-based products[5,10]. In this regard, low density lipoproteins (LDL) extracted from egg yolk, gamma-irradiated egg yolk plasma, pasteurized powdered egg yolk or lecithin from non-animal source like soya were tested as a non-permeable cryoprotectant in extender for deep freezing of farm animals spermatozoa[11-13]. The use of non animal origin chemically defined medium is the method of choice in assisted reproductive technology and semen cryopreservation[14,15]. Substitution of egg yolk with soybean lecithin may reduce hygienic risks in extenders. Recently, there are several studies indicating the valuable effects of soybean lecithin for cryopreservation of sperm in bull[3,16], ram[17,18] and goat[19,20]. A soybean lecithin-based extender has been developed and utilized commercially for bull[3], ram[14,21] and buffalo bull[22,23] semen.
The soybean lecithin is a valuable plant-based phospholipids source that included in commercial extenders used for freezing mammalian sperm without clear levels and adjustments due to trade protection. Therefore, this study was designed to investigate the effect of various levels of soybean lecithin as an alternativefor egg yolk in bull semen extender on post-chilling and post-thaw sperm quality including motility, viability, abnormalities, and plasma membrane integrity and intact acrosome.
2. Materials and methods
2.1. Semen collection and initial evaluation
Five mature genetically improved cattle-bulls with superior quality semen characteristics maintained at The Semen Freezing Center, General Organization for Vet. Services, Ministry of Agriculture, Abbasia, Egypt, were used for this study as semen source. Semen ejaculates were collected from bulls using an artificial vagina at weekly intervals for 5 wk. The semen samples were initially evaluated for volume (in graduated tube), concentration using Thoma rulling of the Neubaur haemocytometer and sperm motility. The neat semen samples with more than 70% motility and 80% morphologically normal spermatozoa were admitted to freezing procedure. The ejaculates were pooled in order to have sufficient semen for a replicate and to eliminate the bull effect. The semen was given a holding time for 10 min at 37 ℃ in a water bath before dilution and reevaluated for sperm motility, viability, total abnormalities, and acrosome and membrane integrities before processing.
2.2. Semen processing
The control cryopreservation extender was Tris-citric acid-egg yolk-fructose (TCFY) diluent[24]. Semen samples were extended gradually 1:7 with TCFY extender (control) and TCFY+different concentration of soya lecithin (0.5%, 1.0%, 1.5%, 2.0%, 2.5%, 3.0%, 3.5% and 4.0%) to ensure 60 million motile spermatozoa/mL, then cooled slowly (approximately for 2 h) up to 5 ℃ and equilibrated for 4 h. Semen was packed into 0.25 mL polyvinyl French straws (IMV, France). After equilibration periods, the straws were placed horizontally on a rack and frozen in a vapor 4 cm above liquid nitrogen for 10 min and were then dipped stored in liquid nitrogen at -196 ℃.
2.3. Assessment of semen quality parameters
Frozen straws were thawed individually at 37 ℃ for 30 s in a water bath for microscopic evaluation[25]. The parameters studied were sperm motility, sperm viability, sperm abnormality, sperm membrane integrity, percent of normal intact acrosome in chilled and frozenthawed semen.
2.3.1. Sperm motility (%)
Subjective motility was observed using phase contrast microscope (Olympus Optical Co. Ltd., Japan). Visual motility was assessed microscopically with closed circuit television[26].
2.3.2. Live and abnormal spermatozoa (%)
The viability and abnormalities % of sperm were evaluated using eosin-Nigrosin stained smear as described by Sidhu et al.[27].
2.3.3. Sperm membrane integrity (%)
Sperm membrane integrity was assessed using the hypoosmotic swelling test[28]. Two hundred spermatozoa were assessed and the percentage of spermatozoa with curled tails (swollen/intact plasma membrane) was calculated.
2.3.4. Intact normal acrosome (%)
Acrosome integrity was evaluated using giemsa stain as described by Watson[29]. The intact acrosome was recorded for 200 spermatozoa that were randomly examined under an immersion objective (×1000) using phase contract microscope.
2.4. Statistical analysis
Output data were analyzed by one-way analysis of variance (ANOVA), followed by Duncan test to determine significant differences in all the parameters among all groups, with SPSS Version 14.0 for Windows[30]. Differences with values of P<0.05 were considered to be statistically significant.
3. Results
3.1. The chilling
Data output in Table 1 referred that the substitution of egg yolk into TCF with soya lecithin at a concentration of 1% significantly (P<0.0 001) ameliorated the maintenance of semen characters (motility, life, and membrane integrity %), meanwhile it had significantly (P<0.0 001) reduced the abnormality % of spermatozoa to its least value compared to some other concentrations in use. In the same consent, the addition of 1.5% of soya lecithin to TCF had maintained non significantly (P<0.0 001) the semen characteristics compared to the control TCFY. The addition of soya lecithin less than 1% or more than 1.5% had lowered significantly (P<0.0 001) the semen characteristics compared to the control TCFY.
The highest mean value of motility% was observed in 1% and 1.5% SL. These values were significantly (P<0.0 001) higher than 0.5%, 2.0% and 2.5%, 3.0%, 3.5% and 4.0% SL post-chilling. Similarly, the highest mean values of life sperm% were observed in 1.0% and 1.5% SL as compared to the control. These values were significantly (P<0.0 001) higher than 0.5%, 2.0% and 2.5%, 3.0%, 3.5% and 4.0% SL post-chilling. The total sperm abnormalities mean values were apparently lower in 0.5%, 1.0% and 1.5% SL as compared to the control. These values were lower than 2.0%, 2.5%, 3.0%, 3.5 and 4.0% post-chilling. The membrane integrity% mean values were significantly higher in 1.0% and 1.5% SL as compared to the control. These values are lower than in 0.5%, 2.0%, 2.5%, 3.0%, 3.5% and 4.0% SL post-chilling (Table 1)
3.2. The post-thawing
Concerning the data output in Table 2, the substitution of egg yolk with 1% soya lecithin showed significantly higher (P<0.0 001) semen characteristics concerning the motility and membrane integrity% compared to control TCFY. The significantly (P<0.0 001) higher mean values of post-thawing sperm motility% were observed in 1% SL as compared to the control. These values were significantly (P<0.0 001) higher than 0.5%, 2.0% and 2.5%, 3.0%, 3.5% and 4.0% SL (Table 2). Meanwhile, the addition of 1.5% soybean lecithin had a similar effect as the egg yolk addition to TCF (Table 2).
The post-thawing life sperm% mean values were significantly (P<0.0 001) lower in 1.5% and 2.0% SL (Table 2) as compared to the control. These values were significantly (P<0.0 001) higher than 0.5%, 1.0%, 2.5%, 3.0%, 3.5% and 4.0% SL (Table 2).
The post-thawing sperm abnormalities% mean values were significantly (P<0.0 001) higher in 0.5%, 1.0% and 1.5% SL (Table 2) compared to the control (18.40%±1.02%). These values were significantly (P<0.0 001) lower than 2.0%, 2.5%, 3.0%, 3.5% and 4% SL (Table 2).
Concerning the acrosome integrity%, mean values were significantly (P<0.0 001) higher in 1% (Table 2) than all SL concentrations.
4. Discussion
Egg yolk is the most widely used advantageous cryoprotectants during freezing and thawing process in the majority of animal species. Although favorable efficiency of egg yolk in extender, the usage of egg yolk facing many protests mainly attributed to hygiene concerns and risk of bacterial contaminations[3,5,14], consequently, its interference with semen quality[31]. The use of non animal origin cryoprotectant instead of egg yolk in semen cryopreservation is of growing interest in the last years[14,15,18,32-34].
The results of the current study revealed that among the wide range (0.5%-4.0%) range of tested concentrations of soya-lecithin, the most beneficial levels of soybean lecithin for cooling and cryopreservation of cattle bull semen were 1.0% and 1.5%. These concentrations showed best semen quality studied parameters after both post-chilling and post-thawing.
In the present experimental work we observed that the range of soy lecithin level from 1.0% to 1.5% in the extender yielded the best semen characteristics post preservation.
The reported optimal concentrations of soybean lecithin in extender used for cryopreservation in the literatures were ranged from 0.8% in canine[35] to 1.0% in ram[17], human[36] and cat[37] and 1.5% in goat[2,34].
These species different may be interrelated to the variations in of sperm plasma membrane composition between species which may determine the required level of soybean lecithin in the extender[38]. Another cause which affected the optimum level of soybean lecithin in the extender is species differences in seminal plasma composition specially the concentration of bovine seminal plasma proteins. The bovine seminal plasma proteins envelop the sperm membrane and stimulate cholesterol and phospholipid efflux and damage the sperm membrane[39].
Our data are in agreement with Salmani et al.[20] in goat, Forouzanfar et al.[17] and Masoudi et al.[34] in ram, Vick et al.[37] in cat, and Reed et al.[36] in human whom reported that 1.0% to 1.5 % SL in extender has beneficial effects and that higher concentrations of soybean lecithin above 1.5% have a harmful and toxic effect on sperm during cryopreservation and resulted in reduction in sperm motility and viability.
Aires et al.[3] reported a significantly higher post-thaw bovine sperm motility in soybean lecithin based extender than Tris-egg yolk diluents. Masoudi et al.[34] recorded higher life sperm% in 1.0% SL contained extender as compared to egg yolk and did not detect any significant variations in sperm motility, membrane and acrosome integrity. However, De Leeuw et al.[40] and Celeghini et al.[41] reported that egg yolk-containing diluents is more efficient in preserving survivability of bull sperm during freezing than diluents containing soybean lecithin. va Wagtendonk-de Leeuw et al.[42] noticed that higher concentrations of lecithin in the freezing medium produced particular debris and increased viscosity and decreased osmotic pressure of extenders which could have adverse effect on semen fertility[42]. Moussa et al.[11] noticed a significant decline in extender osmotic pressure when LDL concentration increase due to the precipitation of fructose and salts included in the extender and this decreased osmotic pressure had a damaging effect to sperm cell. On the contrary, de Paz et al.[18] noticed a significant improvement in frozen-thawed motility when 2.0%-3.5% soybean lecithin was included in ram freezing extender.
In the current study, the sperm motility was significantly improved by addition of the 1.0% and 1.5% SL to TCF medium as compared with TCF with egg yolk. This improvement of sperm motility may be attributed to the low viscosity of these concentrations of soya lecithin as compared with egg yolk[3,42] which may facilitate the movement of spermatozoa. Salmani et al.[20] indicated that soya-lecithin was more efficient in protecting goat sperm against destructive lipid peroxidation during cryopreservation compared to egg yolk which can be attributed to egg yolk composition that is containing more unsaturated fatty acids susceptible to lipid peroxidation. They also noticed a significant reduction in MDA level in the goat semen extender containing different concentrations of soybean lecithin than extender containing egg yolk.
The post-thawing life sperm% mean values were significantly (P<0.0 001) higher in 1.5% and 2.0% SL compared to 0.5%, 1.0%, 2.5%, 3.0%, 3.5% and 4.0% SL. These results are in agreement with Salmani et al.[20] and Masoudi et al.[34] whom notice that SL extender yield a significantly (P<0.0 001) higher live sperm% than egg yolk extender in ram. It appears that SL had extra protective action on plasma membrane of sperm against destruction of intercellular sperm structures[43]. Moreover, Masoudi et al.[34] and Emamverdi et al.[44] reported that SL can prevent apoptotic cascades in goat and ram spermatozoa during cryopreservation.
It has been previously evidenced that the LDL fraction off egg yolk is the main cryoprotective component of egg yolk during the cryopreservation of spermatozoa[11,39,45,46]. The mechanisms for cryoprotective effects of soybean lecithin may be due to an interaction between seminal plasma proteins and LDL in extenders as suggested by Bergeron and Manjunath[45] and also, may be related to the protecting film of lecithin at the surface of spermatozoa membranes against of ice crystal[11]. Furthermore, it has been suggested that exogenous phospholipids could replaces some phospholipids of sperm membrane, refurbishing the damaged plasma membrane[47], and improving tolerance against freezing process[3,11,48].
In conclusion, 1.0% to 1.5% soya lecithin can effectively alternate egg yolk as a cryoprotective additive for cryopreservation extender, without any detrimental effects on post-chilling and post-thaw semen quality in cattle bull. Further studies are necessary for evaluation in vivo fertility of frozen-thawed cattle bull semen in extender including these soybean lecithin concentrations.
Conflict of interest statement
The authors declare that they have no conflict of interest.
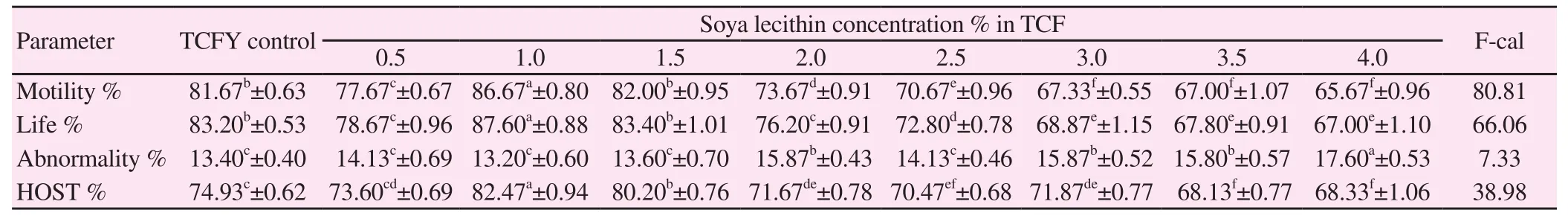
Table 1 Effect of different concentrations of soybean lecithin in extender on post-chilling bull semen characteristics (P<0.0 001).
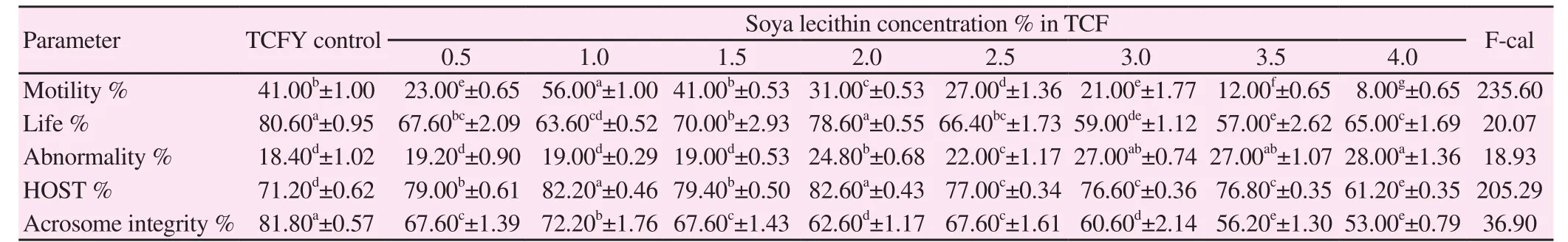
Table 2 Effect of different concentration of soybean lecithin in extender on post-thawing bull semen characteristics (P<0.0001).
[1] Lemma A. Effect of cryopreservation on sperm quality andfertility. In: Manafi M, editor. Artificial insemination in farm animals. In Tech, Croatia; 2011. p. 19-216.
[2] Tasdemir U, Buyukleblebici S, Tuncer PB, Coskun E, Ozgurtas T, Ayd?n FN, et al. Effects of various cryoprotectants on bull sperm quality, DNA integrity and oxidative stress parameters. Cryobiology 2013; 66(1): 38.
[3] Aires VA, Hinsch KD, Muller-schlosser F, Bonger K, Muller-schlosser S, Hinsch E. In vitro and in vivo comparision of egg yolk based and soya bean based extenders for cryopreservation of bovine semen. Theriogenology 2003; 60: 269-279.
[4] Vishwanath R, Shannon P. Storage of bovine semen in liquid and frozen state. Anim Reprod Sci 2000; 62: 23-53.
[5] Bousseau S, Brillard JP, Guienne M, Guerin B, Camus A, Lechat M. Comparison of bacteriological qualities of various egg yolk sources and the in vitro and in vivo fertilizing potential of bovine semen frozen in egg yolk or lecithin-based diluents. Theriogenology 1998; 50: 699-706.
[6] Lipar JL, Ketterson ED, Nolan JrV, Casto JM. Egg yolk layers vary in the concentration of steroid hormones in two avian species. Gen Comp Endocrinol 1999; 115(2): 220-227.
[7] Marco-Jimenez F, Puchades S, Moce E, Viudes-de-Cartro MP, Vicente JS, Rodriguez M. Use of powdered egg yolk vs fresh egg yolk for the cryopreservation of ovine semen. Reprod Dom Anim 2004; 39: 438-441.
[8] Akhter S, Ansari MS, Andrabi SM, Ullah N, Qayyum M. Effect of antibiotics in extender on bacterial and spermatozoal quality of cooled buffalo (Bubalus bubalis) bull semen. Reprod Domest Anim 2008; 43; 272-278.
[9] Althouse GC. Sanitary procedures for the production of extended semen. Reprod Domest Anim 2008; 43: 374-378.
[10] Holt WV. Fundamental aspects of sperm cryobiology: the importance if species and individual differences. Theriogenology 2000; 53; 47-52.
[11] Moussa M, Martinet V, Trimeche A, Tainturier D, Anton M. Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen-thawed bull semen. Theriogenology 2002; 57: 1695-1706.
[12] Amirat L, Tainturier D, Jeanneau L, Thorin C, Gerard O, Courtens JL, et al. Bull semen in vitro fertility after cryopreservation using egg yolk LDL: A comparison with optidyl, a commercial egg yolk extender. Theriogenology 2004; 61: 895-907.
[13] Hu JH, Jiang ZL, Lv RK, Li QW, Zhang SS, Zan LS, et al. The advantages of low-density lipoproteins in the cryopreservation of bull semen. Cryobiology 2011; 62: 83-87.
[14] Fukui Y, Kohno H, Togari T, Hiwasa M, Okabe K. Fertility after artificial insemination using a synthetic semen extender in sheep. J Reprod Develop 2008; 54: 286-289.
[15] Jeyendran R, Acosta V, Land S, Coulam C. Cryopreservation of human sperm in a lecithin-supplemented freezing medium. Fertil Steril 2008; 90: 1263-1265.
[16] Gonzales R, Rosales ABM, Perea CF, Velarde CJ, Soto BE, Palomares ABR, et al. Conception rates using Brahman bull semen frozen in milk based extender containing egg yolk or soybean lipids. A field study in a tropical environment. Fert Develop 2003; 16: 170-171.
[17] Forouzanfar M, Sharafi M, Hosseini SM, Ostadhosseini S, Hajian M, Hosseini L, et al. In vitro comparison of egg yolk-based and soybean lecithin-based extenders for cryopreservation of ram semen. Theriogenology 2010; 73: 480-487.
[18] de Paz P, Esteso MC, Alvarez M, Mata M, Chamorro CA, Anel L. Development of extender based on soybean lecithin for its application in liquid ram semen. Theriogenology 2010; 74: 663-671.
[19] Roof DJ, Bowley S, Price LL, Matsas DJ. Comparison of two commercial extenders for cryopreservation of goat semen without sperm washing. Theriogenology 2012; 77: 412-420.
[20] Salmani H, Towhidi A, Zhandi M, Bahreini M, Sharafi M. In vitro assessment of soybean lecithin and egg yolk based diluents for cryopreservation of goat semen. Cryobiology 2014; 68: 276-280.
[21] Hiwasa M, Kohno H, Togari T, Okabe K, Fukui Y. Fertility after different artificial insemination method using synthetic semen extender in sheep. J Reprod Develop 2009; 55: 50-54.
[22] El Sisy GA, El-Nattat WS, El-Sheshtawy RI, Shalaby SIA. Effect of different extenders on viability of frozen buffalo-bull semen. 3rd International Conference of Veterinary Division, National Research Centre, Giza, Egypt, 5-6 December; 2006. p. 11-22.
[23] Akhter S, Ansari MS, Rakha BA, Andrabi SM, Iqbal S, Ullah N. Cryopreservation of buffalo (Bubalus bubalis) semen in Bioxcell extender. Theriogenology 2010; 74: 951-955.
[24] Foote RH. Fertility of bull semen at high extension rates in Tris buffered extenders. J Dairy Sci 1970; 53: 1475-1477.
[25] Ashrafi I, Kohram H, Naijian H, Bahreini M, Mirzakhani H. Effect of controlled and uncontrolled cooling rate on motility parameters of cryopreserved ram spermatozoa. BMC Res Notes 2011; 4: 547.
[26] Graham EF, Schmehl MKL, Maki-Laurila M. Some physical and chemical methods of evaluating semen. In: Proc. 3rd NAAB Tech Conf Artif Insemin Reprod, 12-14 April Milwaukee, WI. Columbia: National Association of Animal Breeders; 1970. p. 44-48.
[27] Sidhu KS, Guraya SS. Buffalo bull semen morphology, biochemistry, physiology and methodology. Ludhiana: USA Publishers and Distributors; 1985. p. 152-154.
[28] Jeyendran RS, Vander Ven HH, Perez Pelaez M, Crabo BG, Zaneveld LJD. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil 1984; 70: 219-228.
[29] Watson PF. Use of giemsa stain to detect changes in the acrosome of frozen ram spermatozoa. Vet Record 1975; 97: 12-15.
[30] SPSS. v.14.0 for Windows Evaluation Version Release. 2005; 14.0.0.
[31] Ansari MS, Rakha BA, Andrabi SM, Akhter S. Usefulness of powdered and fresh egg yolk for cryopreservation of Zebu bull spermatozoa. Reprod Biol 2010; 10: 235-240.
[32] Papa FO, Felcio GB, Melo-O CM, Alvarenga MA, De Vita B, Trinque C, et al. Replacing egg yolk with soybean lecithin in the cryopreservation of stallion semen. Anim Reprod Sci 2011; 129: 73-77.
[33] Toker MB, Alcay S, Gokce E, Ustuner B. Cryopreservation of ram semen with antioxidant supplemented soybean lecithin-based extenders and impacts on incubation resilience. Cryobiology 2016; 72: 205-209.
[34] Masoudi R, Sharafi M, Shahneh AZ, Towhidi A, Kohram H, Esmaeili VA. Fertility and flow cytometry study of frozen-thawed sperm in cryopreservation medium supplemented with soybean. Cryobiology 2016; 73: 69-72.
[35] Kmenta I, Strohmayer C, Muller-Schlosser F, Schafer-Somi S. Effects of a lecithin and catalase containing semen extender and a second dilution with different enhancing buffers on the quality of cold-stored canine spermatozoa. Theriogenology 2011; 75: 1095-1103.
[36] Reed ML, Ezeh PC, Hamic A, Thompson DJ, Caperton CL. Soya lecithin replaces egg yolk for cryopreservation of human sperm without adversely affecting post thaw motility, morphology, sperm DNA integrity, or sperm binding to hyaluronate. Fertil Steril 2009; 92: 1787-1790.
[37] Vick M, Bateman H, Swanson W. Improved cryopreservation of domestic cat spermatozoa in a soy lecithin-based extender. Reprod Fertil Dev 2010; 23: 153-154.
[38] Bencharif D, Amirat L, Anton M, Schmitt E, Desherces S, Delhomme G, et al. The advantages of LDL (low density lipoproteins) in the cryopreservation of canine semen. Theriogenology 2008; 70: 1478-1488.
[39] Manjunath P, Nauc V, Bergeron A, Menard M. Major proteins of bovine seminal plasma bind to the low-density lipoprotein fraction of hen’s egg yolk. Biol Reprod 2002; 67: 1250-1258.
[40] De Leeuw FE, De Leeuw AM, Den Daas JHG, Colenbrander B, Verkleij AJ. Effects of various crioprotective agents and membrane stabilizing compounds on bull sperm membrane integrity alter cooling and freezing. Crybiology 1993; 30: 32-44.
[41] Celeghini ECC, Arruda RP, Andrade AFC, Nascimento J, Raphael CF, Rodrigues PMH. Effects that bovine sperm cryopreservation using two different extenders has on sperm membranes and chromatin. Anim Reprod Sci 2008; 104; 119-131.
[42] van Wagtendonk-de Leeuw AM, Haring RM, Kaal-Lansbergen LM, den Daas JH. Fertility results using bovine semen cryopreserved with extenders based on egg yolk and soy bean extract. Theriogenology 2000; 54: 57-67.
[43] Holt WV, North RD. Effects of temperature and restoration of osmotic equilibrium during thawing on the induction of plasma membrane damage in cryopreserved ram spermatozoa. Biol Reprod 1994; 51: 414 -424.
[44] Emamverdi M, Zhandi M, Zare Shahneh A, Sharafi M, Akbari-Sharif A. Optimization of ram semen cryopreservation using a chemically defined soybean lecithin-based extender. Reprod Domest Anim 2013; 48(6): 899-904
[45] Bergeron A, Manjunath P. New insights towards understanding the mechanisms of sperm protection by egg yolk and milk. Mol Reprod Dev 2006; 73: 1338-1344.
[46] Bergeron A, Crete MH, Brindle Y, Manjunath P. Low-density lipoprotein fraction from hen’s egg yolk decreases the binding of the major proteins of bovine seminal plasma to sperm and prevents lipid efflux from the sperm membrane. Biol Reprod 2004; 70: 708-717.
[48] Foulkes JA. The separation of lipoproteins from egg yolk and their effect on the motility and the integrity of bovine spermatozoa. J Reprod Fertil 1977; 49: 277-228.
[47] Graham JK, Foote RH. Effect of several lipids, fatty acyl chain length, and degree of unsaturation on the motility of bull spermatozoa after cold shock and freezing. Cryobiology 1987; 24: 42-52.
ment heading
10.1016/j.apjr.2016.10.011
*Corresponding author: Gamal A El-Sisy, Department of Animal Reproduction and Artificial Insemination, Veterinary Division National Research Centre, Egypt.
Tel: (M)+201121410020
E-mail: gelsisy@yahoo.com
Semen
Soybean lecithin
Cryopreservation
 Asian Pacific Journal of Reproduction2016年6期
Asian Pacific Journal of Reproduction2016年6期
- Asian Pacific Journal of Reproduction的其它文章
- The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa
- Prolificity of Portuguese Serrana Goats between 1987 and 2015
- Ultra-structure of testes of rats born to dams treated withhydroxy-progesterone hexanoate
- Hormonal changes and spermatogenesis of male rat puppies born by mothers consuming soybean extract
- Effect of Thaumatococcus daniellii leaf rat-feed on potassium bromate induced testicular toxicity
- Nicotine effect toward the oocyte level of rats(Rattus novergicus)
