A new species of the genus Amolops (Anura: Ranidae) from high-altitude Sichuan, southwestern China, with a discussion on the taxonomic status of Amolops kangtingensis
Liang Fei, Chang-Yuan Ye, Yu-Fan Wang, Ke Jiang,*
1Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu Sichuan610041,China
2State Key Laboratory of Genetic Resources and Evolution,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan650223,China
3Zhejiang Forest Resource Monitoring Center,Hangzhou Zhejiang310020,China
ZOOLOGICAL RESEARCH
A new species of the genus Amolops (Anura: Ranidae) from high-altitude Sichuan, southwestern China, with a discussion on the taxonomic status of Amolops kangtingensis
Liang Fei1, Chang-Yuan Ye1, Yu-Fan Wang2,3, Ke Jiang1,*
1Chengdu Institute of Biology,Chinese Academy of Sciences,Chengdu Sichuan610041,China
2State Key Laboratory of Genetic Resources and Evolution,Kunming Institute of Zoology,Chinese Academy of Sciences,Kunming Yunnan650223,China
3Zhejiang Forest Resource Monitoring Center,Hangzhou Zhejiang310020,China
A new species of the genusAmolopsCope, 1865 is described from Xinduqiao, Kangding, Sichuan. It was previously identified asAmolops kangtingensis, which is synonymized toAmolops mantzorumin this study. The new species,Amolops xinduqiaosp. nov., is distinguished from all other congeners by the following combination of characters: (1) medium body size, adult males SVL 41.2–47.5 mm (n=15, average 43.9 mm), adult females SVL 48.5–56.6 mm (n=15, average 52.5 mm); (2) head length equal to width or slightly wider than long; (3) tympanum small, but distinct; (4) vomerine teeth in two tiny rows, separated by a space about one vomerine teeth row; (5) bony projections on lower jaw absent; (6) dorsolateral folds usually absent; (7) tarsal folds or glands on tarsus absent; (8) circummarginal groove on disc of finger I absent; (9) tibiotarsal articulation reaching nostril or beyond; (10) webs of toe IV reaching to distal articulation, other toes fully webbed to disc; and (11) vocal sac absent in males.
New species; Sichuan; Taxonomy;Amolops xinduqiaosp. nov.;Amolops kangtingensis
INTRODUCTION
The genusAmolopsCope, 1865, which contains 51 species at present (Frost, 2017), is distributed from Nepal, northern India, western and southern China to Malaysia. These species inhabit rocky streams or water falls, from which they receive their common name as cascade frogs. The monophyly of this genus is supported by previous molecular studies (Cai et al., 2007; Stuart, 2008).
Amolops kangtingensis(Liu, 1950) was confused withAmolops mantzorum(David, 1871) for a long time. Liu & Hu (1961) synonymizedA. kangtingensiswithA. mantzorumdue to the difficulty in identification between these two species under preservative condition. Based on karyotyping studies, Wu et al. (1987) supported the validity ofA. kangtingensis, which was followed by Dubois (1992) and Zhao & Adler (1993), but rejected by Fei et al. (2005, 2009b, 2012). Lu et al. (2014) discussed the validity ofA. kangtingensisaccording to molecular data, and Zhang et al. (2015) recognizedA. kangtingensisthrough a combination of morphological, karyotypic, and molecular analyses of different populations of these two species. Based on the species delimitation by Zhang et al. (2015), high altitude (above 3 000 m) populations in the Yalong River Basin are considered different from the mid-high altitude (1 200–2 400 m) populations in the Dadu River Basin. However, the above research supporting the validity ofA. kangtingensisdid not examine the originally designated type locality ofA. kangtingensis. These earlier studies treated Xinduqiao as the type locality; however, according to the original holotype description and information, the type locality ofA. kangtingensiswas limited to Yalagou of Kangding (in the Dadu River Basin) andA. kangtingensisshould be synonymized withA. mantzorum. Thus, herein we describe the Xinduqiao population as a new species.
MATERIALS AND METHODS
Sampling
A total of 133 individuals (51 adult males and 82 adult females) of the new species were collected from four localities, including Xinduqiao (47 males and 75 females), Liuba (one male), Dongeluo (two males and one female), and Pengbuxi (one male and six females) of Kangding, Sichuan, China. Following euthanasia, five recently collected specimens (KIZ 014127–31 from Xinduqiao in 2016) were fixed in 10% formalin solution after sampling of tissues (in 95% ethanol), and transferred to 75% ethanol after fieldwork. Other specimens (collected from Xinduqiao in 1973 and 1980, Liuba and Dongeluo in 1980, and Pengbuxi in 1984) were fixed and deposited in 10% formalin solution. Holotype, allotype, and 126 paratypes were deposited in the museum of the Chengdu Institute of Biology (CIB), Chinese Academy of Sciences (CAS), with another five paratypes (KIZ 014127–31) deposited in the museum of the Kunming Institue of Zoolgy (KIZ), CAS.
Morphological analysis
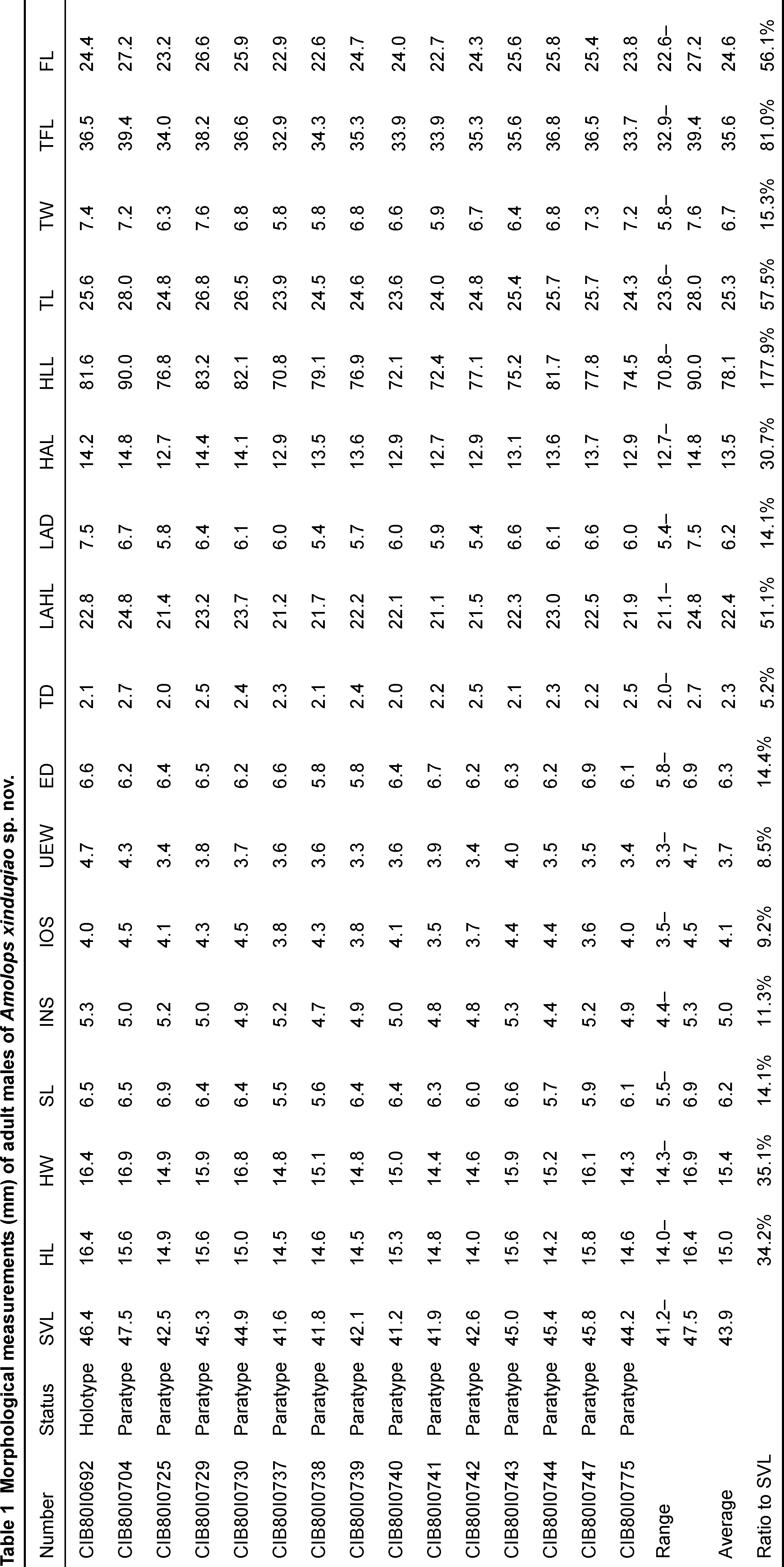
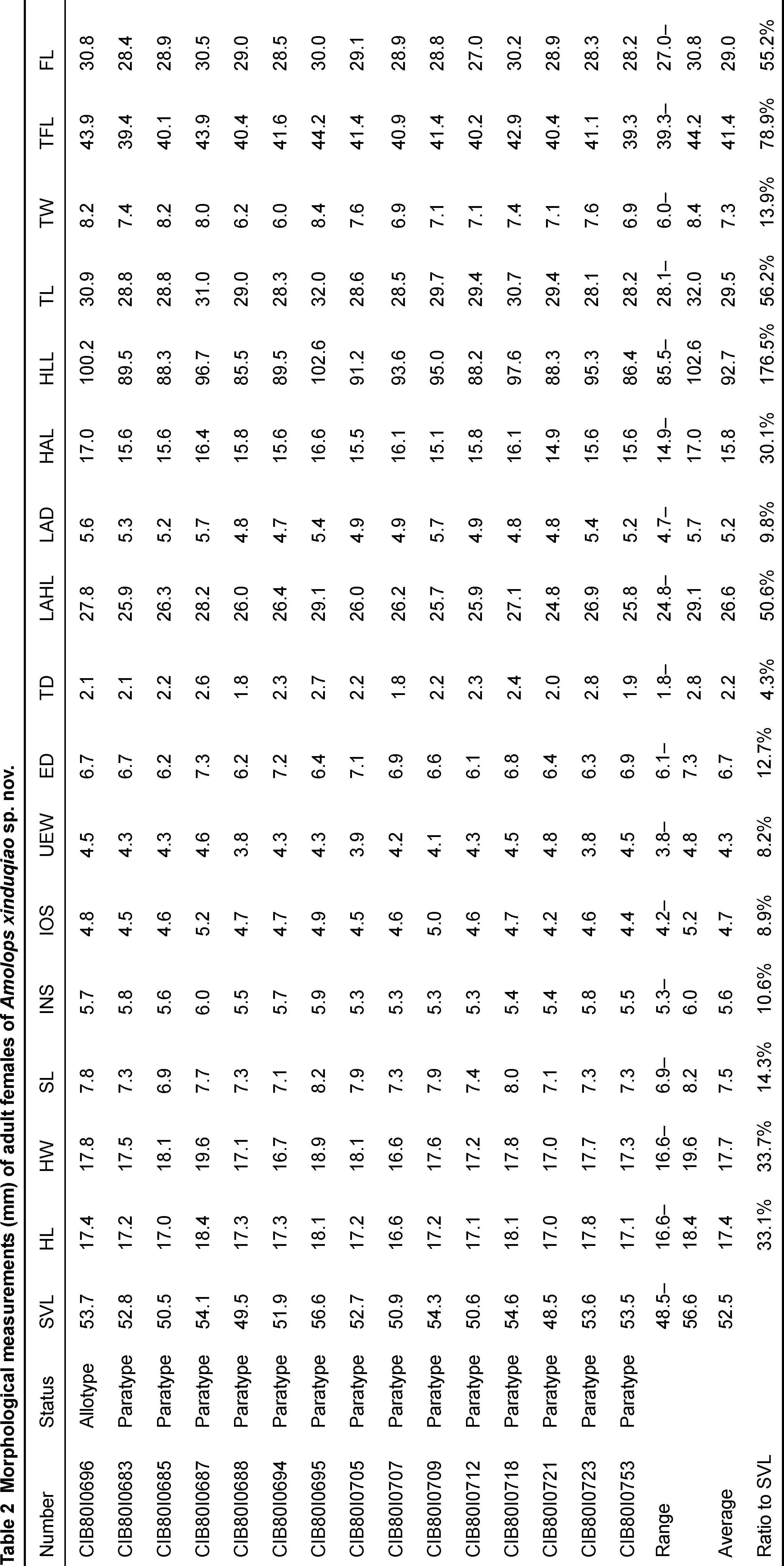
Fifteen males and 15 females from Xinduqiao were measured. All measurements were carried out with slide calipers to the nearest 0.1 mm. Morphological characters used and their measurement methods followed Fei et al. (2009a) and Jiang et al. (2016). The morphological characters and their abbreviations are listed below: SVL, snout-vent length; HL, head length (from posterior corner of mandible to tip of snout); HW, head width (at the angle of the jaw); SL, snout length; INS, internarial space; IOS, interorbital space; UEW, width of upper eyelid; ED, eye diameter (horizontal); TD, tympanum diameter (horizontal); LAHL, length of lower arm and hand; LAD, diameter of lower arm; HAL, hand length; HLL, hindlimb length; TL, tibia length; TW, tibia width; TFL, length of tarsus and foot; FL, foot length.
Morphological data of congeners were obtained from previously published literature (Liu, 1950; Fei et al., 2009b).
RESULTS
According to Fei et al. (2005, 2009b), the morphological data support the Xinduqiao population as a new species of theAmolops mantzorumgroup based on the absence of a dorsolateral fold and the absence of a circummarginal groove on the disc of the first finger. The new species differs from all other species of theA. mantzorumgroup by having a relatively small body size; green or brown-colored dorsum, with relatively small spots, and without spines; and small, but distinct tympanum. Additionally, the molecular data support the differentiation between the high altitude (above 3 000 m) population of western Mt. Zheduo in the Yalong River Basin (including Xinduqiao) and the mid-high altitude (1 200–2 400 m) population of the Dadu River Basin (Lu et al., 2014; Zhang et al., 2015).
Amolops xinduqiaoFei, Ye, Wang, and Jiang, sp. nov. (Figures 1–4)
Staurois kangtingensis: Liu, 1950, Fieldiana: Zool. Mem., 2: 349–353.
Amolopskangtingensis: Wu, Tan, and Zhao, 1987, Acta Herpetol. Sinica, 6(4): 39–41; Lu, Bi, and Fu, 2014, Mol. Phylogenet. Evol., 73: 40–52; Zhang, Yuan, Xia, and Zeng, 2015, Sichuan J. Zool., 34(6): 801–809.
Holotype:CIB 80I0692 (Figure 1 A, B), adult male from Xinduqiao (新都橋, N30.14182°, E101.50044°, altitude 3 400 m), Kangding, Sichuan, PR China, collected by Yongzhao Huang on 14 June 1980.
Allotype:CIB 80I0696 (Figure 1 C, D), adult female, same locality and date as holotype.

Figure 1 Holotype and allotype of Amolops xinduqiao sp. nov. (Photos by Liang Fei)
Paratypes:Xinduqiao (same locality as holotype): 46♂♂, CIB 73I1151–55, 73I1167–69, 73I1173 (11 September 1973), 80I0689, 80I0704, 80I0725–26, 80I0729–30, 80I0736, 80I0738–45, 80I0747, 80I0750–55, 80I0757, 80I0760–61, 80I0765, 80I0767–73, 80I0775 (14 June 1980), 80A0217 (04 August 1980), KIZ 014127, 014130 (31 August 2016); 74♀♀, CIB73I1156–66, 73I1170–1172, 73I1174 (11 September 1973), 80I0683–88, 80I0690–91, 80I0693–95, 80I0697–0703, 80I0705–0724, 80I0727–28, 80I0731–35, 80I0737, 80I0746, 80I0748–49, 80I0759, 80I0762–64, 80I0766, 80I0774 (04 August 1980), 840735 (23 May 1984), KIZ 014128–29, 014131 (31 August 2016). Liuba (六巴), altitude 3 500 m: 1♂, 80I0516 (04 June 1980). Dongeluo (東俄洛), altitude 3 415 m: 2♂♂, 80I0798–99 (15 June 1980); 1♀, 80I0797 (15 June 1980). Pengbuxi (朋布西), altitude 3 300 m: 1♂, 841289 (31 May 1984); 6♀♀, 841290–93 (31 May 1984), 841424–25 (26 May 1984).
Diagnosis:Amolops xinduqiaosp. nov. is distinguished from all other congeners by the following combination of characters: (1) medium body size, adult males SVL 41.2–47.5 mm (n=15, average 43.9 mm), adult females SVL 48.5–56.6 mm (n=15, average 52.5 mm); (2) head length equal to width or slightly wider than long; (3) tympanum small, but distinct; (4) vomerine teeth in two tiny rows, separated by a space about one vomerine teeth row; (5) bony projections on lower jaw absent; (6) dorsolateral folds usually absent; (7) tarsal folds or glands on tarsus absent; (8) circummarginal groove on disc of finger I absent; (9) tibiotarsal articulation reaching nostril or beyond; (10) webs of toe IV reaching to distal articulation, other toes fully webbed to disc; and (11) vocal sac absent in males.
Holotype description:Medium body size, SVL 46.4 mm, slightly compressed in vertical direction. Head length equal to width; snout projecting forward and depressed, slightly pointed at tip; nostril lateral, at middle of snout and eye; canthus rostralis distinct, slightly constricted behind nostrils; loreal region concave and oblique; eye relatively large (ED/HL=0.40); interorbital space less than width of upper eyelid (IOS/UEW=0.85); tympanum small, but distinct, slightly less than one third of eye diameter (TD/ED=0.32); vomerine teeth weakly developed, in two tiny oblique rows between choanae, separated by a space about one vomerine teeth row; tongue pyriform, deeply notched posteriorly; bony projections on lower jaw absent; and vocal sac and vocal sac opening absent.
Forearm robust. Tips of all four fingers expended into discs, disc on finger I smallest, on finger III largest, approximately equal to diameter of tympanum; circummarginal grooves present on tips of outer three fingers, absent on finger I; relative finger length I Hindlimb slender, tibiotarsal articulation reaching nostril, heels overlapping when hind limbs flexed and held perpendicular to body. All five toe tips expanded into discs, relatively smaller than finger discs; relative toe length I Dorsal side smooth, with sparse tubercles on lateral side of head and body, and around vent; supratympanic fold absent; dorsolateral fold absent; ventral surfaces smooth except lightly flat tubercles on basal ventral surface of thigh. Coloration of holotype in preservative:Dorsal and lateral sides of head and body gray-brown, with indistinct gray spots; limbs gray-brown, with indistinct gray transverse bands. Ventral sides uniformly light brown, indistinct gray spots on throat and chest. Second sexual characters:Male without vocal sac or vocal sac opening; inner side of first finger with developed velvety nuptial pad, without spine; forearm of male stronger than forearm of female, and snout-vent length of male smaller than snout-vent length of female. Variation:The type series measurements are summarized in Table 1–2. Coloration of paratype in life (KIZ 014127, adult male) (Figure 2; Figure 4A, B):Dorsal head and body brown, with irregular small green spots; lateral head black, edge of upper lip black, white stripe from tip of snout to anterior joint of shoulder; upper part of lateral body green, with indistinct black spots, lower part of lateral body white, with black spots and large blotches; dorsal side of limbs light brown, with black transverse bands. Ventral head and body cream-white, irregular dark grayspots on throat, chest, and sides of belly; ventral side of limbs flesh-colored, without spots; toe webs gray with yellow; iris light brown, with small black spots. Figure 2 Adult male Amolops xinduqiao sp. nov. (paratype, KIZ014127) (Photos by Yufan Wang) Coloration of paratype in life (KIZ 014129, adult female) (Figure 3; Figure 4C, D):Dorsal head and body green, with reticulate black-brown spots; lateral head green, with black spots, edge of upper lip black, white stripe from tip of snout to anterior of shoulder; upper part of lateral body green, lower part of lateral body white, both with large black blotches; dorsal side of limbs green-yellow, with distinct black transverse bands and spots. Ventral head and body cream-white, irregular gray spots on throat and chest; ventral side of limbs flesh-colored, without spots; toe webs gray with yellow; iris yellow-gray, with small black spots. Figure 3 Adult female Amolops xinduqiao sp. nov. (paratype, KIZ014129) (Photos by Yufan Wang) Figure 4 Ventral view of hand and foot of Amolops xinduqiao sp. nov. Habitat:This frog lives at an altitude of 3 300 to 3 500 m, close to slow-moving mountain rivers or large streams in habitats with a few tall trees and with dense shrubs and weeds. Adult frogs often perch on rocks for foraging at night, but hide under rocks and grass during the day. Male and female frogs were collected during amplexus in a river near Xinduqiao on 11 September 1973, with dissection of the female specimens showing eggs with a diameter of 2.5 mm in the fallopian tube about to be spawned. Dissection of a female specimen collected on 04 June 1980, showed the biggest eggs in the ovaries to be 1.5 mm in diameter, and not yet mature. It is reasoned that the breeding season of this frog might be around September. However, breeding habitats and egg masses have yet to be confirmed. Comparison:According to Fei et al. (2005, 2009b, 2012), five species, i.e.,A. granulosus(Liu & Hu, 1961),A. lifanensis(Liu, 1945),A. loloensis(Liu, 1950),A. mantzorum(David, 1871), andA. viridimaculatus(Jiang, 1983), exist in theA. mantzorumspecies group, which is characterized by the absence of a dorsolateral fold and circummarginal groove at the first finger. Amolops xinduqiaosp. nov. differs fromA. granulosusby having a smooth dorsal surface, without spines, and no vocal sac (v.s.spines on dorsal surface and a pair of internal subgular vocal sacs) (Fei et al., 2009b). Amolops xinduqiaosp. nov. differs fromA. lifanensis,A. loloensis,A. mantzorum, andA. viridimaculatusby having a smaller body size, males SVL 41.2–47.5 mm (n=15), females SVL 48.5–56.6 mm (n=15) (v.s.males SVL 52.0–56.0 mm (n=5), females SVL 61.0–79.0 mm (n=10) inA. lifanensis; males SVL 54.5–62.0 mm (n=19), females SVL 69.5–77.5 mm (n=20) inA.loloensis; males SVL 49.0–57.0 mm (n=10), females SVL 59.0–72.0 mm (n=10) inA. mantzorum; males SVL 72.7–82.3 mm (n=19), females SVL 83.0–94.3 mm (n=10) inA. viridimaculatus) (Fei et al., 2009b). Amolops xinduqiaosp. nov. further differs fromA. lifanensisby having a distinct tympanum (v.s. tympanum indistinct); differs fromA. loloensisandA. viridimaculatusby having no large brown or green spots on dorsum (v.s. large brown spots on dorsum inA. loloensis, large green spots on dorsum inA. viridimaculatus); differs fromA. mantzorumby different dorsal coloration and pattern, dorsum brown, with irregular green spots, or dorsum green, with reticulate black-brown spots (v.s.dorsum brown, with a few large green blotches (Figure 5)) (Fei et al., 2009b). Figure 5 Topotype of Amolops mantzorum, Baoxing, Sichuan (Photos by Liang Fei) Etymology:The specific name “xinduqiao” is named after the type locality Xinduqiao of Kangding, Sichuan. According to the Latin name, we suggest the English common name as“Xinduqiao torrent frog or Xinduqiao cascade frog”, and the Chinese common name as “新都橋湍蛙”. Amolops kangtingensiswas described by Liu (1950). Based on the original designation, the holotype (Figure 6) is a female from“Kangting (=Kangding), Sikang (now part of Sichuan), 8 000 feet (~2 400 m)”, with catalogue number 49412 in the Chicago Natural History Museum (now Field Museum of Natural History), and corresponding to field number 582 (from the collection database of the Field Museum). The holotype (No. 582) measurements were provided in the measurement table on page 351 (Liu, 1950), with body length 74.0 mm. Liu (1950) designated 29 paratypes, with the localities and altitudes provided, including: (1) 16 males, five females, and two young individuals collected with the holotype from Yalakou (=Yalagou) inside Kangting City (with three males and four females measured); four specimens from Tatu River (=Dadu River, altitude 4 500 feet (~1 370 m)) of Luting (=Luding), with the 27 frogs representingA. kangtingensis(=A. mantzorum); and (2) two paratypes from Chuwo (=Zhuwo, altitude 11 000 feet (~3 350 m)) of Luhohsien (=Luhuo) and Hsintuchiao (=Xinduqiao, altitude 11 200 feet (~3 410 m)) representingA. xinduqiaosp. nov. Figure 6 Holotype of Amolops kangtingensis (No. 49412, female) (Provided by Alan Resetar, the Field Museum, USA) According to the molecular data provided by Lu et al. (2014) and Zhang et al. (2015), the high altitude (above 3 000 m) populations of western Mt. Zheduo in the Yalong River Basin (including localities Jiagenba, Pengbuxi, Xinduqiao of Kangding, as well as Luhuo and Yajiang) form a monophyletic group, which should be identified asA. xinduqiaosp. nov. The populations of western Mt. Zheduo differ from the mid-high altitude (1200–2400 m) populations of the Dadu River Basin (including localities Kangding City (county town), Yalagou of Kangding, and Luding), which should be identified asA. mantzorum. Based on the original designation, the holotype ofA. kangtingensiswas collected from Yalagou of Kangding City at an altitude of ~2 400 m, and should be identified asA.mantzorumaccording to Lu et al. (2014) and Zhang et al. (2015). The large body size (female SVL 74.0 mm) of the holotype ofA. kangtingensisalso differs fromA. xinduqiaosp. nov. (females SVL up to 56.6 mm). Additionally, the measurements of the paratypes ofA. kangtingensis(males SVL 53.0–57.0 mm, females SVL 70.0–74.0 mm) provided by Liu (1950) are similar to those of the topotypes ofA. mantzorum(males SVL 49.0–57.0 mm, females SVL 59.0–72.0 mm (Fei et al., 2009b)), but different fromA. xinduqiaosp. nov. Thus, as above, the type locality ofA.kangtingensisis limited to Yalagou of Kangding, andA.kangtingensisshould be synonymized withA. mantzorum. We are grateful to Dr. Jing Che (KIZ) for her kind support in this study. We thank Mr. Qi Liu for fieldwork in Xinduqiao, Prof. Yuezhao Wang, Prof. Yueying Chen, and Mr. Ke Lu (CIB) for their help and permission to examine the related museum specimens, and the Field Museum (USA) for supplying photos of holotype ofAmolops kangtingensis. We also thank the reviewers for their critical reading of this manuscript. Cai HX, Che J, Pang JF, Zhao EM, Zhang YP. 2007. Paraphyly of ChineseAmolops(Anura, Ranidae) and phylogenetic position of the rare Chinese frog,Amolops tormotus.Zootaxa, 1531: 49–55. Dubois A. 1992. Notes sur la classification des Ranidae (Amphibiens Anoures).Bulletin Mensuel de la Société Linnéenne de Lyon, 61 (10): 305–352. Fei L, Ye CY, Huang YZ. 2005. An Illustrated Key to Chinese Amphibians. Chengdu: Sichuan Publishing House of Science and Technology, 1-340. (in Chinese) Fei L, Hu SQ, Ye CY, Huang YZ. 2009a. Fauna Sinica, Amphibia, Vol. 2: Anura. Beijing: Science Press, 1-959. (in Chinese) Fei L, Hu SQ, Ye CY, Huang YZ. 2009b. Fauna Sinica, Amphibia, Vol. 3 Anura Ranidae. Beijing: Science Press, 959-1847. (in Chinese) Fei L, Ye CY, Jiang JP. 2012. Colored Atlas of Chinese Amphibians and Their Distributions. Chengdu: Sichuan Publishing House of Science and Technology, 1-619. (in Chinese) Frost DR. 2017. Amphibian Species of the World: an Online Reference. Version 6.0. [DB/OL]. [2017-03-10]. http://research.amnh.org/herpetology/ amphibia/index.html. Jiang K, Wang K, Yan F, Xie J, Zou DH, Liu WL, Jiang JP, Li C, Che J. 2016. A new species of the genusAmolops(Amphibia: Ranidae) from southeastern Tibet, China.Zoological Research, 37(1): 31-40. Liu CC. 1950. Amphibians of Western China. Fieldiana, Zoology Memoirs, 2: 1-400. Liu CJ, Hu SQ. 1961. Tailless Amphibians of China. Beijing: Science Press, 1-364. (in Chinese) Lu B, Bi K, Fu JZ. 2014. A phylogeographic evaluation of theAmolops mantzorumspecies group: Cryptic species and plateau uplift.Molecular Phylogenetics and Evolution,73: 40-52. Stuart BL. 2008. The phylogenetic problem ofHuia(Amphibia: Ranidae).Molecular Phylogenetics and Evolution,46(1): 49-60. Wu GF, Tan AM, Zhao EM. 1987. Cytoological evidence for the validity ofAmolops kangtingensis.Acta Herpetologica Sinica,6(1): 39-41. (in Chinese) Zhang CH, Yuan SQ, Xia Y, Zeng XM. 2015. Species delimitation ofAmolops kangtingensis.Sichuan Journal of Zoology,34(6): 801-809. (in Chinese) Zhao EM, Adler K. 1993. Herpetology of China. Oxford: Society for the Study of Amphibians & Reptiles, 1-522. 05 March 2017; Accepted: 10 May 2017 s: This study was supported by the Animal Branch of the Germplasm Bank of Wild Species of the Chinese Academy of Sciences (Large Research Infrastructure Funding) *Corresponding author, E-mail: jiangke87615@hotmail.com 10.24272/j.issn.2095-8137.2017.022



DISCUSSION

ACKNOWLEDGEMENTS
REFERENCES

