Introduction to Technical Safety Standards for Cosmetics
Zhao Hua, Yin Yuexuan
Department of Cosmetics, Beijing Technology and Business University, China
Introduction
Technical Safety Standards for Cosmetics (TSSC)came into full effect on 1 December 2016, prohibiting the manufacture and import of any cosmetics that do not comply with the TSSC 2015 regulation.
In order to meet requirement of cosmetics safety supervision, and by following of industry development and improvement of scientific knowledge,[1]China Food and Drug Administration (CFDA) organized the revision work of Hygienic Standard for Cosmetics, and generate the Technical Safety Standard for Cosmetics(2015 Version). On 23 December 2015, CFDA released Notification(No. 268,2015)that Technical Safety Standards for Cosmetics (Version 2015, TSSC) was published upon review of experts from Cosmetics Standard Committee and would come into force since 1 December 2016.The Notification requires prohibiting the production or importation of cosmetics that do not comply with provisions in TSSC, but the related products can be sold until the end of its expiration date. Regarding the change in application conditions and precautions and other related requirements that must be marked on the label,the original product packaging can be used till 30 June 2017, and the related products can be sold till the end of its expiration date.
TSSC provides comprehensive information on the technical standards applied to cosmetics regulation in China. The regulations supply guidance on general safety standards; definitions relating to prohibited,restricted and permitted ingredients; and setting testing methods for the manufacture and operation of cosmetics products in China. The implementation of TSSC can not only direct the new formulation design and new product development,[2]but also create a good industry atmosphere, promote the healthy development of the industry so that China’s cosmetic regulations will be in line with international standards,[3]which play an important guiding role on the development of China’s cosmetics industry.
Evolution of Hygienic Standard for Cosmetics (HSC)
Cosmetic regulations are gradually developing and improving along with the growth of the cosmetic industry. In 1999, the Ministry of Health issued the Hygienic Standard for Cosmetics (HSC), which was compiled based on Cosmetics Directive of the Council European Communities, 76/768/EEC and amendments until 21 November 2005.[4]HSC has played a very important role in cosmetics safety and in promoting the development of the cosmetic industry. With the development of the situation and the improvement in scientific understanding, HSC (Version 2007) cannot fully meet the current needs of the development of the cosmetic industry in some aspects , and need to be revised or supplemented, for example, the expression of the concept,terminology and definition needs to be further improved;part of the detection and evaluation methods may be lagged or missed, and the technical requirements for products and raw materials is too simple or not clear and so on.
HSC was revised again based on the latest global cosmetics safety and supervision standards, which indicates the constant improvement and gradual maturation of China’s cosmetics legalization management system. The new contents include the adjustment of safety risk substances, updated list and testing methods of prohibited and restricted substances.
Main changes
Changes in name and style
The name of the Regulation is changed from HSC(Version 2007) to HSSC (Version 2015), which indicates that objectives and requirements of the administrative departments of cosmetics are more focused on the protection of product safety while highlighting its positioning as a technical standard.
HSC (Version 2007) contains 5 chapters, namely,general principles, toxicology test methods, hygiene safety test methods, microbiological test methods, human safety and efficacy evaluation methods. TSSC contains 8 chapters. The 1st chapter is summary, including scope,terms and definition, general requirements on the safety. The 2nd chapter is the requirement on cosmetics prohibited ingredient list and restricted ingredient list,including 1,388 cosmetics prohibited substances and 47 restricted substances. The 3rd chapter is positive ingredient list, including requirement on 51 preservatives,27 UV filters, 157 colorants and 75 hair dyes. The 4th chapter is physical-chemical testing methods, including 77 testing methods. The 5th chapter is microbiological test methods, including 5 testing methods. The 6th chapter is toxicological test methods, including 16 testing methods. The 7th chapter is human safety test method,including 2 testing methods. The 8th chapter is efficacy evaluation in human, including 3 testing methods.
This version of standard mainly revised following contents based on HSC:
Summary
The Chapter one (summary) of TSSC describes the scope, terminologies, definitions and general requirements of cosmetic safety technology. For example,it clearly explains raw materials (raw materials, new raw materials, prohibited substances, restricted substances and permitted substances), cosmetic products (rinse-off/leave-on products, eye/lips/body/skin products, cosmetic products for children, professional use), packaging materials and substances with safety risk and other terms.The general requirements on the safety of cosmetic include general requirements, requirements on formula,requirements on microbial indexes, and requirements on limit of hazardous substances, requirements on packaging materials, requirements on labeling, requirements on cosmetic products for children, and requirements on raw materials. in which, the name of “fecal coliform”was modified as “thermotolerant coliform bacteria”, and the types and limited requirements of heavy metals and hazardous substances in cosmetics were modified (Table 1).
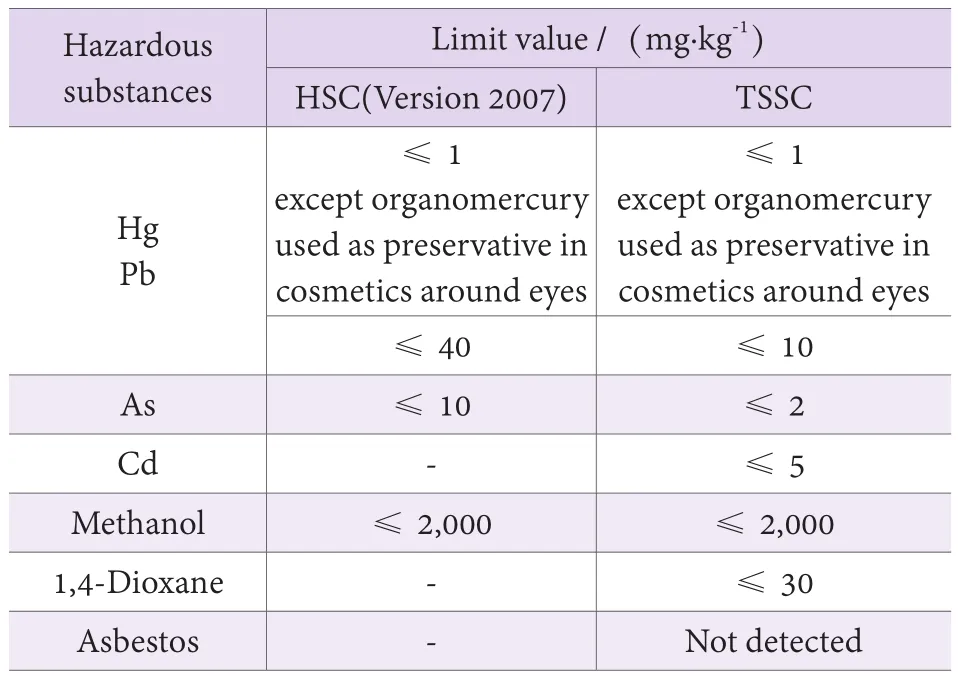
Table1. Limit level of hazardous substances in the cosmetic products
Prohibited substances
The most significant change is the revision of ingredient lists, which requires companies to apply for corresponding modifications or make adjustments regarding their products before 1 December 2016.
Prohibited substances refer to the substances that cannot be used as the raw material of cosmetics product.There are totally 1,388 prohibited substances, within them 133 are supplemented items and 137 are revised items. Some substances such as diethylene glycol, vitamin K-1 and so on are newly added, and more restricted or permitted ingredients are added to the list of prohibited substances due to health concerns. There are totally 47 restricted substances, within them 1 is supplemented item, 31 are revised items, and 27 are deleted items. The restricted substance, cantharidin, originally used in hair growing cosmetics, was listed by EU as a prohibited substance because of its ability to cause immune function decline, redness and foaming on skin and strong irritation.[5,6]So is 4-aminoazobenzene and benzidine.
Prohibited substances include, but not limited to,those prohibited substances in the ingredient table.The listed substances that exist in cosmetic products unintentionally, maybe come from impurities in natural or synthetic raw materials, or from packaging materials,or from the production or storage of the product. Under the production conditions consistent with national mandatory regulations, if the presence of the prohibited substances is technically unavoidable, the general requirements for cosmetic safety should be met, that is to say, the finished product must meet the requirements for not causing harm to the human body under normal,reasonable and foreseeable application conditions.
Restricted substances
Restricted substances are substances that can be used as the raw materials of cosmetic products under the restricted conditions and are prohibited if they exceed this limit. Restricted substances are one of the important cosmetic substances, for instance, α-hydroxy acids and their salts and esters, tri-alkylamine, trialkanolamine and their salts, talc: magnesium silicate hydrate, potassium hydroxide (or sodium hydroxide) are common restricted substances. The restricted substances in TSSC (version 2015) are adjusted from the original 73 categories to 47 categories.[7]
For safety, Cantharidin and trichlorocarban are deleted from restricted substances list, Laureth-9 is added.For the sake of regulatory, some substances in oral hygiene products, toothpaste, soap and artificial nail,including 6-methyl coumarin, benzoyl peroxide and hydroquinone are deleted. Unlike HSC (2007 edition), for the substances that not only can be used as preservatives,but also as restricted ingredients like alkyl (C12-C22)trimethylammonium chloride, benzalkonium chloride,benzalkonium bromide, benzalkonium acetate, benzene formic acid and its sodium salt , phenoxy isopropanol ,salicylic acid , pyrithio zinc , inorganic sulfite and bisulfite,formaldehyde and benzyl alcohol etc. If it is not used as a preservative, the raw material and its function shall be marked on the product label.[8]The inorganic sulfite and bisulfite are defined as sodium sulfite, potassium sulfite, ammonium sulfite, sodium bisulfite, potassium bisulfite, ammonium bisulfite, sodium metabisulfite and pyrosulfite; if salicylic acid is used in the formula, with the product possibly used by children under 3 years of age, and cames in contact with skin, the label must stated“contains salicylic acid; should not be used for children under the age of 3”.
Permitted substances
Preservatives agentsare substances that are added to the cosmetic product for the purpose of inhibiting the growth of microorganisms in cosmetic products.Preservative is an important ingredient of cosmetics.There are totally 51 preservative, within them 14 are revised items and 5 are deleted items. The substances such as chloroacetamide, methyldibromo glutaronitrile,quaternium-15 and sodium iodate, are deleted and prohibited, and the ingredient (urotropine) is also deleted.The other changes are as follows:
4-hydroxybenzoic acid and its salts and esters, commonly named parabens, hydroxyphenyl esters, are a class of widely used preservatives in cosmetics. However,isopropyl 4-hydroxybenzoate (isopropylparaben) and its salts, isobutyl 4-hydroxybenzoate (phenylparaben)and its salts etc. are prohibited used in cosmetics.[9,10]Methylparaben and ethylparaben are currently authorised in ready for use cosmetic preparations at a maximum use concentration of 0.4% (as acid) for a single and 0.8%(as acid) for a mixture of parabens, respectively. The use of propylparaben and butylparaben in such cosmetic products is considered as safe to the consumer, as long as the sum of their individual concentrations does not exceed 0.14% (as acid).[11,12]
Formaldehyde is an effective and inexpensive biocidal preservative that has long been used in the preparation of the cosmetic products. Formaldehyde and formaldehyde-releasing preservatives (FRPs) slowly release small amounts of free formaldehyde over time, and kills microorganisms to protect the cosmetics damage caused by microorganisms. Imidazolidinyl urea, imidazolidinyl urea, DMDM hydantoin, sodium hydroxymethylglycine,formaldehyde and paraformaldehyde and 2-bromo-2 Nitro-1,3-propanediol belong to this kind of preservatives.TSSC stipulates all finished products containing formaldehyde must be labeled with the warning “contains formaldehyde” where the concentration of formaldehyde in the finished product exceeds 0.05%, and shall not be used in spray product.[13,14]
Compared with imported products, iodopropynyl butylcarbamate (IPBC) is widely used as a preservative in domestic cosmetics.[15]TSSC has changed the use range and constraints of different products. It’s maximum concentration in leave-on products is 0.01% and shall not be used in products for children under 3 years of age, or any body cream/milk. According to the Standards, it shall not be used in the products are applied to a large areas of the body, but can be used in the products applied to body parts. In the event that the product (excluding bathroom products & shampoo) is possibly used by children under the age of 3 years, the label must stated “should not be used for children under the age of 3”.
Methyl chlorisothiazolinone(MCI) and methylisothiazolinone(MI) has been used in industrial and consumer products in a 3:1 ratio in a preservative system. MCI/MI is commonly used in cosmetics as preservatives. Skin exposure to high concentrations of MCI/MI can cause cantact dermatitis.[16~22]TSSC suggests that MCI/MI can be only used in rinse-off cosmetic products at a maximum concentration of 0.0015% of a mixture in the ratio3:1 MCI/MI of the two substances, and cannot be used together with MI.
TSSC requires that Containing salicylic acid cosmetics shall be added to the note of warning signs to determine the safety of long-term use and children under 3 years of age also shall not be used.
Sunscreens agentsare substances that are added to the cosmetic products to protect the skin from the harmful effects of certain UV rays or to product the product itself, by means of light absorption, reflection, or scattering. For instance,titanium dioxide, zinc oxide, ethylhexylmethoxycinnamate,butyl methoxydibenzoylmethane, etc. are commonly used in sunscreen products. There are totally 27 UV-filter items, within them 6 are revised items and 1 is deleted item. If the addition of sunscreen to other cosmetics is only to ensure the product stability and protect skin from UV damage, the use of sunscreens is not regulated by TSSC, but the amount of usage must undergo a rigorous evaluation of safety.[23~27]
Colorant agentsrefer to the substances that are added to the cosmetic product to develop a color in the cosmetic products or their application site, by means of absorption or reflection of the visible light. There are totally 157 colorants, within them 1 (galla chinensis extract) is supplemented item and 69 are revised items. Galla chinensis extract must be used in hair dye products with FeSO4.[28]
Hair dyes refer to the substances that are added to the cosmetic products to change the color of hair. There are totally 75 hair dyes, within them 63 are revised items and 21 are deleted items. TSSC requires that the following warning statements must appear on hair dye product label, “Hair color can cause an allergic reactions. Read and follow enclosed instructions on leaflet. Product not intended to be used on children keep out of reach of children. This product must not be used for dyeing the eyelashes or eyebrows. In case of contact with eyes, rinse immediately with plenty of water. Wear gloves when applying hair dye. Do not color if you have had a previous reaction or if scalp is irritated or injured”.[29]

Physical-chemical testing method
As for the testing methods, only the Chapter on physical-chemical testing methods is subject to big changes. The testing methods of strontium, total fluorine and anti-dandruff have been deleted and the testing methods of PH have been revised in addition to adding 60 other testing methods. Other chapters are almost the same. The standards still don’t mention alternatives to animal testing.
Human body efficacy evaluation test method
On 1 June 2016, CFDA issued a Notice concering the Labeling Requirements for the Efficacy of Sunscreen(No.107, 2016), revising the labeling requirements relating to SPF and PFA.
The most significant change to the new regulation is the permission of marking SPF > 50 and PA ++++ on labels. In the past, cosmetics can only be indicated by SPF > 30 and PA +++ even if the actual measured value is higher, which doesn’t align with the international standards.
The Notice requires that SPF should be based on the actual measured SPF value. In case, the actual SPF value of the product is lower than 2, the SPF value should not be labeled; when the actual SPF value ranges from 2 to 50(including 2 and 50, the same below), the actual SPF value should be labeled; in case the measured SPF value of the product is greater than 50, it should be labeled as SPF 50+.The Replies to Relevant Problems on Further Defining the Requirements of Cosmetic Labeling (Document CFDA MC No. 568, [2016] ) issued by CFDA on 15 August 2016,defines the labeling principle once again. In response the CFDA says that any SPF that is measured as being equal or greater than an SPF 2 rating, are subject to rules that include measuring SPFs 2-5 to indicate their actual measures value, and measuring SPF 6-50 according as an agreed calculation of the minimum and maximum tested measure values, the labeling upper limit is actual testing value, and the lower limit is the lower value between lower limit on 95% confidence interval of actual testing value and the maximum integer multiples of 5 which is lower than actual value. While SPFs exceed 50 and the lower limit on 95% confidence interval of actual testing value is higher than 50, the SPF value should be measured as SPF5+. While SPFs exceed 50 and the lower limit on 95%confidence interval of actual testing value is lower than or equivalent to 50, the labeling upper limit is “50+” , and the lower limit on the 95% confidence interval of actual testing value as the labeling lower limit.[30,31]
In case the sun protection cosmetic would like to claim waterproof, it shall meet following requirements:SPF value is reduced by less than 50% after shower in the waterproof capacity test, and both the SPF values before and after shower shall be signed on the label or only the SPF value after shower shall be indicated (only revealing SPF value before shower is not acceptable).
The Notice also requires that when the critical wavelength of sunscreen cosmetics is not less than 370 nm,broad-spectrum sunscreen can be claimed. The labeling of the long-wave ultraviolet (UVA) shall be based on the actual measured PFA value and the UVA protection grade“PA” shall be indicated on the product label. When PFA value is less than 2, the claiming of UVA protection is not allowed. When PFA value ranges from 2 to 3, it shall be labeled as “PA+”. When PFA value ranges from 4 to 7,labeling as “PA++” . When PFA value is between 8 and 15, it shall be labeled as “PA+++”. When PFA value is 16 or more than 16, it shall be labeled as “PA++++”.
Tests of SPF, waterproof performance, critical wavelength, PFA of sunscreen shall be conducted in terms of testing methods on TSSC (2015).
Other
Added and included over 60 newly-issued test methods for the relevant prohibited and restricted substances in cosmetics on the basis of the original test methods as stipulated in the HSC; systematically standardized,classified and composed the texts and styles of the test methods for easy reading and utilizing. Deleted the contents not within the range of standardized version management from HSC, such as the two test methods for strontium and total fluorine; corrected a few mistakes in HSC; performed language standardization and format adjustment to the microbiological and toxicological test methods; revised the test method for human safety and effect assessment, dividing it into the test method for human safety and test method for ergonomics assessment;added the preparation method for high SPF standards (P2 and P3) to the test method for ergonomics SPF evaluation.
The standards deletes all the contents relating to oral care products as the Regulations concerning Supervision and Administration over Cosmetics hasn’t been released yet, which regulates oral care products as cosmetics. After its release, the standards will add the contents. At that time the standard is likely to be overhauled to align with the regulations. Sources confirm that another version of the standards has been completed by National Institutes for Food and Drug Control (NIFDC) and includes a large number of significant changes.
Conclusion
Compared to the previous version, TSSC gives greater consideration to the safety of both products and ingredients and improves evaluation methods, aiming to be a more comprehensive and scientific technical reference which not only sets standards for all cosmetics placed on the Chinese market but also promotes the research and development of cosmetics. Combined with the cutting-edge technologies in global cosmetic development, TSSC have shown China’s cosmetic safety technology research results and maturing cosmetic laws and regulations in recent years. TSSC provides not only stronger protection for the cosmetics, safety, but also references for cosmetic employees.[32]The impact of the implementation of the new laws on the industry is huge and all-round, and has a great significance for each sector of the cosmetic industry, either the main body such as supervisors and regulators, production operators, consumers, or the cosmetic industry chain from raw material selection to product quality and safety control system, product technical requirements,inspection and testing, label identification, even operation. Under TSSC,the manufacture and import of any new cosmetics that fail to comply with its stipulations will be prohibited from entering the Chinese market. In addition to maintaining a scientific nature,advancement and normalization, this edition of TSSC focuses on the administration of the hazardous materials and approved components in cosmetics; it makes full use of the international cosmetics quality & safety control technology and experiences and comprehensively reflects the development of China’s current cosmetics industry and the improvement of China’s inspection and test techniques, thus playing an important role in advancing the scientific supervision of China’s cosmetics, promoting the healthy development of cosmetics industry and raising the authoritativeness and international influence of China’s technical standards of cosmetics.
[1] Wei Shaomin. Status & trends of Chinese cosmetic regulations.Detergent & Cosmetics 2009, 32 (9), 39-41.
[2] José M B. Commission Regulation (EU) No 358 /2014 of 9 April 2014 Amending Annexes II and V to Regulation (EC) No 1223 /2009 of the European Parliament and of the Council on Cosmetic Products, Brussels, Belgium. Official Journal of the European Union, 2014.
[3] Xing Shuxia; Su Ze; Zuo Tiantian; et al. The latest amendment of EU cosmetic regulations and its implications. Chinese Journal of Health Laboratory Technology 2015, 25 (9), 3214-3216.
[4] Qin Yuhui. Cosmetics safety and management regulations.Beijing: Chemical Publishing Industry Press.
[5] Wei Fangchao; Du Juan; Wei Ningning; et al. Present situation and application of cantharidin and its Derivatives. Progress in Modern Biomedicine 2012, 12(8), 1586.
[6] Regulation (EC) No 1223/2009 of the European Parliament and of the Council. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF.
[7] Yang Yanwei; Liu Siran. Investigation on use of restricted substances in cosmetics. Chinese Journal of Health Laboratory Technology 2013, 23 (5), 1298-1299.
[8] Mulholland J.W. The scientific committee on cosmetic products and non-food products intended for consumers opinion concerning. http://pureti.com/content/documents/Full-europeanopinion-approving-nano-TiO2.pdf.
[9] Yin Dawei; Liu Yuting; Liang Gangtao. Improved technology for synthesis of RSM methylparaben. China Condiment 2013,38 (6), 46-50.
[10] David C. Steinberg. Frequency of use of preservatives.Cosmetics & Toiletries 1997, 112 (4), 57-58.
[11] Li Chengbei; Si Xinsheng. Toxicity analysis of components related to preservatives and daily chemical products. Detergent &Cosmetics 2014, 37 (8), 34-40.
[12] Yang Yanwei; Liu Siran; Luo Song; et al. Investigation on use of preservatives in cosmetics. Journal of Environmental Hygiene 2012, 4 (2), 56-58.
[13] Wu Qingmei. Isothiazolinone preservatives -- a cosmetic dermatitis sustained etiology. Foreign Medical (Hygiene Volume) 1990, (3), 191-192.
[14] Jian Longhai; Wen Hongliang. Simultaneous determination of methyl isothiazolinone and CMIT in cosmetics by LC/MS.Chinese Journal of Health Laboratory Technology 2011, 21 (11),2652-2653.
[15] Zhao Yue; Li Qiong. Determination method of iodopropynyl butylcarbamate in preservatives. Journal of Shanghai Institute of Technology (natural science edition) 2011, 3 (1), 18-20.
[16] Sun Jilong; Li Chuanmao; Xiang Qiongbiao. Status and trends of preservatives used in cosmetics. Guangdong Chemical Industry 2015, 42 (4), 57-58.
[17] Si Xinsheng;Yang Junwei. Skin irritation test and mechanism research of fungicidal preservative—isothiazolinones for detergents and cosmetics. Applied Chemical Industry 2011,(10), 1789 -1791.
[18] Zhang Lianxia; Zhu Xiaomin. Cosmetic substances causing skin allergic. Journal of Clinical and Experimental Medicine 2008, (9),165-166.
[19] Zhang Kedong; Li Huiyong. Determination of nine p-aminobenzoic acids and their esters sunscreens by HPLC.Chinese Journal of Analysis Laboratory 2014, 33 (9),1108-1112.
[20] Yu Shujuan; ZhengYubin; Du Jie; et al. A review of the development of sunscreen. China Surfactant Detergent &Cosmetics 2005, 35 (4), 248-251.
[21] Gao Lixue; Wei Qiang. Use frequency analysis of sunscreen in cosmetics. Modern Preventive Medicine 2011, 28 (7), 1324-1326.
[22] Zhao Yue; Li Qiong; Wu Xiaojian; et al. Development of sunscreen in China. China Detergent & Cosmetics 2010, 33(12), 14-17, 31.
[23] Harvey P.W.; Evetett D.J. Parabens detection in different zones of the human breast: consideration of source and implications of findings. J Appl Toxicol 2012, 32 (5), 305-309.
[24] Feng Shiqing; Zhao Hua. Trend of cosmetic preservatives viewing from use frequency. China Detergent & Cosmetics 2006, 29 (11), 30-33.
[25] Luo Meifen; Wu Zhijiang; Tan Jinping. Determination of methyl p-hydroxybenzoate and propyl p-hydroxybenzoate in talcum powder by HPLC. Journal of Shunde Polytechnic 2013,11 (3), 13-15.
[26] Zhen Jinqi; Han Jiayi; Li Huiling. Determination of p - hydroxybenzoate sodium and related substances by HPLC. Chinese Journal of Modern Applied Pharmacy 2012, 29 (1), 73-76.
[27] CAIQ Institute of Chemicals Safety. Studies showing p-hydroxy benzoic acid ester and triclosan may affect fetal growth. Asian Journal of Ecotoxicology 2014, 9 (5),1003.
[28] Yang Yanwei; Zhu Ying; Liu Siran; et al. Investigation on use of colorants in cosmetics. Journal of Environment and Health 2012, 29 (2), 170-172.
[29] Zhu Huijuan; Zhu Ying. Progress in research on safety of hair hye and its detection method. Chinese Journal of Health Laboratory Technology 2006, 7 (16), 888-890.
[30] CFDA. Notice cocerning Labeling Requirements for the Efficacy of Sunscreen (No.107,2016). http://www.sda.gov.cn/WS01/CL0087/154562.html.
[31] Mu Min;, Liu Yang; Liu Hua; et al. A brief introduction to new EU regulations of cosmetics and comparison of cosmetics supervision between China and EU. Flavor Fragrance Cosmetics 2010, 10 (5), 39-42.
[32] Singer H.;Muller S.; Tixier C.; et al. Triclosan: occurrence and fate of a widely used biocide in aquatic environment:field measurements in waste water treatment plants,surface waters, and lake sediments. Environ Sci Technol 2002, 36 (23), 4998-5004.
 China Detergent & Cosmetics2017年1期
China Detergent & Cosmetics2017年1期
- China Detergent & Cosmetics的其它文章
- Are you ready to join in the ITP 2017 ?
- Environmentally Safe Surfactants in Cosmetic/Detergent Formulations:Risk of Microbial Contamination and Possible Solutions
- Regulatory Status of Wet Wipes Used on Human Body in Europe,United States, Canada, Australia and China
- China National Standard—Test Method for Biodegradability of Surfactants (GB/T I5818—2006)
- For Beauty — JALA Group are Here to You
- Development of Chinese Kids Toothpaste Market
