Repurposing cancer drugs to treat neurological diseases – Src inhibitors as examples
PERSPECTIVE
Repurposing cancer drugs to treat neurological diseases – Src inhibitors as examples
Aberrant cell cycle diseases:e cell cycle is an irreversible, ordered set of events that normally leads to cellular division, consisting of quiescent state (G0), the first gap phase (G1), DNA synthetic phase (S), the second gap phase (G2) and mitosis phase (M). Aer the cell has split into its two daughter cells, the new cells enter either G1 or G0. Aberrant cell cycle is one of the hallmarks of many tumor cells in cancers, and also observed in postmortem and/or animal studies of dying neurons in a series of neurological diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), traumatic brain injury (TBI), intracerebral hemorrhage (ICH), epilepsy, cerebral hypoxia-ischemia, and others (Liu and Ander, 2012). Cancer cells keep dividing with uncontrolled cell cycle. By contrast, mature neurons can re-enter cell cycle, but they cannot go through the whole cell cycle processes to regain the G0 state. Since the transitions through the mitotic cell cycle are irreversible processes, the mature neurons that re-enter the cell cycle cannot revert to an earlier G0 either.is presents a critical situation to the neurons from which death may be the only outcome for the mature neurons attempting to complete the cell cycle. Based on these facts, we came up with the new concept of “aberrant cell cycle diseases” that reveals the two different types of diseases - cancers and neurological diseases - are sharing the same mechanism: aberrant cell cycle re-entry (Figure 1) (Liu and Ander, 2012).e similarity of aberrant cell cycle re-entry in cancers and neurological diseases provides a theoretical framework for repurposing cancer drugs to treat neurological diseases. Some cancer drugs that kill tumor cells due to DNA damage or other mechanisms, other than cell cycle inhibition, are beyond the scope of this perspective.
Drug repurposing:Developing a brand-new drug from a promising molecule to market currently takes 12—16 years and costs $2—3 billion on average (Nosengo, 2016). Such enormous amount of time, money and efforts, are mainly due to bottlenecks in the therapeutic development process, during which more than 90% of drugs fail. It is likely an important strategy that repurposing the drugs that have been approved to treat one disease for treating other diseases, as repurposing a drug reduces the time frame to around 6.5 years, and decreases costs to $300 million on average (Nosengo, 2016). A significant advantage of drug repurposing over traditional drug development is that since the repurposed drugs have already passed a significant number of toxicity and other tests in humans, detailed information is available on their safety and the risk of failure for reasons of adverse toxicology are reduced. Repurposing usually builds upon previous research and development efforts; therefore, new candidate therapies could be ready for clinical trials quickly, speeding their review by the FDA and, if approved, their integration into health care.
Cancer drugs that inhibit cell cycle:Under specific environmental and/or pathological conditions, such as exposure of tobacco smoke, benzene, ultraviolet B radiation, mutation of oncogenes/tumor suppressor, and/or enhancement of mitogenic molecules, (e.g., thrombin, integrins, growth factors, cytokines, reactive oxygen species (ROS), and others), abnormal cell cycle re-entry may arise and thus trigger tumorigenesis of normal cells. Although most cancers have multivariate causes, the various causes seem to result in a common outcome - cell cycle re-entry, mediated by mitogenic pathways, such as Src/Ras/Raf/MEK1, 2/ERK1, 2 (Figure 1) (Liu and Ander, 2012). Apart from the core cell cycle molecules- cyclins and cyclin-dependent kinases (CDKs), strategies of interfering with these mitogenic molecules and the signaling pathways have been tested to treat cancers (Liu and Ander, 2012). Although many cancer drugs are able to cause cell cycle arrest, Src inhibitors are focused in this perspective.
Src, Src family members, cell type specificity, structure and activity:Role of the first identified viral oncogene v-Src in oncogenesis led to the discovery of a family of nonreceptor tyrosine kinases-Src family kinases that include at least nine family members: c-Src (the cellular counterpart of v-Src), Fyn, Hck, Lck, Lyn, Blk, Fgr, Yes and Yrk (Parsons and Parsons, 2004; Salter and Kalia, 2004).e expression of Src family members are tissue and cell type specific. Although c-Src, Fyn, Yes and Yrk are expressed ubiquitously in all cell types, others (e.g., Hck, Lck, Lyn, Blk and Fgr) are generally found in brain and hematopoietic cells (Parsons and Parsons, 2004; Salter and Kalia, 2004). Note that brain and platelets express 5—200 fold higher levels of Src kinases than most other cells (Brown and Cooper, 1996). In addition, different Src kinases are often found to compensate for one another, such as c-Src and Fyn (Stein et al., 1994). Structurally, Src family members share a conserved domain structure consisting of consecutive SH3 (polyproline type II helix for protein-protein interaction), SH2 (phosphotyrosine recognition), and SH1 (tyrosine kinase catalytic activity) (Boggon and Eck, 2004).ey also contain a membrane-targeting region at their N-terminus that is followed by a unique domain of 50—70 residues, which is divergent among family members (Boggon and Eck, 2004). Although it is still incompletely clear, Src activity is regulated by tyrosine phosphorylation at two sites (one is at Tyr416 in the SH1 domain, the other at Tyr527 in the short C-terminal tail), but with opposing effects. Tyr416 phosphorylation activates Src, whereas Tyr527 phosphorylation results in inactivation (Boggon and Eck, 2004).
Src up-stream activators, down-stream effectors, and self-initiated inhibitors:Src can be activated by many trans-membrane receptors, such as adhesion receptors, tyrosine kinase receptors, G protein-coupled receptors, cytokine receptors, and others (Tatosyan and Mizenina, 2000).is unique feature of Src makes them a point of convergence that receives initiative signaling from a large number of mitogenic molecules, such as thrombin, integrins, LPS, ROS, and others (Figure 1). Src activation stimulates multiple downstream effectors, including JNK, P38 and Erk mitogen-activated protein kinases (MAPKs), and others (Figure 1). Src can initiate negative feedback to prevent their sustained activationviarecruitment of inhibitory factor C-terminal Src (Csk) (Place et al., 2011).e feedback loop consists of Src activation leading to phosphorylation of Csk binding protein (Cbp), and the phosphorylated Cbp targets Csk to Src and promotes inhibitory Csk phosphorylation of Src (Kaimachnikov and Kholodenko, 2009).
Src in cancer therapy:Due to aberrant activation of Src up-stream activators or mutations in Src or Csk, Src can be activated. Abnormal Src activation has been observed in tumors from colon, liver, lung, breast and the pancreas. Currently, there are several Src inhibitors (e.g., dasatinib, bosutinib, saracatinib, AZD0424) in clinical trials for a variety of solid tumors including breast and lung cancers (Roskoski, 2015), and two Src inhibitors (dasatinib, bosutinib) have been approved as prescription drugs to treat adults who have Philadelphia chromosome-positive chronic myelogenous leukemia but no longer benefit from or did not tolerate other treatments (Roskoski, 2015).
Src in neurological diseases:Recent evidence shows that transient Src activation contributes to cell death of neuronsviacell cycle re-entry following acute brain injury, such as TBI. A large number of studies have revealed that the molecules released following acute brain injury (e.g., adenosine, thrombin, cytokines, ROS) can activate Src, and the over-activated Src causes neurons to enter the aberrant cell cycle and results in post-mitotic death. Apart from acute brain injury, abnormal Src activation has been reported in degenerative neurological disease, such as AD. Moreover, Src inhibitors (PP2, AZD0530) have been tested for the treatment of TBI, AD and other neurological diseases (Liu et al., 2010, 2014; Dhawan and Combs, 2012; Nygaard et al., 2014). In addition to tumorigenesis and neuronal death, the oncogenic kinases (e.g., Src, MAPKs, CDKs, others) also play important roles in proliferation of neural progenitor cells (NPCs) that exist throughout the mammalian brain and serve as asource of newborn brain cells in neurogenesis.erefore, Src inhibitors that prevent tumor growth and neuronal death may have limited benefit because they may impair neurogenesis and lead to cognitive side effects. Early diagnosis and prompt treatment are other challenges to prevent mature neurons from death in neurological diseases, as even the mere re-entry of cell cycle may lead to unavoidable neuronal death, and thus Src inhibitors are required to be administrated prior to the formation of cyclin D/Cdk4 complexes (G0/G1 transition), the first step of cell cycle re-entry.
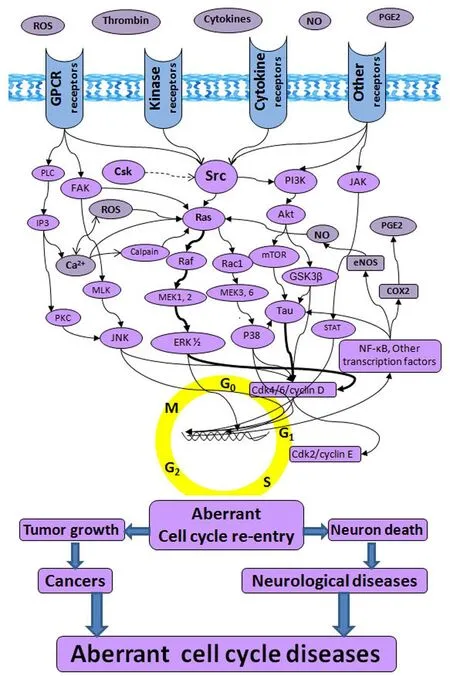
Figure 1e schematic of “aberrant cell cycle diseases”.
Conclusions:Cancer and neurological diseases, two seemingly different disease types, at least in part share the common molecular pathology of cell cycle re-entry.erefore, cancer drugs that block aberrant cell cycle to kill tumor cells (in treatment of cancers) can be repurposed to protect mature neurons from death (in treatment of neurological diseases).is would largely reduce the time frame, decrease costs, and improve success rates in development of new drugs for treating neurological diseases. However, drug repurposing faces some challenges itself since the intellectual property issues of the original drugs may block the repurposed drugs out of the market. Repurposing cancer drugs to treat neurological diseases needs to pay attention to the cognitive side effects, as the cognitive decline has been regarded as one of the major side effects markedly affecting quality of life of patients who receive cancer drugs. Future studies aimed at better understanding the respective cell cycle pathways of tumor cells, mature neurons and NPCs are probably necessary before repurposing cancer drugs for treating certain neurological diseases so as to consider the most effective benefits to the patients without causing severe cognitive and other side effects.
Da Zhi Liu*
Department of Neurology, University of California at Davis, Davis, CA, USA
*Correspondence to:Da Zhi Liu, Ph.D., dzliu@ucdavis.edu.
Accepted:2017-05-05
How to cite this article:Liu DZ (2017) Repurposing cancer drugs to treat neurological diseases – Src inhibitors as examples. Neural Regen Res 12(6):910-911.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Boggon TJ, Eck MJ (2004) Structure and regulation of Src family kinases. Oncogene 23:7918-7927.
Brown MT, Cooper JA (1996) Regulation, substrates and functions of src. Biochim Biophys Acta 1287:121-149.
Dhawan G, Combs CK (2012) Inhibition of Src kinase activity attenuates amyloid associated microgliosis in a murine model of Alzheimer’s disease. J Neuroinflammation 9:117.
Kaimachnikov NP, Kholodenko BN (2009) Toggle switches, pulses and oscillations are intrinsic properties of the Src activation/deactivation cycle. FEBS J 276:4102-4118.
Liu D, Sharp FR, Van KC, Ander BP, Ghiasvand R, Zhan X, Stamova B, Jickling GC, Lyeth BG (2014) Inhibition of Src family kinases protects hippocampal neurons and improves cognitive function aer traumatic brain injury. J Neurotrauma 31:1268-1276.
Liu DZ, Ander BP (2012) Cell cycle inhibition without disruption of neurogenesis is a strategy for treatment of aberrant cell cycle diseases: an update. Scienti ficWorldJournal 2012:491737.
Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, Sharp FR (2010) Blood-brain barrier breakdown and repair by Src aer thrombin-induced injury. Ann Neurol 67:526-533.
Nosengo N (2016) Can you teach old drugs new tricks? Nature 534:314-316.
Nygaard HB, van Dyck CH, Strittmatter SM (2014) Fyn kinase inhibition as a novel therapy for Alzheimer’s disease. Alzheimers Reser 6:8.
Parsons SJ, Parsons JT (2004) Src family kinases, key regulators of signal transduction. Oncogene 23:7906-7909.
Place AT, Chen Z, Bakhshi FR, Liu G, O’Bryan JP, Minshall RD (2011) Cooperative role of caveolin-1 and C-terminal Src kinase binding protein in C-terminal Src kinase-mediated negative regulation of c-Src. Mol Pharmacol 80:665-672.
Roskoski R, Jr. (2015) Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res 94:9-25.
Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5:317-328.
Stein PL, Vogel H, Soriano P (1994) Combined de ficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev 8:1999-2007.
Tatosyan AG, Mizenina OA (2000) Kinases of the Src family: structure and functions. Biochemistry (Mosc) 65:49-58.
10.4103/1673-5374.208569
- 中國神經(jīng)再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease