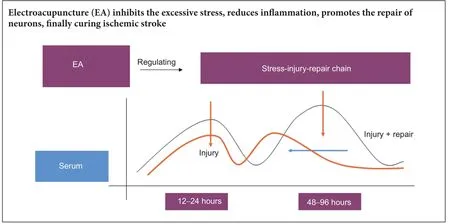
Electroacupuncture regulates the stress-injury-repair chain of events after cerebral ischemia/reperfusion injury
Peng Shi, Lin-lin Sun, Yi-shuo Lee, Ya Tu
School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
Electroacupuncture regulates the stress-injury-repair chain of events after cerebral ischemia/reperfusion injury
Peng Shi#, Lin-lin Sun#, Yi-shuo Lee, Ya Tu*
School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, China
Graphical Abstract
nerve regeneration; electroacupuncture; cerebral ischemia/reperfusion; inflammation; adrenocorticotrophic hormone; heat shock protein 70; Baihui (DU20); Zusanli (ST36); neural regeneration
Introduction
Ischemic stroke, a high-risk disease with high mortality rate, is characterized by slow recovery and high incidence of disability, which seriously harms quality of life. Ischemic stroke accounts for 60—80% of strokes (Tan et al., 2011; Wang and Zeng, 2011). Cerebral ischemia/reperfusion injury triggers a characteristic signaling cascade (Ma et al., 2015). A previous study has shown that early intervention with acupuncture is a good secondary preventing injury aer stroke (Niu, 2011). A clinical investigation of 102 patients showed that the best effects of acupuncture were between 12 hours and 1 week after stroke (Mao, 2011). Our laboratory has conducted animal experiments to investigate acute and chronic stroke combined with hyperlipidemia, and we have shown that electroacupuncture has remarkable effects during the very-early stage of stroke (Jin et al., 2003; Ma et al., 2006; Zhang et al., 2006). These findings indicate that stroke treatment has a rigid time window, and its long-term curative effect is there-fore not always clear.
Release of adrenocorticotrophic hormone (ACTH) from the pituitary gland is a key signal that precedes the body’s stress response. A strong stress response occurs in the acute phase of cerebral ischemia. Once the hypothalamus-pituitary-adrenal axis is activated, the anterior and intermediate lobes of pituitary gland secrete ACTH, which triggers the release of adrenocortical hormone, thus playing a role in the macro regulation of bodily functions (Rizzi et al., 2006). Heat shock protein 70 (Hsp70) is a protein involved in the stress response, and is the most conservative and dominant type of HSP family. Hsp70 is an antioxidant that acts to block apoptosis, can improve the plasticity of neurons, and is involved in neurotropic effects that are mediated by brain-derived neurotrophic factor (Malinverni et al., 2017; Zhang et al., 2017). Hsp70 is an endogenic danger signal that is synthesized when stress occurs in a cell, with expression levels being related to the degree of injury. Once synthesized, it starts a signaling cascade that leads to macrophage activation, and ultimately an innate immune response (Calderwood et al., 2007). Hsp70 can thus help maintain immunological homeostasis of proteins and regulate inflammation (Zheng et al., 2008; Kourtis et al., 2012). Additionally, its expression levels reflect changes in the state of an injury (Yu et al., 2013; Liu et al., 2015b; Zhang et al., 2016).
In this study, we used Sprague-Dawley rats to generate a model of ischemia using Zea Longa’s method, and measured changing levels of ACTH and Hsp70 in the peripheral blood and brain tissue at different time points during the course of the stress-injury-repair sequence of events.
Materials and Methods
Experimental animals
Clean and healthy 8-week-old male Sprague-Dawley rats (n= 130), weighing 275 ± 15 g, were provided by the Experimental Animal Resource Center, China’s Food and Drug Verification Research Institute (license No. SCXK (Jing) 2009-0017). All rats were kept in the Grade II Animal Laboratory in the Institute of Basic Medical Sciences at the Chinese Academy of Medical Sciences. Rats were kept in separate cages for different groups, with 4—5 rats in each cage. The living environment was maintained at 25 ± 3°C, 75% relative humidity, and a 12/12-hour light/dark cycle. All rats were fed adaptively for 1 week with free access to food and water.e experiment strictly adhered to the Guide for Care and Use of Laboratory Animals, and the “3R” principle (Reduction, Replacement, and Refinement).
Rats were randomly divided into three groups: control (n= 10), middle cerebral artery occlusion (the MCAO group) (n= 60), and MCAO with electroacupuncture (the EA group) (n= 60). MCAO and EA groups were subdivided according to the following six time points: postoperative 12, 24, 48, 72, 96, and 144 hours (n= 10 each).
Establishment of the cerebral ischemia/reperfusion model
Rat models were established by intraluminal cerebral ischemia and reperfusion (modified Zea Longa’s method) (Longa et al., 1989). Each was placed on its back and fixed in place after intraperitoneal injection of 10% chloral hydrate (Sinophrm Chemical Reagent Co., Ltd., Shanghai, China; 0.35 mL/100 g) to induce anesthesia. The skin was incised approximately 2 cm at the center of the neck.en, muscle and connective tissue were bluntly dissociated to expose the right common carotid artery, external carotid artery, and internal carotid artery. Excessive traction was avoided to prevent injury to the vagus nerve and blood vessels during the dissection. Aer ligating and cutting offthe external carotid artery, an occlusion line (Beijing Sunbio Biotech Co., Ltd., Beijing, China) was inserted from the stump of the external carotid artery along the internal carotid artery approximately 18 ± 2 cm deep, and the reperfusion was conducted 2 hours aer ischemia.e rats were allowed free access to food and water after waking up in their home cages. Neurological severity score was assessed using a modified Bederson’s 5 grades point system (Wang et al., 2001) to confirm whether the model was successfully established. Rats with Grades 0 and 4 were excluded. In the control group, normal rats were used without any processing.
EA treatment
MCAO rats were taken out of their cage once every day. EA rats were treated with EA at acupointsBaihui(DU20) andZusanli(ST36) (bilateral) (acupuncture depth of 0.2 cm; acupuncture angle of 15°) withHua Tuosterile acupuncture needles (0.30 × 25 mm; Suzhou Medical Supplies Factory Co., Ltd., Suzhou, China). We used a dilatational wave delivered once a day for 20 minutes, with a frequency of 2—100 Hz and an amplitude of 2 mA.e first treatment was given 30 minutes aer removal of the bolt, and the last treatment was given 150 minutes before euthanasia.
Neurological evaluation
At 12, 24, 48, 72, 96, and 144 hours aer MCAO, we again assessed the neurological severity score: Grade 0: no dysfunction; Grade 1: foreleg cannot fully extend; Grade 2: flexion of the leforeleg, resistance decreases when animals were pushed to the le; Grade 3: rat veers to the lewhen crawling; Grade 4: rat loses consciousness, and may not survive another 24 hours.
2,3,5-Triphenyltetrazolium chloride (TTC) staining
Brain infarctions were observed using TTC staining 144 hours aer MCAO. Rats were intraperitoneally anesthetized with 10% chloral hydrate (0.35 mL/100 g). Fresh brain tissue was put into a brain-section mold (East and West Instrument (Beijing) Technology, Beijing, China), cut with a surgical blade into 2 mm sections, then put in 0.1% TTC and incubated in a constant temperature box at 37°C for 30 minutes.
Hematoxylin-eosin staining
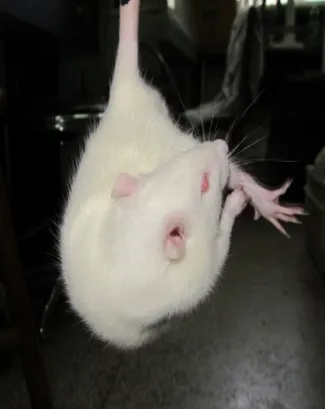
Figure 1 Body torsion of cerebral ischemia/ reperfusion rats.
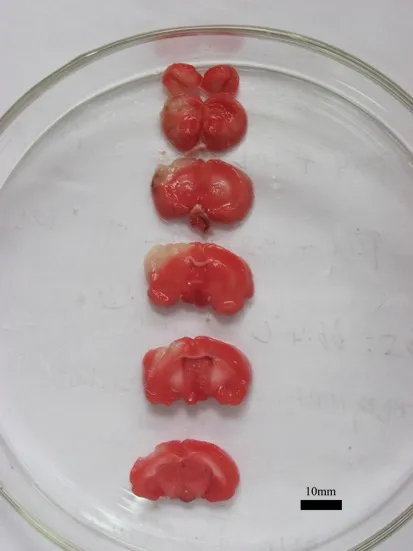
Figure 2 Pathological change of cerebral ischemia/ reperfusion rats (2,3,5-triphenyltetrazolium chloride staining).
Table 1 Neurological score of rats aer model establishment and before execution
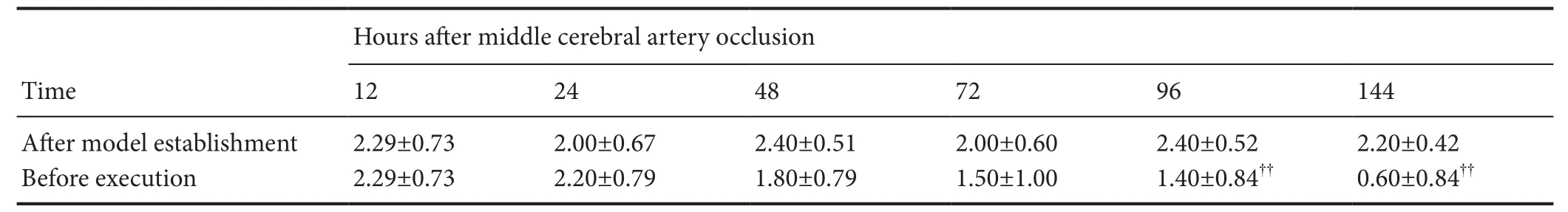
Table 1 Neurological score of rats aer model establishment and before execution
Data are expressed as the mean ± SD (n = 10 rats in each group at each tome point). Normally distributed data with homogeneity variance were compared with one-way analysis of variance among groups. Normally distributed data with heterogeneity variance were compared with approximateftest. Non-normally distributed data were compared with non-parametric test. ??P < 0.01, vs. aer model establishment.
?
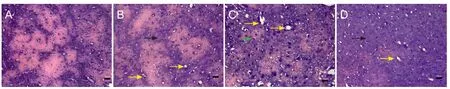
Figure 3 Pathological changes in the frontal lobe and the front of the temporal lobe in rat models of cerebral ischemia/reperfusion injury (hematoxylin-eosin staining, × 200).
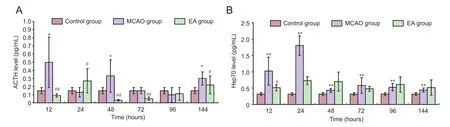
Figure 4 Effect of EA on ACTH (A) and Hsp70 levels (B) in serum of cerebral ischemia/reperfusion rats.
Morphological changes in the brain were observedviahematoxylin-eosin staining 144 hours after MCAO. After collection, brain tissue was rapidly frozen in the liquid nitrogen, and then placed at —80°C in a freezer. The frozensections (2—3 mm in thickness) were placed in hematoxylin for 20 minutes, washed three times with water, treated with hydrochloric acid in alcohol for color separation, and followed by three washes with water. Subsequently, the sections were immersed in 0.5% ammonia. Nucleus staining was observed under a light microscope (Olympus (China) Company, Shanghai, China) until the nucleus turned blue. Aer three washes with water, sections were stained with eosin for 1 minute, washed 3—4 times, and dehydrated with 80% and 95% alcohol (once each), and 100% alcohol twice. Aerward, the sections were permeabilized twice with xylene, mounted with neutral balsam, and observed using a microscope (Olympus (China) Company).
Enzyme linked immunosorbent assay (ELISA)
At 12, 24, 48, 72, 96, and 144 hours after MCAO, 6—7 mL of serum was taken from the leventricle of the heart that was cut with scissors.e levels of ACTH and Hsp70 in the serum were measured by a commercially available ELISA kit for ACTH (Bachem, Bubendorf, Switzerland) and Hsp70 (EnzoLifeSciences, NY, USA) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Normally distributed data of equal variances were compared across groups using one-way analysis of variance (ANOVA). Normally distributed data with unequal variances were compared with an approximateftest. Non-normally distributed data were compared with a non-parametric test. A value ofP< 0.05 was considered statistically significant. Data are expressed as the mean ± SD.
Results
Loss of neurological function in MCAO rats
The control rats did not exhibit any neurological impairment, while those in the MCAO group exhibited different levels of neurologic impairment (Figure 1). In the MCAO group, the neurological score tended to be lower for rats who lived longer. Compared with the newly modeled rats, highly significant differences in neurologic impairment were observed at 96 and 144 hours (allP< 0.01;Table 1).
Pathological changes in MCAO rats
TTC staining
Brain tissue from both hemispheres in the control group was red. In contrast, damaged regions in the right hemispheres of the MCAO rats appeared white (the typical color for damage) 144 hours aer MCAO.is differed significantly from the contralateral normal brain tissue.e white staining was primarily located in the frontal lobe and the anterior region of the temporal lobe, which corresponds to the location of the middle cerebral artery (Figure 2).
Hematoxylin-eosin staining
Brain tissue of the control group was clear and integral.e layout of the neurons was dense and regular and plenty of cytoplasm was lightly stained.e nuclei were located in the middle, and the nucleoli were clear. Glial cells were integral, and arranged densely, with distinct nucleoli.ere was no red staining in cytoplasm. The intercellular substance was compact, and no edema could be seen. In contrast, microstructural changes in the infarct area were observed at 144 hours aer MCAO. In these brains, staining was shallow in the center of the infarct area. We observed fewer cells, shrunken nuclei, and smaller nucleoli. Cell shape was irregular, with interstitial edema and endothelial swelling. Large numbers of central vacuoles could be seen as well as increased number of inflammatory cells at the site of injury (Figure 3).
Thus, the model rats were successfully established using the modified thread-occlusion method. Cerebral infarctions and their locations (middle cerebral artery region) were confirmed through observing neurological impairment, TTC staining, and hematoxylin-eosin staining.
Effects of EA on ACTH and Hsp70 levels in serum of MCAO rats
After cerebral ischemia/reperfusion injury, ACTH expression levels in the MCAO group had two typical peaks.e first was at 12 hours and the second was at 48 hours aer injury. Compared with the control group, ACTH levels in the MCAO group were markedly higher at 12, 48, and 144 hours (P< 0.05). Compared with the control group, ACTH levels in the EA group were significantly lower at 12, 48, 72, and 144 hours (P< 0.01 orP< 0.05;Figure 4A).
The secretion of Hsp70 was limited in the normal rats. After cerebral ischemia/reperfusion injury, its expression in the MCAO group had two typical peaks.e first was at 24 hours and the second was at 72 hours. Compared with the control group, Hsp70 levels in the MCAO group were significantly greater at 12, 24, 48, 72, 96, and 144 hours (P< 0.01). Compared with the MCAO group, Hsp70 levels in the EA group were significantly lower at 12 hours (P< 0.05;Figure 4B).
Discussion
The pathological and physiological mechanism underlying cerebral ischemia/reperfusion injury is now thought to be associated with an injury cascade that includes several sub-mechanisms, such as excitatory toxicity (Lewerenz and Maher, 2015), peripheral depolarization, inflammation (Cuenca-López et al., 2010; Mozaffarian et al., 2015), lipid peroxide reaction caused by oxidative stress (Ying and Xiong, 2010), and programmed cell death.e relationship between these processes is mutually cause-and-effect. Aer the central nervous system is damaged, the sympathetic nervous system and the hypothalamus-pituitary-adrenal axis can be activated by neurotransmitters.is activates the stress response to systemic injury, and inflammatory mediators and chemokines are released to accelerate inflammation.e release of adhesion molecules produces the inflammatory mediators, causes secondary brain injury aer ischemia, and starts the repairing process, thus playing a role in protecting the brain (Taupin, 2008; Denes et al., 2010). An increasingnumber of studies have shown that the main cause of cerebral ischemia/reperfusion injury is inflammation, which can be thought of as a secondary injury aer the initial ischemia (Worthmann et al., 2015; Xu et al., 2016). The activation and aggregation of various cells play indispensable roles in receiving and transmitting the inflammatory signal. These cells include immune cells, mononuclear cells, macrophages, granulocytes, different subtypes of T cells, glial cells, and especially microglia. Adhesion molecules include selectin, immunoglobulin superfamily, integrin and inflammatory mediators, which are highly expressed and participate in the inflammatory response after ischemic injury (Jordan et al., 2008). Studies have shown that inhibiting inflammation aer ischemic injury might be a new strategy for the treatment of ischemic stroke (Jin et al., 2010; Yoo et al., 2016).
Studies have found that acupuncture treatment reduces ischemia/reperfusion injury through multiple paths and targets (Feng et al., 2013; Liu et al., 2015a; Jin et al., 2016), and that it has multiple functions such as reducing inflammation, neuron protection, and promoting the repair of damaged neurons (Yang et al., 2012). EA treatment atBaihuiandRenzhong(DU26) can reduce microglial activation after ischemia/reperfusion in rats. Brain-resuscitation acupuncture can inhibit the production and expression of inflammatory factors that affect rat models of focal cerebral ischemia. Acupuncture atZusanlican affect the functions of lymphocytes and mononuclear macrophages, and reduce the expression of tumor necrosis factor. EA atQuchi(LI11) andZusanliin rat models of ischemia/reperfusion can adjust the secretion levels of inflammatory inhibitors through Toll-like receptor 4/nuclear factor-κB signaling pathway, which improves defective nervous function and reduces the infarct size (Wang et al., 2008; Cheng et al., 2011; Lan et al., 2013).
From the point of view of traditional Chinese medicine, the essential pathogeny of stroke is deficiency ofqiand blood, the imbalance ofYinandYang. Chaos ofqiand blood leads to the paralysis and hemorrhage of the vein, and brings about pathogenic factors that invade the brain. All of these are due largely to a lifestyle that includes worry, anger, weariness, internal injuries, and eating fatty foods.e location of the disease is in the brain, and strongly associated with the heart, liver, spleen and kidney. The nature of disease is asthenia in origin and asthenia in superficiality. Acupuncture has the function of regulating channels, harmonizingqiand blood, and strengthening body resistance to dispel pathogenic factors. According to the meridian theory of traditional Chinese medicine, theBaihuiacupoint is located in the brain, and is an important point related to theDumeridian.eBaihuiacupoint has the following functions: consciousness-restoration, regulatingqiand treating the spirit, and recuperating depletedyangto treat collapse; it is the proximal acupoint. According to the theory that recommends “treating flaccidity and selecting only theYangmingmeridian”, which can be seen in Plain Questions (Su Wen), we know that theYangmingmeridian is a channel with plenty ofqiand blood, and thatZusanliis thehe-sea point for the stomach channel of the footYangming. Acupuncture atZusanlican treat consumption, and is a critical acupoint related to health care.Zusanlican regulateqiand blood, act as a tonic to build up healthful vital energy, and is a distal acupoint.erapeutic effects can be improved with the proximal and distal acupoints, and this is also the characteristic therapy in traditional Chinese medicine.
From our experiment, we found low levels of ACTH and Hsp70 expression in the peripheral blood of normal rats. After the stress response was triggered, their expression levels exhibited bimodal trends. After intervention with EA, the expression of ACTH decreased, reached a peak at 24 hours, and then was maintained at low levels of expression. Hsp70 expression in peripheral blood was significantly lower at the first peak, and maintained a slow level aer the first peak. Aer measuring changes at six time points, we can conclude that the best recovery aer cerebral ischemia/reperfusion injury occurs when EA is administered within 24 hours.e results indicate that cerebral ischemia/ reperfusion injury has a critical time-window for effective treatment with EA. This evidence indicates that cerebral ischemia/reperfusion injury can be relieved by EA by suppressing excessive stress responses, reducing inflammatory injury, promoting the contact between cells, and starting the repair process. This curative effect depending on the timing of the EA. Studying more time points and signals in future experiments will hopefully positively influence clinical treatment.
Author contributions:YT designed the research. LLS and YSL performed the experiment and analyzed the data. PS performed the experiment as an assistant, and wrote this paper. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Calderwood SK, Mambula SS, Gray PJ,eriault JR (2007) Extracellular heat shock proteins in cell signaling. FEBS Lett 581:3689-3694.
Cheng J, Li ZR, Zhu Y, Shen MH, Jing DD, Li C, Pan JL, Wu WZ (2011) Effects of electroacupuncture on expression of calmodulin in the hippocampus of rats with cerebral ischemia-reperfusion injury. Zhongguo Zhen Jiu 31:1015-1019.
Cuenca-López MD, Brea D, Segura T, Galindo MF, Antón-Martínez D, Agulla J, Castillo J, Jordán J (2010) In flammation as a therapeutic agent in cerebral infarction: cellular in flammatory response and inflammatory mediators. Rev Neurol 50:349-359.
Feng X, Yang S, Liu J, Huang J, Peng J, Lin J, Tao J, Chenl(2013) Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med Rep 7:1516-1522.
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukocyte Biol 87:779-789.
Jin XL, Li PF, Zhang CB, Wu JP, Feng XL, Zhang Y, Shen MH (2016) Electroacupuncture alleviates cerebral ischemia and reperfusion injury via modulation of the ERK1/2 signaling pathway. Neural Regen Res 11:1090-1098.
Jin ZX, Hao JD, Lu J, Tu Y (2003) Influence of electroacupuncure at different therapeutic time windows on calmodulin content of the brain tissue in focal cerebral ischemic injury rats. Zhen Ci Yan Jiu 28:178-181.
Jordan J, Segura T, Brea D, Galindo MF, Castillo J (2008) Inflammation as therapeutic objective in stroke. Curr Pharm Des 14:3549-3564.
Kourtis N, Nikoletopoulou V, Tavernarakis N (2012) Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature 490:213-218.
Lan L, Tao J, Chen A, Xie G, Huang J, Lin J, Peng J, Chenl(2013) Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. Int J Mol Med 31:75-80.
Lewerenz J, Maher P (2015) Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front Neurosci 9:469.
Liu F, Jiang YJ, Zhao HJ, Yao LQ, Chen LD (2015a) Electroacupuncture ameliorates cognitive impairment and regulates the expression of apoptosis-related genes Bcl-2 and Bax in rats with cerebral ischaemia-reperfusion injury. Acupunct Med 33:478-484.
Liu YX, Gao DK, Ma YL, Gao Q, Liu Q (2015b) A study on the protective effect and its mechanism of ulinastatin in cerebral ischemia reperfusion injury. Xiandai Shengwu Yixue Jinzhan 15:4462-4466.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Ma HF, Tu Y, Ma WZ, Guo CQ, Hao JD, Wu JH (2006) Effect of acupuncture of twelve Jing (well)-points on cerebral and serum tnf-α contents in rats with regional cerebral ischemia. Zhen Ci Yan Jiu 31:35-37.
Ma JN, Gao JW, Hou BR, Ren HJ, Liu JX, Chen SH, Yan GZ (2015) Establishing an animal model of ischemic stroke with photothrombosis method. Zhongguo Zuzhi Gongcheng Yanjiu 19:7951-7957.
Malinverni D, Jost Lopez A, De Los Rios P, Hummer G, Barducci A (2017) Modeling Hsp70/Hsp40 interaction by multi-scale molecular simulations and co-evolutionary sequence analysis. Elife 6. pii: e23471.
Mao P (2011) Study of time window in early ischemic stroke rehabilitation cured by acupuncture. Beijing: China Academy of Chinese Medical Sciences.
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, et al. (2015) Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circ J 131:e29-e322.
Niu YG (2011) Modern clinical literature research of stroke treated by acupuncture. Qingdao: Shangdong University of Traditional Chinese Medicine.
Rizzi M, Ferrera F, Filaci G, Indiverif(2006) Disruption of immunological tolerance: Role of AIRE gene in autoimmunity. Autoimmun Rev 5:145-147.
Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PTH, Jeyaseelan K (2011) microRNAs in stroke pathogenesis. Curr Mol Med 11:76-92.
Taupin P (2008) Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int J Med Sci 5:127-132.
Wang CX, Yang Y, Yang T, Shuaib A (2001) A focal embolic model of cerebral ischemia in rats: introduction and evaluation. Brain Res Brain Res Protoc 7:115-120.
Wang HH, Zeng H (2011) General overview of ischemic cerebrovascular disease. Zhongguo Linchuang Yisheng 39:7-10.
Wang J, Yu ZS, Jia SQ, Zhou HY, Wang YS (2008) Effect of electroacupuncture on inflammatory factors in blood of rat with cerebral Ischemia reperfusion. Zhongguo Shiyong Yiyao 3:4-5.
Worthmann H, Tryc AB, Dirks M, Schuppner R, Brand K, Klawonn F, Lichtinghagen R, Weissenborn K (2015) Lipopolysaccharide binding protein, interleukin-10, interleukin-6 and C-reactive protein blood levels in acute ischemic stroke patients with post-stroke infection. J Neuroinflammation 12:13.
Xu DS, Min HM, Bao CF, Jia YJ, Cheng X, Min LQ, Hu LL (2016) Effect of procyanidins on TNF-α and HSP70 in cerebral tissue of rats with focal cerebral ischemia injury. Zhongfeng yu Shenjing Jibing Zazhi 33:699-702.
Yang ZX, Chen PD, Yu HB, Luo WS, Wu YG, Pi M, Peng JH, Liu YF, Zhang SY, Gou YH (2012) Research advances in treatment of cerebral ischemic injury by acupuncture of conception and governor vessels to promote nerve regeneration. Zhong Xi Yi Jie He Xue Bao 10:19-24.
Ying W, Xiong ZG (2010) Oxidative stress and NAD+in ischemic brain injury: current advances and future perspectives. Curr Med Chem 17:2152-2158.
Yoo KY, Kim IH, Cho JH, Ahn JH, Park JH, Lee JC, Tae HJ, Kim DW, Kim JD, Hong S, Won MH, Kang IJ (2016) Neuroprotection of Chrysanthemum indicum Linne against cerebral ischemia/reperfusion injury by anti-inflammatory effect in gerbils. Neural Regen Res 11:270-277.
Yu NQ, Dou YH, Sun P (2013) Research of the Nerve Protective Effect of HSP70 During Craniocerebral Injury. Shandong Yiyao 53:91-93.
Zhang T, Lu Q, Su C, Yang Y, Hu D, Xu Q (2017) Mercury induced oxidative stress, DNA damage, and activation of antioxidative system and Hsp70 induction in duckweed (Lemna minor). Ecotoxicol Environ Saf 143:46-56.
Zhang HM, Fei YT, Shi YJ, Jia BH, Tu Y (2006) Effects of acupuncture of “Baihui” (GV 20) and “Taiyang” (EX-HN 5) on functions of vascular endothelial cells in cerebral ischemia injury rats. Zhen Ci Yan Jiu 31:67-72.
Zhang XM, Tang JG, Xie J, Lu G, Zhang Y (2016) Changes of apelin-13 and stress related protein levels of patients with acute cerebral infarction at different periods. Zhongguo Quanke Yixue 19:2302-2306.
Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA (2008) Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 28:53-63.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Shi P, Sun LL, Lee YS, Tu Y (2017) Electroacupuncture regulates the stress-injury-repair chain of events aer cerebral ischemia/reperfusion injury. Neural Regen Res 12(6):925-930.
Funding: This work was supported by a grant from the Major Science and Technology Project “Major New Drug Created” Funding, No. 2009ZX09103-707.
*Correspondence to:
Ya Tu, tuyab@263.net.
orcid:
0000-0001-8576-3311
(Ya Tu)
10.4103/1673-5374.208574
Accepted: 2017-05-24
- 中國神經(jīng)再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease