Metabolite changes in the ipsilateral and contralateral cerebral hemispheres in rats with middle cerebral artery occlusion
Lei Ruan, Yan Wang, Shu-chao Chen, Tian Zhao Qun Huang Zi-long Hu Neng-zhi Xia Jin-jin Liu Wei-jian Chen Yong Zhang, Jing-liang Cheng, Hong-chang Gao, Yun-jun Yang, Hou-zhang Sun
1 Department of Radiology, First Af filiated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
2 Department of Radiology, First Af filiated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
3 School of Pharmacy, Wenzhou Medical University, Wenzhou, Zhejiang Province, China
Metabolite changes in the ipsilateral and contralateral cerebral hemispheres in rats with middle cerebral artery occlusion
Lei Ruan1,#, Yan Wang1,#, Shu-chao Chen1,#, Tian Zhao1, Qun Huang1, Zi-long Hu1, Neng-zhi Xia1, Jin-jin Liu1, Wei-jian Chen1, Yong Zhang2, Jing-liang Cheng2, Hong-chang Gao3, Yun-jun Yang1,*, Hou-zhang Sun1,*
1 Department of Radiology, First Af filiated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, China
2 Department of Radiology, First Af filiated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
3 School of Pharmacy, Wenzhou Medical University, Wenzhou, Zhejiang Province, China
Graphical Abstract
Cerebral ischemia not only causes pathological changes in the ischemic areas but also induces a series of secondary changes in more distal brain regions (such as the contralateral cerebral hemisphere).e impact of supratentorial lesions, which are the most common type of lesion, on the contralateral cerebellum has been studied in patients by positron emission tomography, single photon emission computed tomography, magnetic resonance imaging and diffusion tensor imaging. In the present study, we investigated metabolite changes in the contralateral cerebral hemisphere aer supratentorial unilateral ischemia using nuclear magnetic resonance spectroscopy-based metabonomics.e permanent middle cerebral artery occlusion model of ischemic stroke was established in rats. Rats were randomly divided into the middle cerebral artery occlusion 1-, 3-, 9- and 24-hour groups and the sham group.1H nuclear magnetic resonance spectroscopy was used to detect metabolites in the leand right cerebral hemispheres. Compared with the sham group, the concentrations of lactate, alanine, γ-aminobutyric acid, choline and glycine in the ischemic cerebral hemisphere were increased in the acute stage, while the concentrations of N-acetyl aspartate, creatinine, glutamate and aspartate were decreased.is demonstrates that there is an upregulation of anaerobic glycolysis (shown by the increase in lactate), a perturbation of choline metabolism (suggested by the increase in choline), neuronal cell damage (shown by the decrease in N-acetyl aspartate) and neurotransmitter imbalance (evidenced by the increase in γ-aminobutyric acid and glycine and by the decrease in glutamate and aspartate) in the acute stage of cerebral ischemia. In the contralateral hemisphere, the concentrations of lactate, alanine, glycine, choline and aspartate were increased, while the concentrations of γ-aminobutyric acid, glutamate and creatinine were decreased.is suggests that there is a difference in the metabolite changes induced by ischemic injury in the contralateral and ipsilateral cerebral hemispheres. Our findings demonstrate the presence of characteristic changes in metabolites in the contralateral hemisphere and suggest that they are most likely caused by metabolic changes in the ischemic hemisphere.
nerve regeneration; brain injury; cerebral ischemia; middle cerebral artery occlusion model; ischemic hemisphere; contralateral hemisphere; metabonomics;1H nuclear magnetic resonance; lactate; choline; γ-aminobutyric acid; diaschisis; neural regeneration
Introduction
Cerebral ischemia is a common cerebrovascular disease with high rates of disability and mortality (Anuncibay-soto et al., 2016). Metabolic disturbance plays an important role in ischemic brain injury, and an understanding of the underlying mechanisms is essential for the development of effective treatments (Yang et al., 2012). Focal brain lesions can have major effects on distal brain regions. A network perspective suggests that physiological effects of brain injury are best assessed over entire networks rather than just locally at the site of structural damage (He et al., 2007; Honey and Sporns, 2008; Carter et al., 2010).
Following the seminal report on the phenomenon of diaschisis, defined as a functional inhibition of the brain distant from the original site of injury (Igarashi et al., 2001), researchers started to investigate changes in brain regions far from the ischemic cerebrum, such as the cerebellum and thalamus. Diaschisis has been shown to involve perturbations in glucose and oxygen metabolism as well as decreases in cerebral blood flow in adjacent brain regions (Enager et al., 2004). Arango-Davila et al. (2016) evaluated transcallosal changes aer local ischemic injury in rats using the neuronal nuclear marker NeuN to examine interhemispheric diaschisis. Magnetic resonance imaging demonstrated that remote changes post-stroke are readily measurable in patients (Yassi et al., 2015). Cerebellar diaschisis (diaschisis between the supratentorial lesion and the contralateral cerebellar hemisphere), the most common form of diaschisis in patients with infarction of the deep middle cerebral artery territory, has been previously investigated by various research groups (Nguyen and Botez, 1998; Liu et al., 2007a; Lin et al., 2009; Madai et al., 2011). Another type of diaschisis, interhemispheric diaschisis, has also been studied, but to a lesser extent. It remains unclear whether the metabolism of the contralateral hemisphere is affected by the ischemic side.
1H nuclear magnetic resonance (1H NMR) spectroscopy is able to detect molecules based on their chemical shi. Many metabolites can be detectedin vitroby1H NMR, and it has been used to study changes in brain biochemistry associated with ischemic neuropathologic processes (Graham et al., 1992). In the present study, using1H NMR, we investigated whether metabolite changes in the ischemic hemisphere impact metabolite changes in the contralateral hemisphere in the rat middle cerebral artery occlusion (MCAO) model of brain ischemia. Metabolic analysis may be a valuable approach for understanding the biochemical mechanisms of stroke and the associated diaschisis.
Materials and Methods
Animals
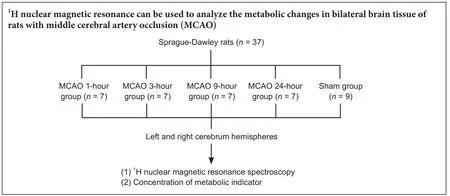
Thirty-eight male Sprague-Dawley rats, weighing 250—320 g and 8—9 weeks of age, were purchased from the Shanghai Laboratory Animal Co., Ltd., Shanghai, China.e rats were regularly fed and allowed free access to water in a quiet room at 25—26°C and 70% humidity at the Experimental Animal Center of Wenzhou Medical University, China (license No. SYXK (Zhe) 2015-0009). This study was approved by the Ethics Committee of Wenzhou Medical University of China (wydw2015-0094).
Rats were randomly divided into the MCAO group (n= 28) and the sham group (n= 9).e MCAO group was subdivided into the 1-hour, 3-hour, 9-hour and 24-hour subgroups (n= 7 for each), according to the duration of cerebral ischemia.
Permanent MCAO surgery
The MCAO model was established as previously described with some minor modifications (Longa et al., 1989). Rats were anesthetized with chloral hydrate (0.3 mL/100 g) and then placed in the supine position. Aer disinfection of the skin, a midline incision of 3—4 cm was made along the neck.en, the lecommon carotid artery, vagus nerve, external carotid artery and internal carotid artery were successively separated.e common carotid and external carotid arteries were ligated with a silk suture and then an aneurysm clip was placed across the internal carotid artery. A V-shaped cut was made on the common carotid artery with microscissors, and a tip-rounded 3-0 monofilament nylon suture (Beijing Sunbio Biotech Co., Ltd., Beijing, China) was inserted into the stump of the external carotid artery. Mild resistance indicated that the filament was inserted 1.6—1.8 cm into the internal carotid artery and blood flow was blocked at the middle cerebral artery origin. The left common carotid artery was ligated at the proximal end to fix the nylon suture. Finally, the skin was sutured and each rat was transferred to a heating blanket to recover from anesthesia. Rats in the sham group were subjected to the same manipulation, but without insertion of the monofilament nylon suture.
Neurological score assessment
Neurological deficit was graded using Longa’s scoring system (Longa et al., 1989) blindly by one experimenter.e scoring scale was as follows: 0, no apparent neurological deficit; 1, contralateral forelimb flexion; 2, circling motion toward the paretic side when attempting to walk; 3, falling to the lateral side when pushed gently; 4, no spontaneous locomotion and depressed levels of consciousness. Rats with a neurological score of 0 were excluded from further experiments.
Preparation of samples, acquisition of1H NMR spectra and data analysis
Seven rats from each group were decapitated at 1, 3, 9 and 24 hours aer MCAO. Nine rats in the sham group were sacrificed as control. Both the leand right cerebral tissues were quickly removed, snap-frozen in liquid nitrogen and stored at -80°C for further processing.e brain tissue was weighed and mixed with distilled water (0.85 mL/g), ice-cold methanol (4 mL/g) and chloroform (2 mL/g).e supernatant was extracted and lyophilized for approximately 24 hours. The metabolite mixture obtained was then weighed and dissolved in 500 μL of 99.5% D2O for NMR spectroscopy.
All1H NMR experiments were carried out on a Bruker AVANCE III 600 MHz NMR spectrometer (Bruker BioSpin, Rheinstetten, Germany), with a spectral width of 12 kHz.e acquisition time was 2.66 seconds per scan, with an additional 8-second relaxation delay to ensure full relaxation.e number of scans was 128.e spectra were zero-filled to 64 K, and an exponential line-broadening function of 0.3 Hz was applied to the free induction decay prior to Fourier transformation.e pre-processed NMR data were then imported into the SIMCA-P+ 12.0 software package (Umetrics, Ume?, Sweden) for analysis and visualization by multivariate statistical methods. Data were mean-centered, and quantitative values of metabolite relative concentrations were obtained.e data were Pareto-scaled prior to partial least squares-discriminant analysis (PLS-DA). Finally, scatter plots and loading plots were acquired.e metabolite concentrations were determined from the spectra and normalized to the weight of the freeze-dried metabolite mixture.
Statistical analysis
Spectra data were statistically analyzed using SPSS 13.0 so-ware (SPSS, Chicago, IL, USA). Data were expressed as the mean ± SD. Independentt-tests were used for comparisons between groups. AP-value of < 0.05 was considered statistically significant.
Results
Neurological assessment
Rats before MCAO surgery and those in the sham group had no neurological deficits, with a score of 0.e scores in the MCAO 1-, 3-, 9- and 24-hour groups ranged from 1 to 4.
1H NMR spectra
Representative1H NMR spectra of cerebrum samples from rats in the sham and MCAO 1-, 3-, 9- and 24-hour groups are shown inFigure 1.
1H NMR spectra and PLS-DA in the ipsilateral (le) ischemic hemisphere
Changes in concentrations of metabolites in the ipsilateral (ischemic, le) hemisphere
Metabolite concentrations in the left cerebral hemisphere at different ischemic time points (1, 3, 9 and 24 hours) were compared with the sham group. Concentrations of lactate, alanine, γ-aminobutyric acid (GABA) and glycine were all higher in the MCAO groups than in the sham group. Lac-tate concentrations steadily increased along with increasing length of the ischemic period (from 25.99 ± 1.72 at 1 hour to 39.82 ± 0.82 at 24 hours). Alanine and glycine increased nonlinearly, and reached a peak (5.15 ± 0.80 and 2.82 ± 0.21) at 24 hours of cerebral ischemia. GABA concentrations reached a maximum (9.94 ± 1.38) at 3 hours after ischemia. Conversely, N-acetyl aspartate (NAA) and creatinine continually decreased. Glutamate and aspartate reached a minimum (19.31 ± 0.97 and 2.43 ± 0.20, respectively) at 24 hours of ischemia. Choline increased at 1 hour (2.62 ± 0.64), 3 hours (2.60 ± 0.38) and 24 hours (2.59 ± 0.33) of ischemia.ese changes were all statistically significant (P< 0.05) (Tables 1and2).
Table 1 Metabolite concentrations in the ipsilateral (le) hemisphere in each group

Table 1 Metabolite concentrations in the ipsilateral (le) hemisphere in each group
Values are expressed as the mean ± SD (n = 7 for each of the MCAO groups and n = 9 for the sham group; independent t-test). *P < 0.05, vs. sham group. MCAO: Middle cerebral artery occlusion; NAA: N-acetyl aspartate; GABA: γ-aminobutyric acid.
Metabolites Sham group MCAO 1-hour group MCAO 3-hour group MCAO 9-hour group MCAO 24-hour group Lactate 23.30±0.78 25.99±1.72* 29.55±4.45* 36.97±3.43* 39.82±0.82*Alanine 1.95±0.12 3.44±0.44* 4.01±0.22* 3.39±0.64* 5.15±0.80*NAA 14.99±0.20 14.93±0.38 14.16±0.44* 11.89±0.73* 8.20±1.23*GABA 6.40±0.30 7.58±1.09* 9.94±1.38* 7.14±1.25 7.62±1.08*Glutamate 24.21±0.62 21.33±1.00* 21.12±1.37* 21.50±1.34* 19.31±0.97*Aspartate 3.00±0.14 3.02±0.32 2.45±0.26* 2.57±0.24* 2.43±0.20*Creatine 16.30±0.74 16.20±0.47 15.33±0.67* 14.69±0.77* 13.67±0.73*Choline 1.89±0.13 2.62±0.64* 2.60±0.38* 1.78±0.42 2.59±0.33*Glycine 1.81±0.12 2.24±0.18* 2.74±0.26* 2.36±0.32* 2.82±0.21*
Table 2 Comparison of metabolite concentrations at various ischemic time points in the ipsilateral (le) hemisphere
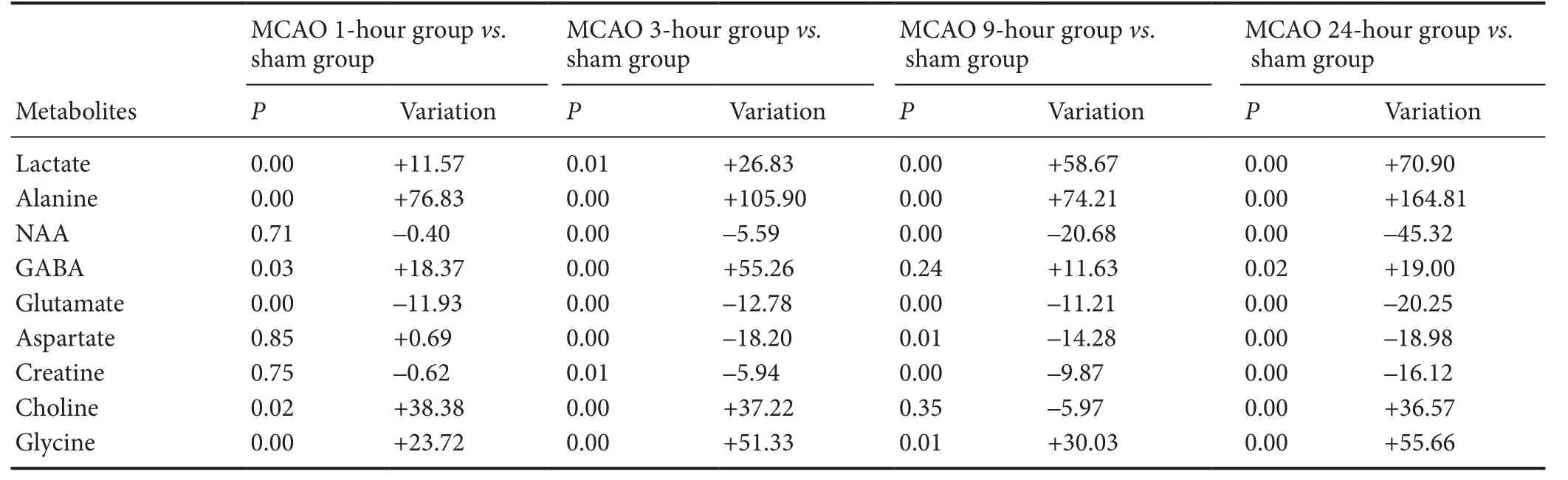
Table 2 Comparison of metabolite concentrations at various ischemic time points in the ipsilateral (le) hemisphere
Changes are shown as an elevation (+) or decline (—) in metabolite concentration in the MCAO groups compared with the sham group. 0.00 indicates P < 0.01. P < 0.05 is considered statistically significant.e significance of metabolite changes was determined by independent t-test (P < 0.05). MCAO: Middle cerebral artery occlusion; NAA: N-acetyl aspartate; GABA: γ-aminobutyric acid.
MCAO 24-hour group vs. sham group P Variation P Variation P Variation P Variation Lactate 0.00 +11.57 0.01 +26.83 0.00 +58.67 0.00 +70.90 Alanine 0.00 +76.83 0.00 +105.90 0.00 +74.21 0.00 +164.81 NAA 0.71 –0.40 0.00 –5.59 0.00 –20.68 0.00 –45.32 GABA 0.03 +18.37 0.00 +55.26 0.24 +11.63 0.02 +19.00 Glutamate 0.00 –11.93 0.00 –12.78 0.00 –11.21 0.00 –20.25 Aspartate 0.85 +0.69 0.00 –18.20 0.01 –14.28 0.00 –18.98 Creatine 0.75 –0.62 0.01 –5.94 0.00 –9.87 0.00 –16.12 Choline 0.02 +38.38 0.00 +37.22 0.35 –5.97 0.00 +36.57 Glycine 0.00 +23.72 0.00 +51.33 0.01 +30.03 0.00 +55.66 MCAO 1-hour group vs. sham group MCAO 3-hour group vs. sham group MCAO 9-hour group vs. sham group Metabolites

Table 3 Metabolite concentrations in the contralateral (right) hemisphere in each group
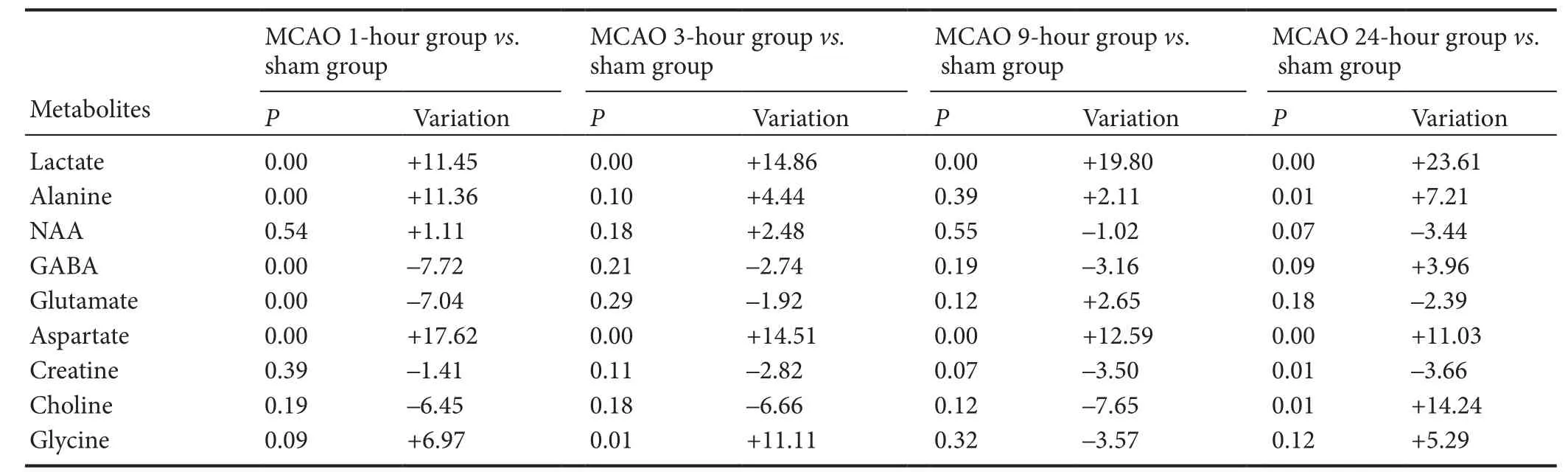
Table 4 Comparison of metabolite concentrations at the various ischemic time points in the contralateral (right) hemisphere
Changes in metabolite concentrations in the contralateral (right) hemisphere
Metabolite concentrations in the right cerebral hemisphere were also compared with the sham group. Concentrations of lactate continually increased from 25.79 ± 0.62 at 1 hour to 28.61 ± 1.61 at 24 hours. Glycine and choline were only increased at 3 hours (glycine: 2.05 ± 0.15) and 24 hours (choline: 2.20 ± 0.21), respectively. Alanine was elevated at 1 hour (2.12 ± 0.09) and 24 hours (2.04 ± 0.08), GABA and glutamate were decreased at 1 hour (5.93 ± 0.20, 22.51 ± 0.85), and creatinine was reduced at 24 hours (15.82 ± 0.32).ese changes were all statistically significant (P< 0.05) (Tables 3and4).
Discussion
Damage induced by MCAO is not confined to the infarct; secondary injuries may spread to other areas with a normal blood supply, such as the cerebellum and contralateral cerebrum (Stenset et al., 2007). The term “transhemispheric diaschisis” was first introduced in 1987. Hoedt-Rasmussen et al. (1964) reported a bilateral reduction of hemispheric blood flow in patients with unilateral cerebral infarction.ey found that hemispheric blood flow was reduced on the healthy side as well, and they hypothesized that unilateral infarction decreased metabolism in the contralateral hemisphere. Accordingly, we hypothesized that the infarct might trigger changes in metabolites in the hemisphere contralateral to the damage.
NMR-based metabonomics, combined with1H NMR spectroscopy, is a novel approach for rapidly identifying changes in global metabolite profiles of biological samples and has been applied in disease studies, such as stroke and diabetes (Nicholson et al., 2002; Yang et al., 2012; Guan et al., 2013). Metabolites reflect the integrative information of cellular function, and understanding changes in neurochemical metabolites may help identify region-specific biomarkers and advance our understanding of the molecular pathogenesis of brain lesions (Shen et al., 2014). In the present study, we examined changes in metabolites in both cerebral hemispheres in rats with MCAO using a1H NMR-based metabonomics approach.
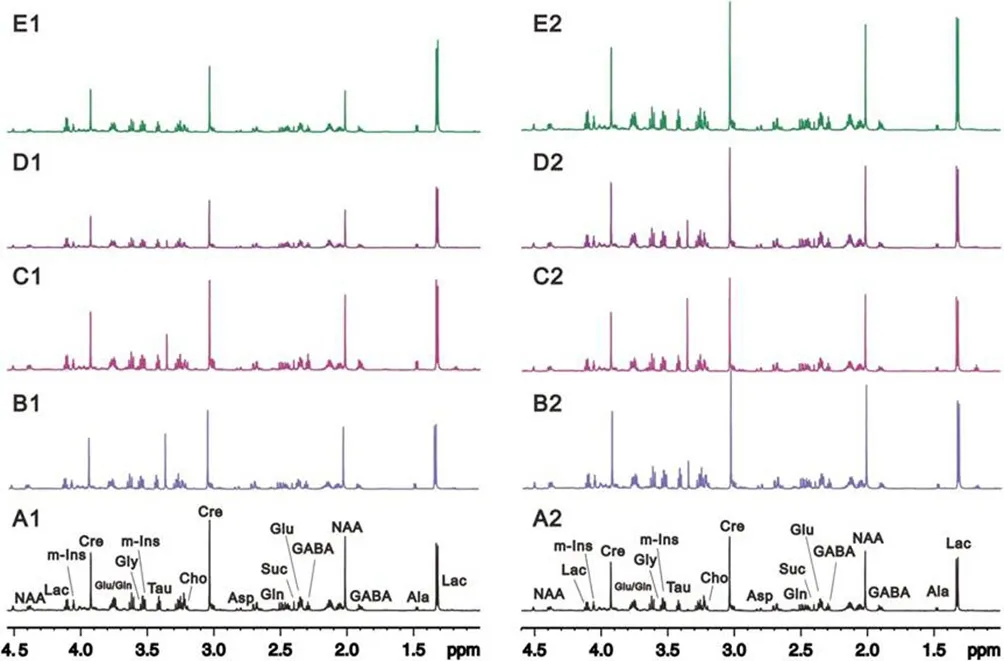
Figure 1 Representative1H NMR spectra of the cerebrum.
In the present experiment, we found that the first principal components in the infarcted cerebral hemisphere were significantly different among the four MCAO groups (1, 3, 9 and 24 hours) and between these groups and the sham group. As the duration of ischemia increased, the differences in the first principal components (i.e., between the 3, 9 and 24-hour groups and the 1-hour group) became greater.
Lactate and alanine levels in the ischemic hemisphere were significantly increased at the different time points, consistent with previous reports (Lanfermann et al., 1995; Igarashi et al., 2003). Lactate is present in the ischemic brain and indicates a switch from oxidative metabolism to anaerobic glycolysis (Graham et al., 1992; Saunders, 2000). Lactate remains sequestered in necrotic tissue and leaves the region of injury only very slowly by passive diffusion after cell lysis, likely accounting for the gradual but sustained increase in the metabolite in the ipsilateral tissue. Lactate could also arise from a shift toward anaerobic glycolysis in viable cells that continue to metabolize glucose under locally hypoxic conditions (Graham et al., 1992).
GABA and glycine were also increased and remained elevated, compared with sham-operated rats, in the ipsilateral ischemic side. GABA levels probably increase due to a combination of factors, including an initial increase in GABA production from glutamate by glutamate decarboxylase, which can proceed without functioning mitochondria, diminished GABA breakdown by GABA transaminase, and reduced astrocytic uptake and metabolism of GABA. GABA release is also increased during ischemia, with an initial exocytotic Ca2+-dependent release followed by a non-vesicular release. Increased activation of GABAergic receptors may be neuroprotective by reducing glutamate release (Haberg et al., 1998, 2001; Phillis and O’Regan, 2003; Saransaari and Oja, 2005; Ouyang et al., 2007; Hertz, 2008).e role of glycine in ischemia is unclear. Some studies suggest that glycine may contribute to the development of ischemic injury (Katsuki et al., 2007; Oda et al., 2007), whereas other studies suggest a neuroprotective effect of glycine (Zhao et al., 2005; Liu et al., 2007; Tanabe et al., 2010). Yao et al. (2012) observed that high levels of glycine exert neuroprotective effects by activating the glycine receptor and the differential regulation of N-methyl-D-aspartate receptor subunit components, leading them to suggest that glycine receptors are a potential target for the clinical treatment of stroke.
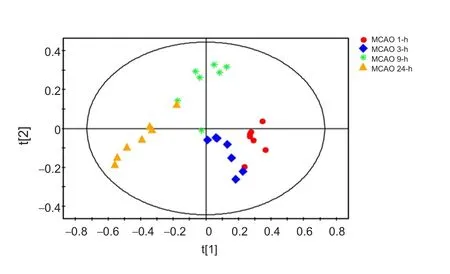
Figure 3 PCA score plot for the MCAO 1-h (red), MCAO 3-h (blue), MCAO 9-h (green) and MCAO 24-h (orange) groups.
The role of NAA in the brain is unclear, although it has been used as a neuronal marker (Castillo et al., 1996). Decreased NAA can reflect the severity of metabolic perturbation in the acute stage. Severely impaired synthesis and accelerated hydrolysis of NAA during ischemia may underlie the drastic decrease in the metabolite in the ipsilateral ischemic hemisphere.is steep reduction in NAA in the acute stage of ischemia is thought to reflect dynamic metabolic changes rather than the density of surviving neurons (Igarashi et al., 2001; Igarashi et al., 2003).
In the present study, we also observed increased GABA and glycine combined with decreased glutamate and aspartate in the ischemic hemisphere in the acute stage. Glutamate and aspartate are two major excitatory amino acids and they may play an important role in the pathways leading to cell death. Inhibitory amino acids, such as GABA and glycine, can inhibit the release of glutamate. It is widely accepted that an imbalance between excitatory and inhibitory amino acids underlies cerebral ischemic damage (Kato and Kogure, 1999; Bogaert et al., 2000; Nishizawa, 2001; Wang et al., 2014). Choline is a constituent of the phospholipid membranes of cells and reflects membrane turnover, and it is a precursor of acetylcholine and phosphatidylcholine (Miller, 1991).erefore, increased choline likely reflects elevated membrane synthesis and/or a higher number of cells (Castillo et al., 1996).
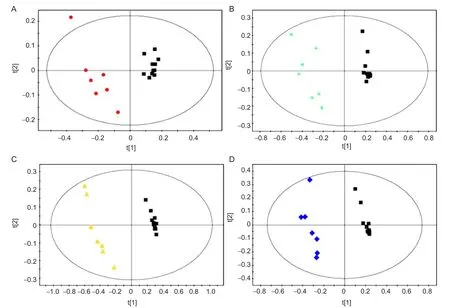
Figure 2 PCA score plots derived from1H NMR spectra of extracts from the le(ipsilateral) cerebral tissue of rats in the MCAO (colorized) and sham (black) groups.
The concentration of metabolites in the contralateral cerebral tissues was also compared with the sham group in this study. The continuous increase in lactate may imply an increase in anaerobic glycolysis in the contralateral cerebral hemisphere.e increase in aspartate and glycine and the reduction in GABA and glutamate may indicate a change in the balance between excitation and inhibition in the contralateral hemisphere. It is likely that the excitability of the contralateral cerebral hemisphere is influenced by the ipsilateral ischemic hemisphere mainlyviathe corpus callosum (Imbrosci et al., 2015). Creatinine serves as a major energy source when ATP is lacking, helping to maintain energy supply in cells. The slight reduction in creatinine levels in the contralateral cerebrum may indicate a perturbation in energy metabolism caused by the ischemic injury. Because the two cerebral hemispheres are connected by the large mass of neural fibers forming the corpus callosum, this type of diaschisis is referred to as transcallosal diaschisis (Reggia, 2004). It is widely thought that the primary mechanism responsible for transcallosal diaschisis is a loss of excitatory inputs from the damaged cerebral hemisphere that are conveyed by the corpus callosum to the intact contralateral cerebral cortex (Berlucchia, 1983; Caselli, 1991; Fiorelli et al., 1991; Meyer et al., 1993). Animal models have also demonstrated that the contralateral effects of an acute hemispheric infarct are reduced or abolished by prior sectioning of the corpus callosum (Kempinsky, 1958; Meyer, 1982). Although changes in metabolite concentrations in the contralateral cerebral hemisphere were detected in the current study, the underlying cellular and molecular mechanisms remain unknown, and further studies are required.
In conclusion,1H NMR-based metabonomics is a powerful tool for analyzing metabolic changes in the ipsilateral and contralateral cerebral hemispheres in rats with ischemic injury. Our findings provide further support for transhemispheric diaschisis. However, further studies are needed to clarify the complex mechanisms underlying this phenomenon.
Author contributions:HCG, YJY and HZS contributed to experimental design. TZ, QH, ZLH, and JJL contributed to animal experiments. NZX, WJC, YZ and JLC contributed to sample collection and metabolomics data acquisition. LR, YW and SCC participated in data analysis, result interpretation and writing. All authors have read, revised and approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Anuncibay-Soto B, Santos-Galdiano M, Fernández-López A (2016) Neuroprotection by salubrinal treatment in global cerebral ischemia. Neural Regen Res 11:1744-1745.
Arango-Davila CA, Munoz Ospina BE, Castano DM, Potes L, Umbarila Prieto J (2016) Assessment transcallosal diaschisis in a model of focal cerebral ischemia in rats. Colomb Med (Cali) 47:87-93.
Berlucchia G (1983) Two hemispheres but one brain. Behav Brain Sci 6:171-172.
Bogaert L, Scheller D, Moonen J, Sarre S, Smolders I, Ebinger G, Michotte Y (2000) Neurochemical changes and laser Doppler flowmetry in the endothelin-1 rat model for focal cerebral ischemia. Brain Res 887:266-275.
Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M (2010) Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance aer stroke. Ann Neurol 67:365-375.
Caselli RJ (1991) Bilateral impairment of somesthetically mediated object recognition in humans. Mayo Clin Proc 66:357-364.
Castillo M, Kwock L, Mukherji SK (1996) Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 17:1-15.
Enager P, Gold L, Lauritzen M (2004) Impaired neurovascular coupling by transhemispheric diaschisis in rat cerebral cortex. J Cereb Blood Flow Metab 24:713-719.
Fiorelli M, Blin J, Bakchine S, Laplane D, Baron JC (1991) PET studies of cortical diaschisis in patients with motor hemi-neglect. J Neurol Sci 104:135-142.
Geoffrey A Donnan MF, Macleod M, DavisSM ( 2008) Stroke. Lancet 373:1612-1623.
Graham GD, Blamire AM, Howseman AM, Rothman DL, Fayad PB, Brass LM, Petroff OA, Shulman RG, Prichard JW (1992) Proton magnetic resonance spectroscopy of cerebral lactate and other metabolites in stroke patients. Stroke 23:333-340.
Guan M, Xie L, Diao C, Wang N, Hu W, Zheng Y, Jin L, Yan Z, Gao H (2013) Systemic perturbations of key metabolites in diabetic rats during the evolution of diabetes studied by urine metabonomics. PLoS One 8:e60409.
Haberg A, Qu H, Haraldseth O, Unsgard G, Sonnewald U (1998) In vivo injection of [1-13C]glucose and [1,2-13C]acetate combined with ex vivo 13C nuclear magnetic resonance spectroscopy: a novel approach to the study of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 18:1223-1232.
Haberg A, Qu H, Saether O, Unsgard G, Haraldseth O, Sonnewald U (2001) Differences in neurotransmitter synthesis and intermediary metabolism between glutamatergic and GABAergic neurons during 4 hours of middle cerebral artery occlusion in the rat: the role of astrocytes in neuronal survival. J Cereb Blood Flow Metab 21:1451-1463.
He BJ, Shulman GL, Snyder AZ, Corbetta M (2007) The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol 20:655-660.
Hertzl(2008) Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 55:289-309.
Hoedt-Rasmussen K SE (1964) Transneural depression of the cerebral hemispheric metabolism in man. Acta Neurol Scand 40:41-46.
Honey CJ, Sporns O (2008) Dynamical consequences of lesions in cortical networks. Hum Brain Mapp 29:802-809.
Igarashi H, Kwee IL, Nakada T, Katayama Y, Terashi A (2001) 1 h magnetic resonance spectroscopic imaging of permanent focal cerebral ischemia in rat: longitudinal metabolic changes in ischemic core and rim. Brain Res 907:208-221.
Igarashi H, Kwee IL, Okubo S, Nakada T, Katayama Y (2003) Predicting the pathological fate of focal cerebral ischemia using 1 h-magnetic resonance spectroscopic imaging. Int Congress Series 1252:341-344.
Imbrosci B, Wang Y, Arckens L, Mittmann T (2015) Neuronal mechanisms underlying transhemispheric diaschisis following focal cortical injuries. Brain Struct Funct 220:1649-1664.
Kato H, Kogure K (1999) Biochemical and molecular characteristics of the brain with developing cerebral infarction. Cell Mol Neurobiol 19:93-108.
Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81:740-749.
Kempinsky WH (1958) Experimental study of distant effects of acute focal brain injury; a study of diaschisis. AMA Arch Neurol Psychiatry 79:376-389.
Lanfermann H, Kugel H, Heindel W, Herholz K, Heiss WD, Lackner K (1995) Metabolic changes in acute and subacute cerebral infarctions: findings at proton MR spectroscopic imaging. Radiology 196:203-210.
Lin DD, Kleinman JT, Wityk RJ, Gottesman RF, Hillis AE, Lee AW, Barker PB (2009) Crossed cerebellar diaschisis in acute stroke detected by dynamic susceptibility contrast MR perfusion imaging. AJNR Am J Neuroradiol 30:710-715.
Liu Y, Karonen JO, Nuutinen J, Vanninen E, Kuikka JT, Vanninen RL (2007a) Crossed cerebellar diaschisis in acute ischemic stroke: a study with serial SPECT and MRI. J Cereb Blood Flow Metab 27:1724-1732.
Liu YT, Wong TP, Aarts M, Rooyakkers A, Liu LD, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT (2007) NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci 27:2846-2857.
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84-91.
Madai VI, Altaner A, Stengl KL, Zaro-Weber O, Heiss WD, von Samson-Himmelstjerna FC, Sobesky J (2011) Crossed cerebellar diaschisis aer stroke: can perfusion-weighted MRI show functional inactivation? J Cereb Blood Flow Metab 31:1493-1500.
Meyer JS (1982) Changes in local CBF and lambda values following regional cerebral infarction in the baboon. Adv Biosci 43:153-165.
Meyer JS, Obara K, Muramatsu K (1993) Diaschisis. Neurol Res 15:362-366.
Miller BL (1991) A review of chemical issues in 1 h NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 4:47-52.
Nguyen DK, Botez MI (1998) Diaschisis and neurobehavior. Can J Neurol Sci 25:5-12.
Nicholson JK, Connelly J, Lindon JC, Holmes E (2002) Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Discov 1:153-161.
Nishizawa Y (2001) Glutamate release and neuronal damage in ischemia. Life Sci 69:369-381.
Oda M, Kure S, Sugawara T, Yamaguchi S, Kojima K, Shinka T, Sato K, Narisawa A, Aoki Y, Matsubara Y, Omae T, Mizoi K, Kinouchi H (2007) Direct correlation between ischemic injury and extracellular glycine concentration in mice with genetically altered activities of the glycine cleavage multienzyme system. Stroke 38:2157-2164.
Ouyang C, Guo L, Lu Q, Xu X, Wang H (2007) Enhanced activity of GABA receptors inhibits glutamate release induced by focal cerebral ischemia in rat striatum. Neurosci Lett 420:174-178.
Phillis JW, O’Regan MH (2003) Characterization of modes of release of amino acids in the ischemic/reperfused rat cerebral cortex. Neurochem Int 43:461-467.
Reggia JA (2004) Neurocomputational models of the remote effects of focal brain damage. Med Eng Phys 26:711-722.
Saransaari P, Oja SS (2005) GABA release modified by adenosine receptors in mouse hippocampal slices under normal and ischemic conditions. Neurochem Res 30:467-473.
Saunders DE (2000) MR spectroscopy in stroke. Br Med Bull 56:334-345.
Shen Y, Gao H, Shi X, Wang N, Ai D, Li J, Ouyang L, Yang J, Tian Y, Lu J (2014) Glutamine synthetase plays a role in D-galactose-induced astrocyte aging in vitro and in vivo. Exp Gerontol 58:166-173.
Stenset V GR, Reinvang I, Hessen E, Cappelen T, Bj?rnerud, A GL, Fladby T. (2007) Diaschisis after thalamic stroke: a comparison of metabolic and structural changes in a patient with amnesic syndrome. Acta Neurol Scand 115:68-71.
Tanabe M, Nitta A, Ono H (2010) Neuroprotection via strychnine-sensitive glycine receptors during post-ischemic recovery of excitatory synaptic transmission in the hippocampus. J Pharmacol Sci 113:378-386.
Wang T, Li Y, Zhao P, Wang J, Zhang X, Hao Y, Du J, Zhao C, Sun T, Yu J, Zhou R, Jin S (2014) Effects of oxysophoridine on amino acids aer cerebral ischemic injury in mice. Ann Indian Acad Neurol 17:313-316.
Yang M, Wang S, Hao F, Li Y, Tang H, Shi X (2012) NMR analysis of the rat neurochemical changes induced by middle cerebral artery occlusion. Talanta 88:136-144.
Yao W, Ji F, Chen Z, Zhang N, Ren SQ, Zhang XY, Liu SY, Lu W (2012) Glycine exerts dual roles in ischemic injury through distinct mechanisms. Stroke 43:2212-2220.
Yassi N, Malpas CB, Campbell BC, Moffat B, Steward C, Parsons MW, Desmond PM, Donnan GA, Davis SM, Bivard A (2015) Contralesional thalamic surface atrophy and functional disconnection 3 months aer ischemic stroke. Cerebrovasc Dis 39:232-241.
Zhao P, Qian H, Xia Y (2005) GABA and glycine are protective to mature but toxic to immature rat cortical neurons under hypoxia. Eur J Neurosci 22:289-300.
Zhong Q, Zhou Y, Ye W, Cai T, Zhang X, Deng DY (2012) Hypoxia-inducible factor 1-alpha-AA-modified bone marrow stem cells protect PC12 cells from hypoxia-induced apoptosis, partially through VEGF/PI3K/Akt/FoxO1 pathway. Stem Cells Dev 21:2703-2717.
Copyedited by Patel B, Raye W, Wang J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Ruan L, Wang Y, Chen SC, Zhao T, Huang Q, Hu ZL, Xia NZ, Liu JJ, Chen WJ, Zhang Y, Cheng JL, Gao HC, Yang YJ, Sun HZ (2017) Metabolite changes in the ipsilateral and contralateral cerebral hemispheres in rats with middle cerebral artery occlusion. Neural Regen Res 12(6):931-937.
*Correspondence to:
Hou-zhang Sun or Yun-jun Yang, M.D., shzlxm@163.com or wzfskyyj2011@163.com.
orcid:
0000-0001-5463-8358
(Hou-zhang Sun)
0000-0002-4493-8477
(Yun-jun Yang)
10.4103/1673-5374.208575
Accepted: 2017-05-21
- 中國神經(jīng)再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease