High-frequency and brief-pulse stimulation pulses terminate cortical electrical stimulation-induced afterdischarges
Zhi-wei Ren, Yong-jie Li, Tao Yu, Duan-yu Ni, Guo-jun Zhang, Wei Du, Yuan-yuan Piao, Xiao-xia Zhou
Beijing Institute of Functional Neurosurgery, Xuanwu Hospital of Capital Medical University, Beijing, China
High-frequency and brief-pulse stimulation pulses terminate cortical electrical stimulation-induced afterdischarges
Zhi-wei Ren, Yong-jie Li*, Tao Yu, Duan-yu Ni, Guo-jun Zhang, Wei Du, Yuan-yuan Piao, Xiao-xia Zhou
Beijing Institute of Functional Neurosurgery, Xuanwu Hospital of Capital Medical University, Beijing, China
Graphical Abstract
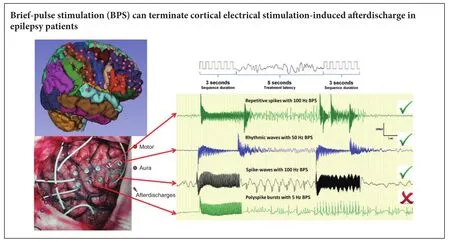
Brief-pulse stimulation at 50 Hz has been shown to terminate aerdischarges observed in epilepsy patients. However, the optimal pulse stimulation parameters for terminating cortical electrical stimulation-induced aerdischarges remain unclear. In the present study, we examined the effects of different brief-pulse stimulation frequencies (5, 50 and 100 Hz) on cortical electrical stimulation-induced aerdischarges in 10 patients with refractory epilepsy. Results demonstrated that brief-pulse stimulation could terminate cortical electrical stimulation-induced aerdischarges in refractory epilepsy patients. In conclusion, (1) a brief-pulse stimulation was more effective when the aerdischarge did not extend to the surrounding brain area. (2) A higher brief-pulse stimulation frequency (especially 100 Hz) was more likely to terminate an aerdischarge. (3) A low current intensity of brief-pulse stimulation was more likely to terminate an aerdischarge.
nerve regeneration; cortical electrical stimulation; aerdischarges; intractable epilepsy; functional brain mapping; high frequency stimulation; low frequency stimulation; neuromodulation; neural regeneration
Introduction
Resective surgery is used to treat patients with intractable partial epilepsy, although some patients with multifocal epilepsy or epileptic foci and eloquent cortex overlap are not optimal surgical candidates (Fong et al., 2011; Forcadas-Berdusan et al., 2011). Moreover, some patients with non-lesional neocortical epilepsy may not have the same seizure-free result aer surgery as patients with mesial temporal sclerosis or lesional epilepsy (Asadi-Pooya et al., 2016; Goldenholz et al., 2016). Neurostimulation (especially cortical stimulation) for epilepsy treatment offers distinct benefits, while avoiding the potential toxic, cognitive and idiosyncratic side effects of medication.e responsive neurostimulation closed-loop system is designed to stimulate the brain shortly aer seizure onset, and terminate a seizure before it evolves into an uncontrollable seizure. Although responsive neurostimulation has benefits, the optimal stimulation parameters remain unclear (Sun and Morrell, 2014).
Functional mapping studies in patients with subdural grids indicate that stimulation may produce aerdischarges, which are an inevitable side effect of electrical cortical stimulation, while a subsequent stimulation can terminate the afterdischarges (Lesser et al., 1999; Motamedi et al., 2002).e majority of human studies on aerdischarges have investigated theirtermination using a 50 Hz stimulation frequency. Further, a lower risk of inducing afterdischarges was reported when using 50 Hz stimulation compared with 100 Hz (Motamedi et al., 2007). However, to our knowledge, detailed testing of 5 Hz to 100 Hz frequencies for terminating aerdischarges has not been reported, and there are only a few studies describing the effects of varying frequencies in humans.us, in the present study, we examined the effects of low and high frequency pulse stimulation of the human cortex on inhibition of aerdischarges induced by cortical electrical stimulation.
Subjects and Methods
Subjects
We selected 10 sequential patients who received treatment from November 2013 to March 2014 in Beijing Institute of Functional Neurosurgery of China based on the following inclusion criteria: (1) diagnosed with medically intractable epilepsy (Berg, 2006; Go and Snead, 2008); (2) underwent subdural electrode placement for an invasive evaluation; (3) was able to cooperate during cortical electrical stimulation for functional brain mapping; and (4) afterdischarges were observed during cortical stimulation. Other patients were excluded because of low cortical excitability resulting in no identifiable aerdischarges, or because of high cortical excitability resulting in recurrent epilepsy seizures during cortical stimulation (Figure 1).
To estimate the group size, we first measured the terminating rate for the three different frequency groups in 10 patients.e terminating rates of the different groups were 19.4% (5 Hz), 41.9% (50 Hz) and 53.3% (100 Hz). Power analysis usingα= 0.05, two-tailed and a power of 80% indicated that 30 trials per group were required to show group differences. In the 10 enrolled patients, the trial numbers of different groups were 31 (5 Hz), 43 (50 Hz) and 30 (100 Hz).
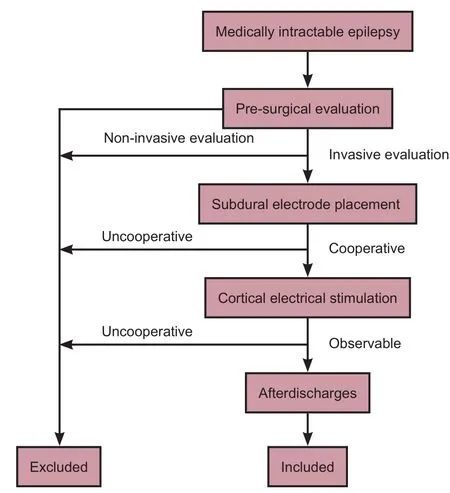
Figure 1 Flow chart of the study.
This study followed theDeclaration of Helsinkiand the relevant set of ethical principles. All patients (or parent/ guardian where appropriate) provided written informed consent.e Ethics Review Committee of the Xuanwu Hospital, Capital Medical University and the Ministry of Health, China approved this study (approval number: [2015]001). All measurements performed in the present study were in compliance with the current laws and regulations in China.
Pre-surgical evaluation
All patients underwent an individualized presurgical evaluation, including a history interview, seizure semiology, 3.0-T magnetic resonance imaging (Siemens Verio, Erlangen, Germany) and scalp video-electroencephalogram (EEG) monitoring, which captured at least three spontaneous seizures. Visual and neuropsychological examinations were conducted if necessary. An invasive subdural electrode or stereotactic deep electrode implantation was performed if there were inconsistent findings or the epileptogenic zone was closely related to the eloquent cortex. Electrode placement was guided by non-invasive exams and intraoperative electrocorticography. If the patients’ epileptogenic zones were near the eloquent cortex, we performed preoperative cortical electrical stimulation for functional mapping.
Intracranial electrodes
The subdural electrodes were 1.5-mm-thick soft silastic sheets embedded with platinum-iridium disc electrodes (3 mm total diameter, 2.5 mm diameter exposed to the cortical surface, 90% platinum and 10% iridium) that were equally spaced with 10 mm center-to-center distances in a rectangular or linear array (HKHS, Beijing, China).e electrodes were arranged in either a grid (4 × 8 to 8 × 8) or a strip (1 × 8 to 2 × 8).
Intracranial video-EEG monitoring and functional mapping
Interictal and ictal EEGs were recorded using a video-EEG monitoring system (BRAIN QUICK, Micromed; Treviso, Italy) that could simultaneously record up to 128 channels, with 1,024 samples per second per channel. Electrical stimulation for functional mapping was conducted to localize the sensory, motor and language areas aer EEGs were recorded at least twice to capture the clinical seizures, which usually occurred 3—4 days aer electrode implantation. For electrical stimulation, 0.2-ms bipolar monophasic square wave pulses were repeated at 50 Hz and presented in trains lasting 3 seconds, with a 20-second interval (SD LTM STIM; Micromed, Treviso, Italy). Stimuli at each electrode (using the adjacent electrode as a reference) started at 1.0 mA and increased in 0.5—1.0 mA increments subsequently until a functional alteration was achieved and an aerdischarge was recorded or 12 mA was reached (Guojun et al., 2014).
We used previous afterdischarge definitions, descriptions and classifications (Lesser et al., 1999; Blume et al., 2004),and we revised the morphological classification as repetitive spikes, rhythmic waves, spike-waves or polyspike bursts. We used a viewer (SystemPLUS; Micromed) to review EEGs that simultaneously displayed up to 128 channels, which allowed us to precisely mark the afterdischarge location and other events. Data were reviewed using the following settings: 400—800 μV/cm sensitivity, 100 Hz low pass filter, 0.5 Hz high pass filter and speeds of 1—10 second/screen. Only epileptiform discharges that were clearly distinguishable from the underlying EEG activity aer cortical stimulation were considered as aerdischarges.e aerdischarge frequency was calculated based on the mean interpeak distance.
Several individuals performed the preliminary assessments of portions of the recordings, but one board certified electroencephalographer (Yuanyuan Piao, Beijing Institute of Functional Neurosurgery) performed the final markings of all recordings, and marked the entire data set twice.e kappa coef ficient for the two reviews was good (κ = 0.95).
Brief-pulse stimulation (BPS)
Occasionally, we used a BPS to terminate afterdischarges during cortical electrical stimulation. When aerdischarges occurred, we oen stimulated the same pair again using the same parameters. In the present study, we assessed whether a BPS was more effective at a higher frequency or lower frequency. To study the effects of different BPS parameters, especially the effect of different frequencies on aerdischarge termination, we repeated the pulse stimulations at three frequencies (5 Hz, 50 Hz and 100 Hz) at one electrode pair. For example, we repeated stimulations three times at one electrode pair, and if each trial induced an aerdischarge, then the BPS was applied at three different frequencies (from 5 Hz to 100 Hz sequentially). However, if a trial did not induce an aerdischarge, then we moved to the next frequency. Aer the functional mapping, we repeated the electrical stimulation on the electrode pairs that previously generated aerdischarges using a slightly lower intensity, and then continued to increase the stimulation intensity until the aerdischarges recurred or 12 mA was reached. Once afterdischarges recurred, we used a BPS at the same electrode pair.
To avoid side effects, we did not repeatedly stimulate after seizure onset or repeatedly stimulate the electrode pairs that were located at the eloquent cortex. For analytical convenience, we used the same pulse type, pulse width and sequence duration, with a treatment latency of 5 seconds (Figures 2,3). In addition, we used a low frequency (5 Hz), intermediate frequency (50 Hz) and a high frequency (100 Hz), and then recorded and evaluated the aerdischarge response to a BPS. When an aerdischarge was not observed aer a BPS, or when what appeared to be aerdischarge termination was observed within the first 2 seconds aer a BPS, we considered that the BPS was effective (Lesser et al., 1999).
Statistical analysis
All statistical analyses were performed with statistical so-ware (SPSS 19.0; IBM Corporation, Armonk, NY, USA). We calculated the number and percentage of electrodes that placed, stimulated and produced aerdischargesviathe electrode location in the frontal lobe, central lobe, parietal-occipital lobe and temporal lobe.e ef ficacy of different BPS frequency groups on aerdischarges was analyzed using chisquare tests.e mean threshold and aerdischarge extension were analyzed by one-way analysis of variance on the effective and ineffective groups. A logistic regression was used to examine whether there was an increase or decrease in the overall probability of afterdischarges terminated by BPSs for some characteristics of afterdischarges and BPSs, including aerdischarge extension, aerdischarge frequency, BPS frequency and BPS intensity.
A kappa (κ) statistic was calculated to evaluate the consistency of the afterdischarges observed between the two reviews performed by the board-certified electroencephalographer. The consistency was poor if κ < 0.2, fair if κ = 0.21—0.40, moderate if κ = 0.41—0.60, good if κ = 0.61—0.80 and excellent if κ ≥ 0.81. The kappa coefficient for the two reviews (κ = 0.95) was excellent.
AP-value of < 0.05 was considered statistically significant. Based on the logistic regression model analysis, we reported odds ratios, 95% confidence intervals andP-values.
Results
General characteristics and cortical electrical stimulation outcome
For the 10 patients, seven were men, three were women, the mean age at surgery was 11.4 years (range, 9—31 years), seizure duration was 10.1 years (range, 3.5—22 years) and the mean number of intracranial electrodes was 72 (range, 64—96). The number of trials per patient ranged from 77 to 424; a total of 2,662 trials were included in the analysis, and 375 trials (14.1%) elicited aerdischarges. A total of 720 electrodes were placed in the subdural space, 132 electrodes (18.3%) evoked afterdischarges and the number of electrodes that covered the frontal, central, parietal-occipital and temporal lobes were 163, 149, 234 and 174, respectively. We stimulated 79, 148, 116 and 42 sites in the frontal, central, parietal-occipital and temporal lobes, respectively, of which 36, 35, 50 and 12 sites evoked aerdischarges, respectively.e basic patient data and the distribution of aerdischarges are listed inTable 1.
Characteristics of aerdischarges

Table 1 Basic patient information
Table 2 Effect of BPS on terminating different aerdischarge waveforms and frequencies, and different BPS frequencies on terminating aerdischarge
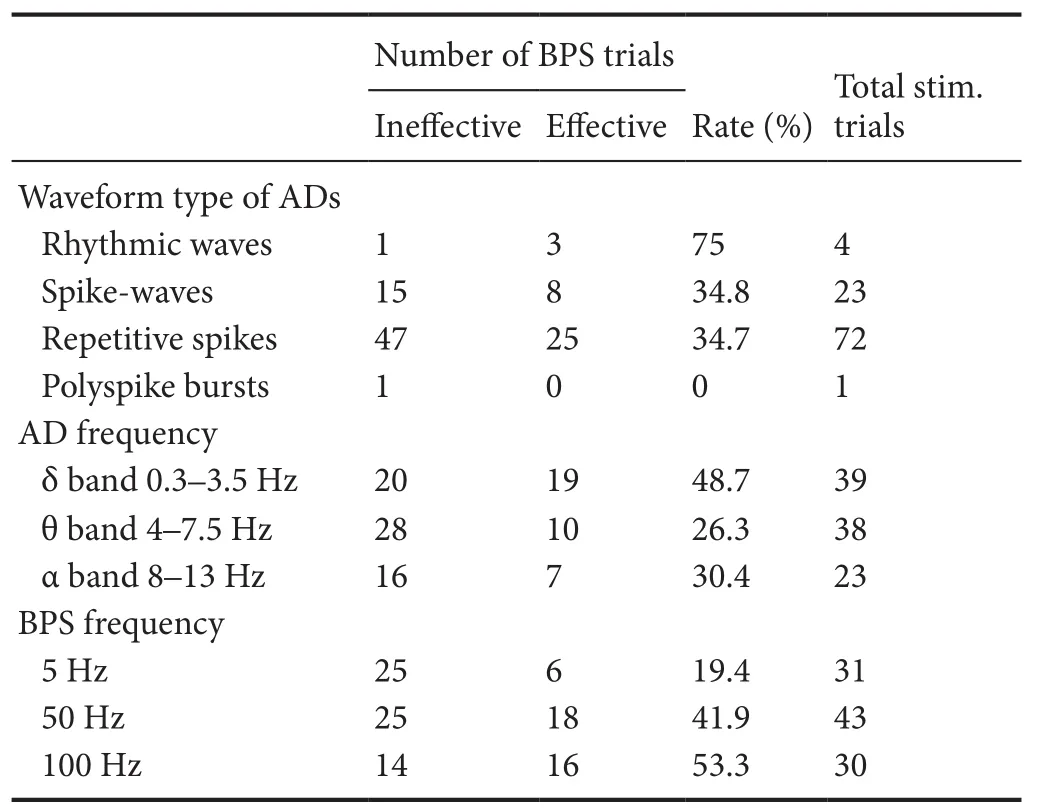
Table 2 Effect of BPS on terminating different aerdischarge waveforms and frequencies, and different BPS frequencies on terminating aerdischarge
Total stim. trials:e total number of effective and ineffective BPS trials in each group; BPS: brief-pulse stimulation; AD: aerdischarge.
Number of BPS trials Ineffective Effective Waveform type of ADs Rhythmic waves 1 3 75 4 Spike-waves 15 8 34.8 23 Repetitive spikes 47 25 34.7 72 Polyspike bursts 1 0 0 1 AD frequency δ band 0.3–3.5 Hz 20 19 48.7 39 θ band 4–7.5 Hz 28 10 26.3 38 α band 8–13 Hz 16 7 30.4 23 BPS frequency 5 Hz 25 6 19.4 31 50 Hz 25 18 41.9 43 100 Hz 14 16 53.3 30 Rate (%) Total stim. trials
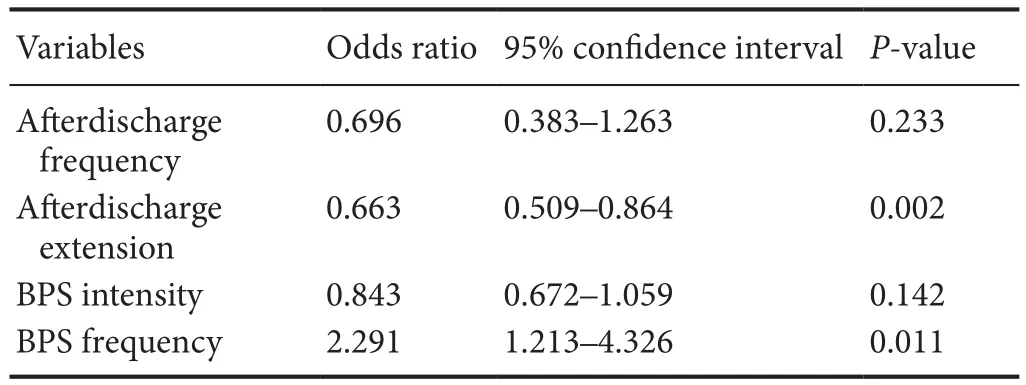
Table 3 Predictor variables in the logistic regression model

Figure 4 Effect of BPS on AD extension and BPS intensity.
Within the 132 electrode pairs that could elicit aerdischarges, we repeated the stimulation at the same parameter as prior to the aerdischarge for 63 pairs, and 51 pairs showed re-induced afterdischarges. Among the 51 electrode pairs, BPSs were applied and 47 pairs had stable afterdischarges in 104 trials. In 40 trials (38.5%), afterdischarges were terminated immediately or within the first 2 seconds. The afterdischarge extension was smaller in the BPS effective group (range, 1—7; mean: 3.0 ± 1.5) compared with the BPS ineffective group (range, 1—12; mean: 4.4 ± 2.3;F= 6.37,P= 0.019;Figure 4A). Of the four types of afterdischarge waveforms, rhythmic waves were the most likely (75%) to be terminated by the BPS, while polyspikes were the least likely (0%;Table 2). Among the three different aerdischarge frequency bands, the δ bands were the most likely (54.1%) to be terminated by the BPS, while the θ bands were the least likely (26.2%;Table 2). A significant difference was observed in the termination ef ficiencies among three band groups (χ2= 6.537,P= 0.038).
BPS characteristics and aerdischarge termination
Predictor variables in a logistic regression model
Next, we performed a logistic regression to identify the factors that determined the BPS effect. Five variables were included in regression model, and aerdischarge extension and BPS frequency were found as predictive variables (P< 0.05;Table 3).
Discussion
The results of the present study confirmed those of a preliminary study showing that a stimulation pulse can terminate cortical-stimulated aerdischarges (Lesser et al., 1999;Motamedi et al., 2002). A higher frequency stimulation of 100 Hz was particularly effective at aerdischarge termination during functional mapping in epilepsy patients, even though the stimulation pulse was not administered shortly aer seizure onset, as with the responsive neurostimulation system (Bergey, 2008; Sun and Morrell, 2014). The present study was designed to investigate the optimal stimulation frequency, and to explore the underlying mechanisms of BPS efficacy. Our main findings were: (1) a BPS was more effective when the aerdischarge did not extend into the surrounding brain area; (2) a higher BPS frequency was more likely to terminate an afterdischarge; (3) a BPS was more likely to terminate the aerdischarge when administered at a low current intensity; and (4) a BPS was more effective when the aerdischarge frequency was in the δ-band range. With respect to the capacity of an electrical stimulus to terminate an aerdischarge, we found significant differences for aerdischarge extension, aerdischarge frequency, BPS intensity and BPS frequency, although logistic regression model analysis revealed a predictive value for only aerdischarge extension and BPS frequency.
Our data suggest that use of a BPS to control an aerdischarge following a BPS is more effective if the aerdischarge does not evolve over an extensive area or far from the primary site;i.e., seizure control is easier by pulse stimulation when the epileptic activity is localized into a small area. In support, the responsive neurostimulation system can stimulate the brain shortly after a seizure onset, and terminate one seizure before it evolves into an uncontrollable seizure (Bergey, 2008; Sun and Morrell, 2014).
A preliminary study in human subjects used only a 50 Hz stimulation pulse (Lesser et al., 1999), while in the present study we used three different BPS frequencies (5 Hz, 50 Hz and 100 Hz) and compared their efficacy in afterdischarge suppression. Our findings are consistent with those in studies using deep brain stimulation and spinal cord stimulation, where high frequency stimulation had an inhibitory effect, mimicking the effects of making a lesion (Lee, 2009). By contrast, low frequency stimulation was reported to have an excitatory effect (Linderoth, 2009), although the underlying mechanism remains unclear.
For deep brain stimulation of the subthalamic nucleus in patients with Parkinson’s disease, a stimulation frequency of 130 Hz or higher is generally used (Benabid, 2003). By contrast, deep brain stimulation in the pedunculopontine tegmentum uses a stimulation frequency of 30 Hz (Mazzone et al., 2005). In many regions of the brain, including the amygdala, hippocampus and cerebral cortex, rapid kindling using high frequency (50 Hz) stimulation as a model of epileptogenesis allows for the acceleration of epilepsy induction (Sankar et al., 2010).erefore, a number of studies have applied low frequency stimulation to those regions to suppress epileptic activity (Zhong et al., 2012). Indeed, low frequency stimulation (1 Hz for 15 minutes) after kindling of the amygdala and hippocampus inhibited the development and expression of amygdala-kindled seizures (Weiss et al., 1995, 1998), suppressed the aerdischarge duration (Velisek et al., 2002) and increased the aerdischarge threshold (Bragin et al., 2002). However, in a rat model of genetic absence epilepsy, very high frequencies (500—1,000 Hz) were more effective at shortening seizure durations (Nelson et al., 2011), while a comparison of low (1 Hz)versushigh (100 Hz) frequency stimulation in rat hippocampal brain slices found that both frequency stimulations suppressed epileptiform activity (Albensi et al., 2004). Human studies have also shown that electrical cortical stimulation of the seizure onset zone using both low (0.9 Hz) and high (50 Hz) frequencies suppressed epileptogenesis (Kinoshita et al., 2005). Our data indicate that high frequency stimulation may play an important role in acute seizure termination in humans.
A potential limitation of our study is that we only investigated the effects of three stimulation frequencies that were less than 100 Hz, while we did not investigate the effects of higher frequencies (> 300 Hz) or other parameters, such as pulse width and waveform type, duration and pattern.
In conclusion, high frequency and low intensity (< 7 mA) stimulation pulses can effectively terminate afterdischarges during cortical stimulation.
Author contributions:YJL, ZWR and TY participated in study design. ZWR and TY participated in literature research. ZWR, TY, DYN and GJZ performed the epilepsy surgery. ZWR, WD, YYP and XXZ conducted data acquisition and analysis. ZWR and YYP participated in statistical analysis. ZWR and TY prepared the paper. YJL, ZWR and TY edited the paper. GJZ obtained the funding. All authors participated in manuscript revision/review, and approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:
Declaration of patient consent:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Albensi BC, Ata G, Schmidt E, Waterman JD, Janigro D (2004) Activation of long-term synaptic plasticity causes suppression of epileptiform activity in rat hippocampal slices. Brain Res 998:56-64.
Asadi-Pooya AA, Nei M, Sharan A, Sperling MR (2016) Historical risk factors associated with seizure outcome aer surgery for drug-resistant mesial temporal lobe epilepsy. World Neurosurg 89:78-83.
Benabid AL (2003) Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol 13:696-706.
Berg AT (2006) Defining intractable epilepsy. Adv Neurol 97:5-10.
Bergey GK (2008) Responsive neurostimulation for the treatment of epileptic seizures. In: Seizure Prediction in Epilepsy (Schelter B, Timmer J, Schulze-Bonhage A, eds), pp 299-306. Weinheim: Wiley-VCH Press.
Blume WT, Jones DC, Pathak P (2004) Properties of aer-discharges from cortical electrical stimulation in focal epilepsies. Clin Neurophysiol 115:982-989.
Bragin A, Wilson CL, Engel J Jr (2002) Increased aerdischarge threshold during kindling in epileptic rats. Exp Brain Res 144:30-37.
Fong JS, Jehi L, Najm I, Prayson RA, Busch R, Bingaman W (2011) Seizure outcome and its predictors aer temporal lobe epilepsy surgery in patients with normal MRI. Epilepsia 52:1393-1401.
Forcadas-Berdusan MI, Bustos-Sanchez JL, Valle-Quevedo E, Aurrecoechea Obieta J, Mateos Goni B, Martinez-Indart L, Molano Salazar A, Gomez-Esteban JC, Garamendi-Ruizi(2011) Predictive factors for a good prognosis following surgery for temporal lobe epilepsy: a cohort study in Spain. Epileptic Disord 13:36-46.
Go C, Snead OC, 3rd (2008) Pharmacologically intractable epilepsy in children: diagnosis and preoperative evaluation. Neurosurg Focus 25:E2.
Goldenholz DM, Jow A, Khan OI, Bagic A, Sato S, Auh S, Kua C, Inati S, Theodore WH (2016) Preoperative prediction of temporal lobe epilepsy surgery outcome. Epilepsy Res 127:331-338.
Guojun Z, Duanyu N, Fu P, Lixin C, Tao Y, Wei D, Liang Q, Zhiwei R (2014)e threshold of cortical electrical stimulation for mapping sensory and motor functional areas. J Clin Neurosci 21:263-267.
Kinoshita M, Ikeda A, Matsuhashi M, Matsumoto R, Hitomi T, Begum T, Usui K, Takayama M, Mikuni N, Miyamoto S, Hashimoto N, Shibasaki H (2005) Electric cortical stimulation suppresses epileptic and background activities in neocortical epilepsy and mesial temporal lobe epilepsy. Clin Neurophysiol 116:1291-1299.
Lee KH (2009) Mechanisms of action of deep brain stimulation: a review. In: Neuromodulation (Krames ES, Peckham PH, Rezai A, eds), pp 157-169. Oxford: Academic Press.
Lesser RP, Kim SH, Beyderman L, Miglioretti DL, Webber WR, Bare M, Cysyk B, Krauss G, Gordon B (1999) Brief bursts of pulse stimulation terminate aerdischarges caused by cortical stimulation. Neurology 53:2073-2081.
Linderoth B (2009) Mechanisms of spinal cord stimulation in neuropathic and ischemic pain syndromes. In: Neuromodulation (Krames ES, Peckham PH, Rezai A, eds), pp 345-354. Oxford: Academic Press.
Mazzone P, Lozano A, Stanzione P, Galati S, Scarnati E, Peppe A, Stefani A (2005) Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport 16:1877-1881.
Motamedi GK, Okunola O, Kalhorn CG, MostofiN, Mizuno-Matsumoto Y, Cho YW, Meador KJ (2007) Aerdischarges during cortical stimulation at different frequencies and intensities. Epilepsy Res 77:65-69.
Motamedi GK, Lesser RP, Miglioretti DL, Mizuno-Matsumoto Y, Gordon B, Webber WR, Jackson DC, Sepkuty JP, Crone NE (2002) Optimizing parameters for terminating cortical aerdischarges with pulse stimulation. Epilepsia 43:836-846.
Nelson TS, Suhr CL, Freestone DR, Lai A, Halliday AJ, McLean KJ, Burkitt AN, Cook MJ (2011) Closed-loop seizure control with very high frequency electrical stimulation at seizure onset in the GAERS model of absence epilepsy. Int J Neural Syst 21:163-173.
Sankar R, Auvin S, Kwon YS, Pineda E, Shin D, Mazarati A (2010) Evaluation of development-specific targets for antiepileptogenic therapy using rapid kindling. Epilepsia 51 Suppl 3:39-42.
Velisek L, Veliskova J, Stanton PK (2002) Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. Neurosci Lett 326:61-63.
Weiss SR, Eidsath A, Li XL, Heynen T, Post RM (1998) Quenching revisited: low level direct current inhibits amygdala-kindled seizures. Exp Neurol 154:185-192.
Weiss SR, Li XL, Rosen JB, Li H, Heynen T, Post RM (1995) Quenching: inhibition of development and expression of amygdala kindled seizures with low frequency stimulation. Neuroreport 6:2171-2176.
Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, Li XM, Zhang SH, Chen Z (2012) Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis 48:20-26.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Ren ZW, Li YJ, Yu T, Ni DY, Zhang GJ, Du W, Piao YY, Zhou XX (2017) High-frequency and brief-pulse stimulation pulses terminate cortical electrical stimulation-induced aerdischarges. Neural Regen Res 12(6):938-944.
*Correspondence to:
Yong-jie Li, geekai@163.com.
orcid:
0000-0001-8889-3568
(Yong-jie Li)
10.4103/1673-5374.208576
Accepted: 2017-05-11
- 中國神經再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease