Monosialoganglioside 1 may alleviate neurotoxicity induced by propofol combined with remifentanil in neural stem cells
Jiang Lu, Xue-qin Yao, Xin Luo, Yu Wang Sookja Kim Chung, He-xin Tang Chi Wai Cheung Xian-yu Wang Chen Meng Qing Li
1 Anesthesiology Research Institute of Hubei University of Medicine, Shiyan, Hubei Province, China
2 Department of Anesthesiology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei Province, China
3 Department of Anesthesiology, Li Ka Shing Faculty of Medicine,e University of Hong Kong, Hong Kong Special Administrative Region, China
4 Department of Anatomy, Li Ka Shing Faculty of Medicine,e University of Hong Kong, Hong Kong Special Administrative Region, China
5 Laboratory and Clinical Research Institute for Pain, Li Ka Shing Faculty of Medicine,e University of Hong Kong, Hong Kong Special Administrative Region, China
Monosialoganglioside 1 may alleviate neurotoxicity induced by propofol combined with remifentanil in neural stem cells
Jiang Lu1,2,*,#, Xue-qin Yao1,2,#, Xin Luo3,5,#, Yu Wang2, Sookja Kim Chung4, He-xin Tang1, Chi Wai Cheung1,3,5, Xian-yu Wang1,2, Chen Meng1,2, Qing Li1,2,*
1 Anesthesiology Research Institute of Hubei University of Medicine, Shiyan, Hubei Province, China
2 Department of Anesthesiology, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei Province, China
3 Department of Anesthesiology, Li Ka Shing Faculty of Medicine,e University of Hong Kong, Hong Kong Special Administrative Region, China
4 Department of Anatomy, Li Ka Shing Faculty of Medicine,e University of Hong Kong, Hong Kong Special Administrative Region, China
5 Laboratory and Clinical Research Institute for Pain, Li Ka Shing Faculty of Medicine,e University of Hong Kong, Hong Kong Special Administrative Region, China
Graphical Abstract
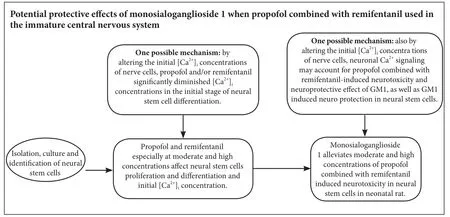
Monosialoganglioside 1 (GM1) is the main ganglioside subtype and has neuroprotective properties in the central nervous system. In this study, we aimed to determine whether GM1 alleviates neurotoxicity induced by moderate and high concentrations of propofol combined with remifentanil in the immature central nervous system. Hippocampal neural stem cells were isolated from newborn Sprague-Dawley rats and treated with remifentanil (5, 10, 20 ng/mL) and propofol (1.0, 2.5, 5.0 μg/mL), and/or GM1 (12.5, 25, 50 μg/mL). GM1 reversed combined propofol and remifentanil-induced decreases in the percentage of 5-bromodeoxyuridine(+) cells and also reversed the increase in apoptotic cell percentage during neural stem cell proliferation and differentiation. However, GM1 with combined propofol and remifentanil did not affect β-tubulin(+) or glial fibrillary acidic protein(+) cell percentage during neural stem cell differentiation. In conclusion, we show that GM1 alleviates the damaging effects of propofol combined with remifentanil at moderate and high exposure concentrations in neural stem cells in vitro, and exerts protective effects on the immature central nervous system.
nerve regeneration; monosialoganglioside 1; propofol; remifentanil; neural stem cells; neurotoxicity; neuroprotection; proliferation; dierentiation; apoptosis; [Ca2+]i; neural regeneration
Introduction
A recent clinical study found that children exposed to general anesthesia from 0 to 4 years old may suffer from a higher risk of cognitive or functional limitations, especially in aspects of language and abstract reasoning (Lee et al., 2009; Atkins and Mandel, 2013; Beers et al., 2014; Wang et al., 2014; Gleich et al., 2015; Zhang et al., 2015; Sun, 2016; Ward et al., 2016; Jevtovic-Todorovic et al., 2017; McCann and de Graaf f, 2017; Walters and Paule, 2017). Consequently, neurotoxicity of anesthetic agents, especially at moderate or high concentrations is potentially a concern for neonatal anesthesia. Propofol and remifentanil are widely used drugs in total intravenous anesthesia, because they potentiate each other for effectively controlling intraoperative anesthesia responses and facilitating rapid emergence from anesthesia (Hogue et al., 1996; Kuroyanagi et al., 2015; O’Connor et al., 2016). However, combined application of propofol and remifentanil in general anesthesia of infants may still be a concern in terms of potential adverse neurodevelopmental effects (Lee et al., 2009; van Kralingen et al., 2011; Wei et al., 2011; Atkins and Mandel, 2013; Wang et al., 2014; Zhang et al., 2015; Satomoto and Makita, 2016; Sun, 2016; Ward et al., 2016; Cho et al., 2017; Dalton et al., 2017; Jevtovic-Todorovic et al., 2017; McCann and de Graaf f, 2017; Scott et al., 2017; Vutskits and Davidson, 2017; Walters and Paule, 2017). Our previous studies and those of others have shown that remifentanil, propofol, and other general anesthetic agents can have damaging effects on the immature central nervous system, including neuronal apoptosis and changes in dendritic morphology, particularly at high exposure concentrations (Davidson, 2011; Braz et al., 2012; Creeley et al., 2013; Li et al., 2014; Van Biesen et al., 2015; Satomoto and Makita, 2016; Ward et al., 2016; Earley et al., 2017; Jevtovic-Todorovic et al., 2017; McCann and de Graaf f, 2017; Vutskits and Davidson, 2017; Walters and Paule, 2017; Zanghi and Jevtovic-Todorovic, 2017).
Monosialoganglioside 1 (GM1) is a major ganglioside subtype that is abundant in the outer leaflet of the plasma membrane of neuronal cells of the central nervous system (Skaper et al., 1991; Baek et al., 2010; Colsch et al., 2011; Ji et al., 2015; Dalton et al., 2017). Further, GM1 exhibits neuroprotective properties in the central nervous system (Baek et al., 2010; Kreutz et al., 2011; Bilotta et al., 2013; Kreutz et al., 2013; Scheller, 2014; Ji et al., 2015; Schneider et al., 2015), with GM1 preventing lead-induced impairment of synaptic activity and oxidative damage in the hippocampusin vivo(She et al., 2009). Hence, we determined whether GM1 alleviates the neurotoxicity of propofol combined with remifentanil, especially at moderate and high exposure concentrations, in the immature central nervous system using anin vitromodel of hippocampal neural stem cells (NSCs) derived from neonatal rats.
Materials and Methods
Ethics statement
Fourteen specific-pathogen-free Sprague-Dawley rats (7 males and 7 females, postnatal day 1) were provided by Hubei University of Medicine and Af filiated Hospital of Taihe, China (license No. SCXK (E) 2005-0008). This manuscript was prepared in accordance with the “Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals” by the International Committee of Medical Journal Editors.e experimental procedures were approved by the Animal Ethics Committee, Hubei University of Medicine and Affiliated Taihe Hospital of Hubei University of Medicine, China (2013-07).
Isolation, proliferation, and differentiation of NSCs
NSCs were harvested from rats following a previously described protocol (Lu et al., 2012a, b; Li et al., 2014).e original NSC culture medium was proliferation medium plus 50 IU/mL penicillin and 50 μg/mL streptomycin. Pure proliferation of NSCs was induced by 10 ng/mL basic fibroblast growth factor (Promega, Madison, WI, USA) and 10 ng/mL epidermal growth factor (Promega) in basic culture medium, which contained Dulbecco’s Modified Eagle Media: Nutrient Mixture F12 (DMEM/F12, 1:1, v/v, Gibco, Grand Island, NY, USA), 2% B27 (Gibco), 1% N2 (Gibco) supplement, 0.5 mM L-glutamine, and 0.5 mM non-essential amino acid (Gibco). Pure differentiation culture medium mainly contained 1% fetal bovine serum (Gibco) and 1% serum replacement (Gibco) in basic culture medium without basic fibroblast growth factor and epidermal growth factor (Vescovi and Snyder, 1999; Wen et al., 2002, 2007; Yu et al., 2007; Lu et al., 2012a, b; Li et al., 2014; SanMartin et al., 2014). In each treatment group, different concentrations of GM1 and/or propofol combined with remifentanil were added into pure proliferation or differentiation culture medium at the beginning, and co-cultured during the entire proliferation or differentiation process.
Identification of NSCs
NSCs were identified by immunocytochemistry and reverse transcription polymerase chain reaction (RT-PCR) following a previously described protocol, with identification results also shown in our previous studies (Lu et al., 2012a, b; Li et al., 2014).
Drug exposure
After culture in proliferation culture medium or differentiation culture medium for 8 hours, NSCs were exposed at the same time to propofol (AstraZeneca, London, UK) and remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, Hubei Province, China), and/or GM1 (TRB Pharmaceutical Co., Buenos Aires, Argentina) for 30 minutes. Low, moderate, and high doses of propofol combined with remifentanil were 1, 2—2.5, and 4—5 times respectively, the effective clinical drug blood concentration (Van’t Veer et al., 2009). There were 11 experimental groups (n= 5 wells in each treatment), seeTable 1for details.
NSC proliferation assay
Proliferation of NSCs was assessed by immunocytochemistry using our previously described protocol (Lu et al., 2012a, b; Li et al., 2014). From 6 to 8 hours after drug exposure,
NSCs were incubated with 5-bromodeoxyuridine (BrdU) (5 μM; Sigma, St. Louis, MO, USA) for 20 minutes.
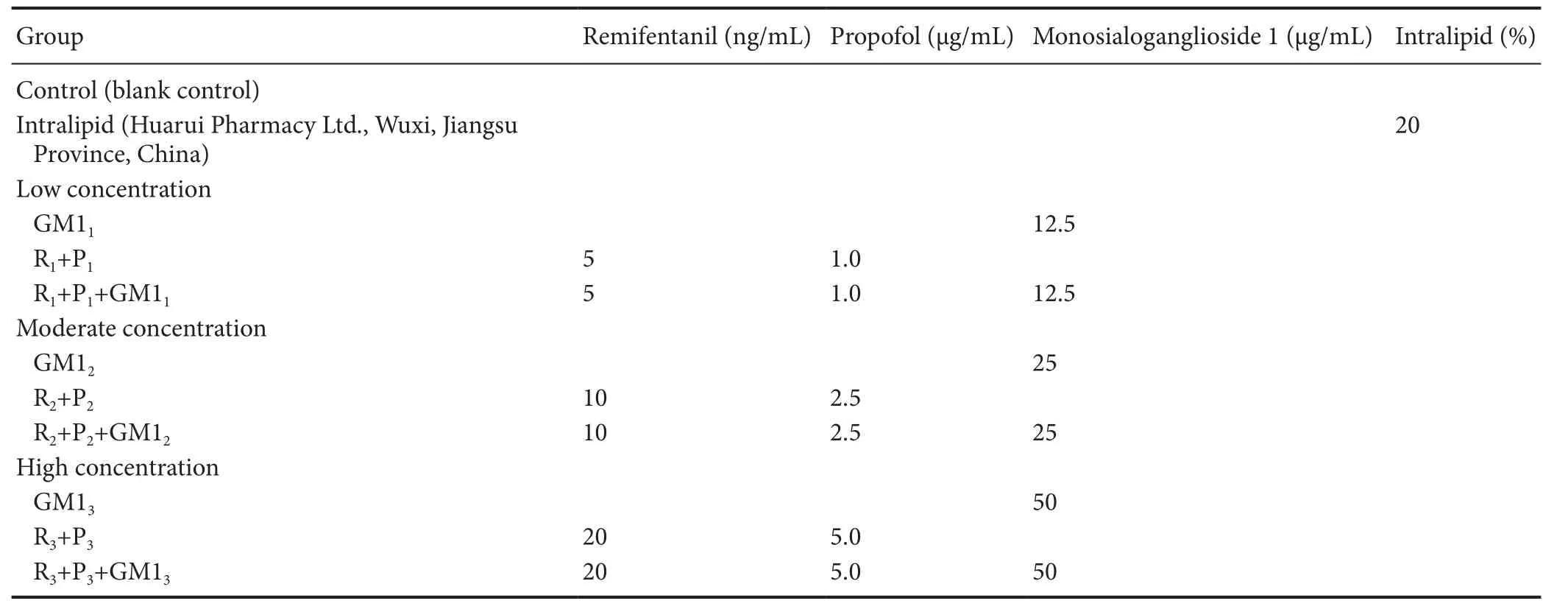
Table 1 Drug exposure paradigm
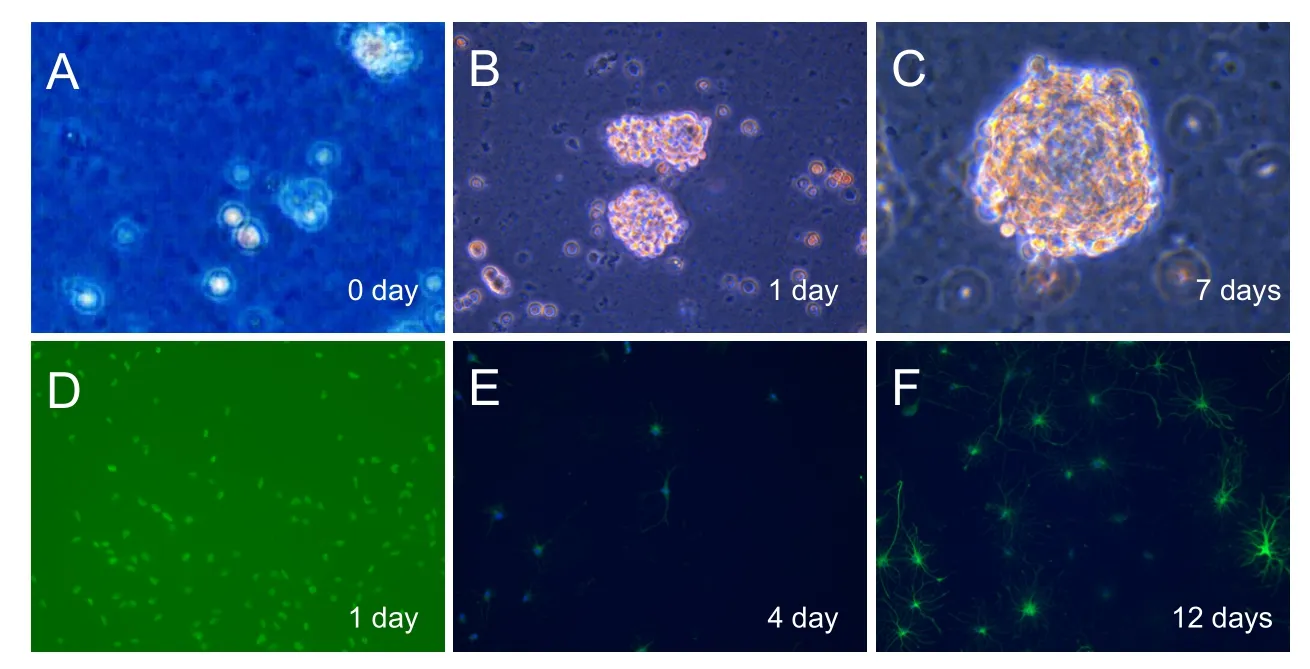
Figure 1 Isolation, proliferation, and differentiation of NSCs (× 200).
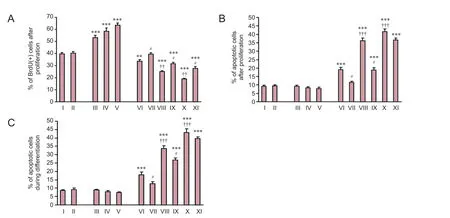
Figure 2 Effect of propofol, remifentanil, and/or GM1 on NSCs: BrdU(+) and apoptosis during proliferation and differentiation.
NSC apoptosis assay
The protocol was described in our previous studies (Lu et al., 2012a, b; Li et al., 2014). At 48 hours aer drug exposure, NSCs were washed with phosphate buffered saline (PBS) and digested. NSCs were gently dissociated using a pipette and centrifuged at 2,000 r/min for 5 minutes. NSCs were then re-suspended in 400 μL binding buffer at a density of 1 × 106cells/mL, and treated with 5 μL Annexin V-FITC and propidium iodide at 2—8°C in the dark. Stained cells were evaluated by flow cytometry (Beckman-Coulter, Miami, FL, USA) at a wavelength of 488 nm. Fluorescein isothiocyanate (FITC) and propidium iodide emissions were measured. Apoptotic analysis was represented by the ratio of apoptotic cells to total cells.
NSC differentiation assay
Determination of intracellular calcium ([Ca2+]i) concentration in NSCs
Statistical analysis
Data are expressed as the mean ± SEM and were analyzed using SPSS 15.0 soware (SPSS, Chicago, IL, USA). One-way analysis of variance and Bonferronipost hoctests were used. A value ofP< 0.05 was considered statistically significant.
Results
Morphology and identification of NSCs
Hippocampal cells were isolated from neonatal rats (postnatal day 1) and cultured in NSC culture medium (Figure 1A). Suspended single NSCs formed small neurospheres (composed of 10—20 cells) on day 1 (Figure 1B), and large neurospheres (composed of 50—100 cells) on day 7 aer culture (Figure 1C). NSCs were split from large neurospheres into suspended NSCs (almost single cells), and cultured in differentiation culture medium (Figure 1D). Differentiation of NSCs to astroglia (marked with GFAP) and neurons (marked with β-tubulin) (Lu et al., 2012a, b; Li et al., 2014) was observed on days 4 and 12 aer culture.
Effect of propofol, remifentanil, and GM1 on NSC proliferation
Effect of propofol, remifentanil, and GM1 on NSC apoptosis during proliferation and differentiation
The effect of propofol, remifentanil, and GM1 on NSC apoptosis was examined during both proliferation and differentiation. In proliferation culture medium, intralipids and GM1 did not affect the apoptotic percentage of NSCs compared with the control group (P> 0.05;Figure 2B). Combined treatment of propofol (1 μg/mL) and remifentanil (5 ng/mL) increased the percentage of apoptotic cells compared with the control group (P< 0.001). Treatment with both propofol and remifentanil induced NSC apoptosis in a dose-dependent manner (Figure 2B). Co-treatment of GM1 with propofol and remifentanil decreased the percentage of apoptotic cells compared with propofol and remifentanil at low and moderate doses (P< 0.05;Figure 2B). However, co-treatment of GM1 (at 50 μg/mL) with propofol and remifentanil did not significantly decrease the percentage of apoptotic NSCs compared with propofol (5.0 μg/mL) and remifentanil (20 ng/mL) at high doses (P> 0.05;Figure 2B).
In differentiation culture medium, intralipids and GM1 did not affect the percentage of apoptotic cells (P> 0.05). Combined propofol and remifentanil induced NSC apoptosis in a dose-dependent manner (P< 0.001). Co-treatment of GM1 (at 12.5 μg/mL or 25 μg/mL) with propofol and remifentanil decreased the percentage of apoptotic NSCscompared with propofol and remifentanil at the same dose (P< 0.05;Figure 2C). Nevertheless, co-treatment of GM1 (at 50 μg/mL) with propofol and remifentanil did not signif icantly decrease the percentage of apoptotic NSCs compared with propofol (5.0 μg/mL) and remifentanil (20 ng/mL) at high doses (P> 0.05;Figure 2C).
Effect of propofol, remifentanil, and/or GM1 on NSC differentiation
The effect of drug treatment on NSC differentiation was examined by measuring the percentage of cells labeled with GFAP or β-tubulin. Intralipids and GM1 did not affect GFAP or β-tubulin cell percentage (P> 0.05). Compared with the control group, combined propofol (5 μg/mL) and remifentanil (20 ng/mL) decreased β-tubulin(+) cell percentage (P< 0.05) and increased GFAP(+) cell percentage (P< 0.05). Moreover, the other groups showed no significant changes in β-tubulin(+) or GFAP(+) cell percentage (Figures 3A,B,and 4).
Effect of propofol, remifentanil, and/or GM1 on intracellular Ca2+([Ca2+]i) concentration of NSCs
Incubation of intralipids and GM1 did not affect [Ca2+]iof NSCs. Moreover, combined propofol (1 μg/mL) and remifentanil (5 ng/mL) decreased [Ca2+]iin NSCs compared with propofol (1 μg/mL) (P< 0.05). Co-treatment of propofol and remifentanil decreased NSC [Ca2+]ilevels in a dose-dependent manner (P< 0.01 or 0.001). Co-treatment of GM1 with propofol and remifentanil increased [Ca2+]ilevels compared with propofol and remifentanil at the same dosage (P< 0.05;Figure 3C).
Discussion
Major hippocampal functions in the brain include memory formation and navigation. Proliferation and differentiation of these NSCs and hippocampal neural progenitor cells are associated with behavioral and cognitive states, including memory and learning (Deng et al., 2010; Shetty, 2014; Blaya et al., 2015; Gu et al., 2015; Iggena et al., 2017).erefore, NSCs and hippocampal neural progenitor cells are widely used asin vitromodels in anesthesia-induced neurotoxicity studies. Previously, our research and other studies have shown that general anesthesia exerts damaging effects on the immature central nervous system, especially at moderate and high concentrations (Davidson, 2011; Braz et al., 2012; Creeley et al., 2013; Li et al., 2014; Van Biesen et al., 2015; Satomoto and Makita, 2016; Ward et al., 2016; Earley et al., 2017; Jevtovic-Todorovic et al., 2017; McCann and de Graaff, 2017; Vutskits and Davidson, 2017; Walters and Paule, 2017; Zanghi and Jevtovic-Todorovic, 2017). In rats at postnatal day 14, but not adult (postnatal day 60), repeated isoflurane infusion causes impairment of reversal learning and object recognition, a decrease in the hippocampal stem cell pool, and neurogenesis reductionin vivo. Furthermore, such deficits and impairments induced by isoflurane exposure become worse when affected rats get older (Zhu et al., 2010; Cheng and Levy, 2014; Schenning et al., 2017). Consequently, we used anin vitromodel of NSCs derived from neonatal rat (postnatal day 1) in this study.
GM1 is the main constituent of lipid rafts in the outer plasma membrane layer of neurons and is distributed in various brain areas (Skaper et al., 1991; Baek et al., 2010; Colsch et al., 2011; Ji et al., 2015; Dalton et al., 2017). In the immature hippocampus, GM1 signaling plays important roles in neural activities, such as axonal growth and neuronal differentiation. Plasma membrane ganglioside sialidase is essential for synthesis of endogenous GM1 and is expressed at high abundance in the developing hippocampus (Rodriguez et al., 2001; Iwamori et al., 2005; Schneider et al., 2015). Neurons overexpressing plasma membrane ganglioside sialidase show enhanced axon growth and accelerated polarizationin vitro(Rodriguez et al., 2001; Iwamori et al., 2005; Schneider et al., 2015). Moreover, GM1 has neuroprotective properties in the central nervous system under various pathological conditions. Exogenous GM1 prevents loss of neurogenesis induced by D-galactose in the mouse hippocampus (Zhang et al., 2005). Transgenic mice without GM1 synthesis show impaired neuronal activity, including dysfunction of calcium regulation, loss of neuroprotection, and enhanced susceptibility to kainate-induced neuronal apoptosis (Wu et al., 2005; Huang et al., 2007; Wu et al., 2016; Zanghi and Jevtovic-Todorovic, 2017). Recently, studies also found a potential association between GM1 signaling and anesthesia-induced neurotoxicity. Accordingly, in the current study, we first investigated the neuroprotective effects of GM1 on NSCs against neurotoxicity induced by propofol combined with remifentanil.
The calcium signaling pathway plays modulative roles in neurogenesis and differentiation of NSCs derived from the immature brain, while dysregulation of intracellular calcium is associated with developmental disorders (Slusarski and Pelegri, 2007; U et al., 2014; Deng et al., 2015; Wang et al., 2016). Here, we found that propofol combined with remifentanil decreased [Ca2+]ilevels in NSCs in a dose-dependent manner. Direct evidence for a modulative role of GM1 in neuronal calcium signaling has not yet been found.e present results suggest that neuronal calcium signaling may account for combined propofol and remifentanil-induced neurotoxicity, as well as GM1 induced neuroprotection of NSCs.
GM1 appears to alleviate combined propofol and remifentanil-induced neurotoxicity, especially at moderate and high concentrations, in NSCsin vitro. Neuronal calcium signaling may account for the combined propofol and remifentanil-induced neurotoxicity and neuroprotective effects of GM1. Our findings raise concerns about the potential central nervous system adverse effects of propofol and remifentanil in neonatal anesthesia.e protective role of GM1 in the immature central nervous system warrants further investigation.
Author contributions:JL, QL and CWC were responsible for study conception and paper authorization. QL and JL designed, performed and completed this work, analyzed the data and wrote the paper. XL and XQY took part in analyzing the data and modifying the paper. SKC, CM, HXT, YW, XYW and CWC took part in modifying the paper. QL and JL con-tributed to the funds. All authors approved the final version of the paper.
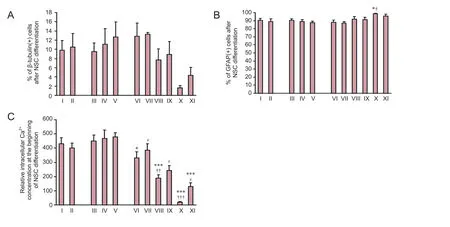
Figure 3 Effect of propofol, remifentanil, and/or GM1 on neural stem cell (NSC) differentiation.
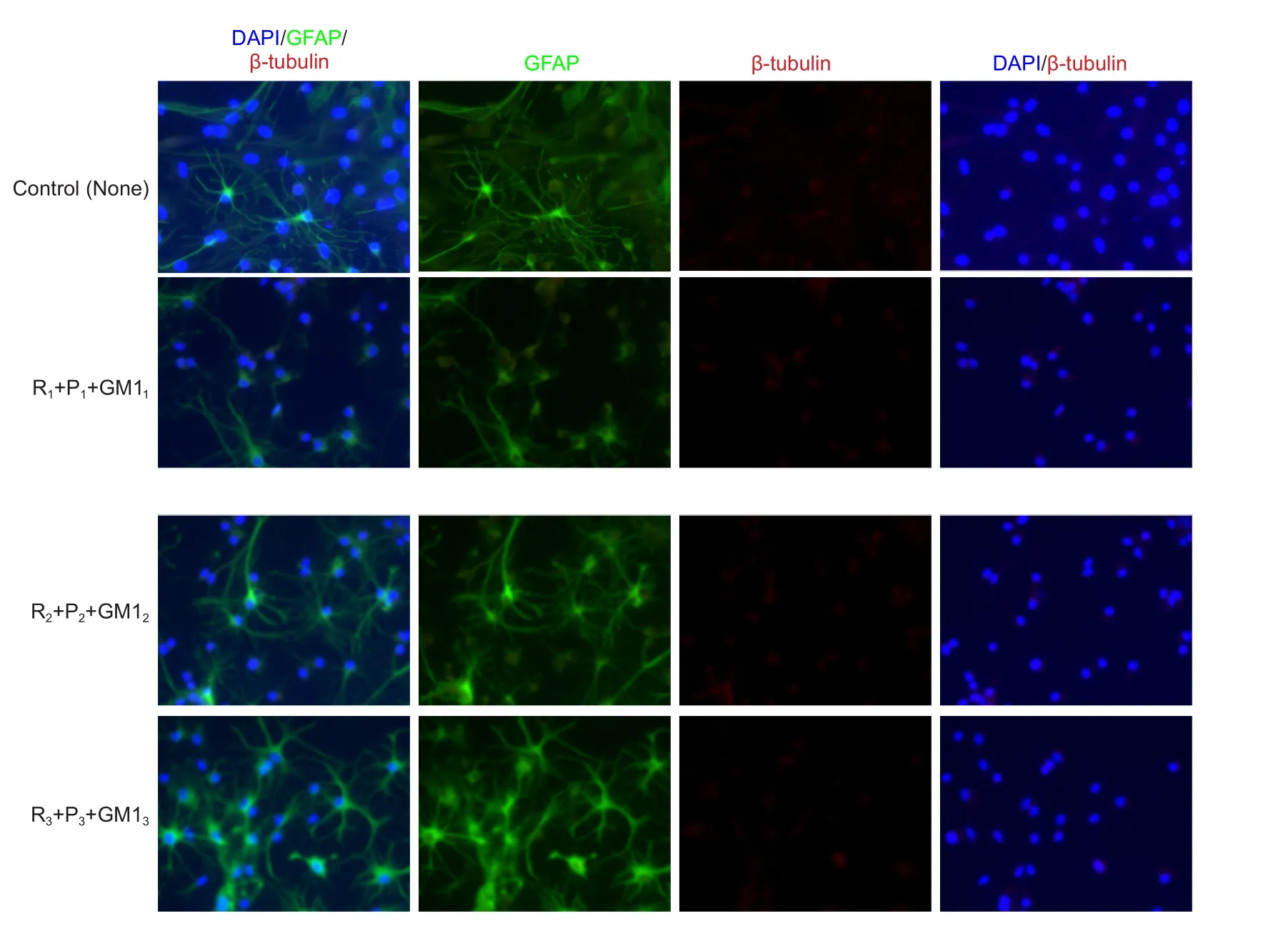
Figure 4 Effects of different concentrations of propofol combined with remifentanil and monosialoganglioside 1 (GM1) on neural stem cell (NSC) morphology (immunofluorescence staining, × 400).
Conflicts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Atkins JH, Mandel JE (2013) Abdominal relaxation during emergence from general anesthesia with propofol and remifentanil. J Clin Anesth 25:106-109.
Baek RC, Broekman ML, Leroy SG, Tierney LA, Sandberg MA, d’Azzo A, Seyfried TN, Sena-Esteves M (2010) AAV-mediated gene delivery in adult GM1-gangliosidosis mice corrects lysosomal storage in CNS and improves survival. PLoS One 5:e13468.
Beers SR, Rofey DL, McIntyre KA (2014) Neurodevelopmental assessment aer anesthesia in childhood: review of the literature and recommendations. Anesth Analg 119:661-669.
Bilotta F, Gelb AW, Stazi E, Titi L, Paoloni FP, Rosa G (2013) Pharmacological perioperative brain neuroprotection: a qualitative review of randomized clinical trials. Br J Anaesth 110 Suppl 1:i113-120.
Blaya MO, Tsoulfas P, Bramlett HM, Dietrich WD (2015) Neural progenitor cell transplantation promotes neuroprotection, enhances hippocampal neurogenesis, and improves cognitive outcomes aer traumatic brain injury. Exp Neurol 264:67-81.
Braz MG, Braz LG, Mazoti MA, Pinotti MF, Pardini MI, Braz JR, Salvadori DM (2012) Lower levels of oxidative DNA damage and apoptosis in lymphocytes from patients undergoing surgery with propofol anesthesia. Environ Mol Mutagen 53:70-77.
Cheng Y, Levy RJ (2014) Subclinical carbon monoxide limits apoptosis in the developing brain aer isoflurane exposure. Anesth Analg 118:1284-1292.
Cho YJ, Kim TK, Hong DM, Seo JH, Bahk JH, Jeon Y (2017) Effect of desflurane-remifentanil vs. Propofol-remifentanil anesthesia on arterial oxygenation during one-lung ventilation for thoracoscopic surgery: a prospective randomized trial. 17:9.
Colsch B, Jackson SN, Dutta S, Woods AS (2011) Molecular microscopy of brain gangliosides: illustrating their distribution in hippocampal cell layers. ACS Chem Neurosci 2:213-222.
Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A (2013) Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br J Anaesth 110 Suppl 1:i29-38.
Dalton G, An SW, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, Hilgemann DW, Xie J, Luby-Phelps K, Kohler JJ, Birnbaumer L, Huang CL (2017) Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci U S A 114:752-757.
Davidson AJ (2011) Anesthesia and neurotoxicity to the developing brain: the clinical relevance. Paediatr Anaesth 21:716-721.
Deng S, Hou G, Xue Z, Zhang L, Zhou Y, Liu C, Liu Y, Li Z (2015) Vitamin E isomer delta-tocopherol enhances the efficiency of neural stem cell differentiation via L-type calcium channel. Neurosci Lett 585:166-170.
Deng W, Aimone JB, Gage FH (2010) New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci 11:339-350.
Earley MA, Pham LT, April MM (2017) Scoping review: Awareness of neurotoxicity from anesthesia in children in otolaryngology literature. Laryngoscope doi: 10.1002/lary.26485.
Gleich SJ, Flick R, Hu D, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson A, Buenvenida S, Wilder RT, Sprung J, Voigt RG, Paule MG, Chelonis JJ, Warner DO (2015) Neurodevelopment of children exposed to anesthesia: design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemp Clin Trials 41:45-54.
Gu G, Zhang W, Li M, Ni J, Wang P (2015) Transplantation of NSC-derived cholinergic neuron-like cells improves cognitive function in APP/PS1 transgenic mice. Neuroscience 291:81-92.
Hogue CW, Jr., Bowdle TA, O’Leary C, Duncalf D, Miguel R, Pitts M, Streisand J, Kirvassilis G, Jamerson B, McNeal S, Batenhorst R (1996) A multicenter evaluation of total intravenous anesthesia with remifentanil and propofol for elective inpatient surgery. Anesth Analg 83:279-285.
Huang F, Liu Z, Liu H, Wang L, Wang H, Li Z (2007) GM1 and NGF modulate Ca2+ homeostasis and GAP43 mRNA expression in cultured dorsal root ganglion neurons with excitotoxicity induced by glutamate. Nutr Neurosci 10:105-111.
Iggena D, Winter Y, Steiner B (2017) Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J Pineal Res 62.
Iwamori M, Kaido T, Iwamori Y, Ohta Y, Tsukamoto K, Kozaki S (2005) Involvement of the C-terminal tail of Arthrobacter ureafaciens sialidase isoenzyme M in cleavage of the internal sialic acid of ganglioside GM1. J Biochem 138:327-334.
Jevtovic-Todorovic V, Bushnell PJ, Paule MG (2017) Introduction to the special issue “Developmental neurotoxicity associated with pediatric general anesthesia: Preclinical findings”. Neurotoxicol Teratol 60:1.
Ji J, Yan X, Li Z, Lai Z, Liu J (2015)erapeutic effects of intrathecal versus intravenous monosialoganglioside against bupivacaine-induced spinal neurotoxicity in rats. Biomed Pharmacother 69:311-316.
Kreutz F, Frozza RL, Breier AC, de Oliveira VA, Horn AP, Pettenuzzo LF, Netto CA, Salbego CG, Trindade VM (2011) Amyloid-beta induced toxicity involves ganglioside expression and is sensitive to GM1 neuroprotective action. Neurochem Int 59:648-655.
Kreutz F, Scherer EB, Ferreira AG, Petry Fdos S, Pereira CL, Santana F, de Souza Wyse AT, Salbego CG, Trindade VM (2013) Alterations on Na(+),K(+)-ATPase and acetylcholinesterase activities induced by amyloid-beta peptide in rat brain and GM1 ganglioside neuroprotective action. Neurochem Res 38:2342-2350.
Kuroyanagi A, Nagata O, Matsunaga A, Terashi T, Kanmura Y (2015)e effect of remifentanil on the estimated target effect-site concentration (esTEC) of propofol during total intravenous anesthesia. Masui 64:116-122.
Lee U, Mashour GA, Kim S, Noh GJ, Choi BM (2009) Propofol induction reduces the capacity for neural information integration: implications for the mechanism of consciousness and general anesthesia. Conscious Cogn 18:56-64.
Li Q, Lu J, Wang X (2014) Propofol and remifentanil at moderate and high concentrations affect proliferation and differentiation of neural stem/progenitor cells. Neural Regen Res 9:2002-2007.
Lu J, Li D, Lu K (2012a) Distribution and localization of fibroblast growth factor-8 in rat brain and nerve cells during neural stem/progenitor cell differentiation. Neural Regen Res 7:1455-1462.
Lu J, Lu K, Li D (2012b) Changes in expression and secretion patterns of fibroblast growth factor 8 and Sonic Hedgehog signaling pathway molecules during murine neural stem/progenitor cell differentiation in vitro. Neural Regen Res 7:1688-1694.
McCann ME, de Graaff J (2017) Current thinking regarding potential neurotoxicity of general anesthesia in infants. Curr Opin Urol 27:27-33.
O’Connor S, Zhang YL, Christians U, Morrison JE, Jr., Friesen RH (2016) Remifentanil and propofol undergo separation and layering when mixed in the same syringe for total intravenous anesthesia. Paediatr Anaesth 26:703-709.
Rodriguez JA, Piddini E, Hasegawa T, Miyagi T, Dotti CG (2001) Plasma membrane ganglioside sialidase regulates axonal growth and regeneration in hippocampal neurons in culture. J Neurosci 21:8387-8395.
SanMartin A, Johansson F, Samuelson L, Prinz CN (2014) Microarray analysis reveals moderate gene expression changes in cortical neural stem cells cultured on nanowire arrays. J Nanosci Nanotechnol 14:4880-4885.
Satomoto M, Makita K (2016) Anesthesia-induced neurotoxicity in an animal model of the developing brain: mechanism and therapies. Neural Regen Res 11:1407-1408.
Scheller C (2014) Pharmacological perioperative brain neuroprotection: nimodipine? Br J Anaesth 112:178-179.
Schenning KJ, Noguchi KK, Martin LD, Manzella FM, Cabrera OH, Dissen GA, Brambrink AM (2017) Isoflurane exposure leads to apoptosis of neurons and oligodendrocytes in 20- and 40-day old rhesus macaques. Neurotoxicol Teratol 60:63-68.
Schneider JS, Seyfried TN, Choi HS, Kidd SK (2015) Intraventricular sialidase administration enhances GM1 ganglioside expression and is partially neuroprotective in a mouse model of parkinson’s disease. PLoS One 10:e0143351.
Scott HB, Choi SW, Wong GT, Irwin MG (2017)e effect of remifentanil on propofol requirements to achieve loss of response to command vs. loss of response to pain. Anaesthesia 72:479-487.
She JQ, Wang M, Zhu DM, Tang M, Chen JT, Wang L, Ruan DY (2009) Monosialoanglioside (GM1) prevents lead-induced neurotoxicity on long-term potentiation, SOD activity, MDA levels, and intracellular calcium levels of hippocampus in rats. Naunyn Schmiedebergs Arch Pharmacol 379:517-524.
Shetty AK (2014) Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection? Epilepsy Behav 38:117-124.
Skaper SD, Mazzari S, Vantini G, Facci L, Toffano G, Leon A (1991) Monosialoganglioside GM1 and modulation of neuronal plasticity in CNS repair processes. Adv Exp Med Biol 296:257-266.
Slusarski DC, Pelegrif(2007) Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol 307:1-13.
Sun LS (2016) Introduction to the Proceedings of the Fifth PANDA Symposium on “Anesthesia and Neurodevelopment in Children”. J Neurosurg Anesthesiol 28:349.
U KP, Subramanian V, Nicholas AP, Thompson PR, Ferretti P (2014) Modulation of calcium-induced cell death in human neural stem cells by the novel peptidylarginine deiminase-AIF pathway. Biochim Biophys Acta 1843:1162-1171.
Van’t Veer A, Du Y, Fischer TZ, Boetig DR, Wood MR, Dreyfus CF (2009) Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res 87:69-78.
Van Biesen S, Van de Velde M, Rex S (2015) Anesthesia and neurotoxicity in the developing brain: A non-systematic review. Acta Anaesthesiol Belg 66:67-79.
van Kralingen S, van de Garde EM, van Dongen EP, Diepstraten J, Deneer VH, van Ramshorst B, Knibbe CA (2011) Maintenance of anesthesia in morbidly obese patients using propofol with continuous BIS-monitoring: a comparison of propofol-remifentanil and propofol-epidural anesthesia. Acta Anaesthesiol Belg 62:73-82.
Vescovi AL, Snyder EY (1999) Establishment and properties of neural stem cell clones: plasticity in vitro and in vivo. Brain Pathol 9:569-598.
Vutskits L, Davidson A (2017) Update on developmental anesthesia neurotoxicity. Curr Opin Anaesthesiol doi: 10.1097/ ACO.0000000000000461.
Walters JL, Paule MG (2017) Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol 60:2-23.
Wang C, Liu F, Patterson TA, Paule MG, Slikker W, Jr. (2016) Relationship between ketamine-induced developmental neurotoxicity and NMDA receptor-mediated calcium influx in neural stem cell-derived neurons. Neurotoxicology doi: 10.1016/j.neuro.2016.04.015.
Wang X, Xu Z, Miao CH (2014) Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: an updated systematic review with meta-regression. PLoS One 9:e85760.
Ward CG, Hines SJ, Maxwell LG (2016) Neurotoxicity, general anesthesia in young children, and a survey of current pediatric anesthesia practice at US teaching institutions. Paediatr Anaesth 26:60-65.
Wei LX, Deng XM, Wang L, Sui JH, Zhang YM, Tong SY, Tang GZ, Xu KL (2011) Induction of tracheal intubation without muscle relaxant by target controlled infusion of propofol combined with remifentanil in children. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 33:440-444.
Wen T, Bao K, Li H (2007) Blocking BE301622 gene expression by RNAi initiates differentiation of neural stem cells in rat. Cell Biochem Funct 25:775-779.
Wen T, Gu P, Minning TA, Wu Q, Liu M, Chen F, Liu H, Huang H (2002) Microarray analysis of neural stem cell differentiation in the striatum of the fetal rat. Cell Mol Neurobiol 22:407-416.
Wu G, Lu ZH, Andre S, Gabius HJ, Ledeen RW (2016) Functional interplay between ganglioside GM1 and cross-linking galectin-1 induces axon-like neuritogenesis via integrin-based signaling and TRPC5-dependent Ca(2)(+) influx. J Neurochem 136:550-563.
Wu G, Lu ZH, Wang J, Wang Y, Xie X, Meyenhofer MF, Ledeen RW (2005) Enhanced susceptibility to kainate-induced seizures, neuronal apoptosis, and death in mice lacking gangliotetraose gangliosides: protection with LIGA 20, a membrane-permeant analog of GM1. J Neurosci 25:11014-11022.
Yu Y, Gu S, Huang H, Wen T (2007) Combination of bFGF, heparin and laminin induce the generation of dopaminergic neurons from rat neural stem cells both in vitro and in vivo. J Neurol Sci 255:81-86.
Zanghi CN, Jevtovic-Todorovic V (2017) A holistic approach to anesthesia-induced neurotoxicity and its implications for future mechanistic studies. Neurotoxicol Teratol 60:24-32.
Zhang H, Du L, Du Z, Jiang H, Han D, Li Q (2015) Association between childhood exposure to single general anesthesia and neurodevelopment: a systematic review and meta-analysis of cohort study. J Anesth 29:749-757.
Zhang Q, Huang Y, Li X, Cui X, Zuo P, Li J (2005) GM1 ganglioside prevented the decline of hippocampal neurogenesis associated with D-galactose. Neuroreport 16:1297-1301.
Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K (2010) Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 30:1017-1030.
Copyedited by James R, Haase R, Yu J, Li CH, Qiu Y, Song LP, Zhao M
How to cite this article: Lu J, Yao XQ, Luo X, Wang Y, Chung SK, Tang HX, Cheung CW, Wang XY, Meng C, Li Q (2017) Monosialoganglioside 1 may alleviate neurotoxicity induced by propofol combined with remifentanil in neural stem cells. Neural Regen Res 12(6):945-952.
*Correspondence to:
Qing Li, M.D. or Jiang Lu, M.D., liqing8801@163.com or lulujiangjiang@hotmail.com.
orcid:
0000-0003-4557-5839
(Qing Li)
0000-0001-7553-1277
(Jiang Lu)
10.4103/1673-5374.208589
Accepted: 2017-03-05
- 中國神經(jīng)再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease