Brain injury in combination with tacrolimus promotes the regeneration of injured peripheral nerves
Xin-ze He, Jian-jun Ma, Hao-qi Wang, Tie-min Hu, Bo Sun Yun-feng Gao Shi-bo Liu Wei Wang, Pei Wang
1 Department of Hand and Foot Surgery, Af filiated Hospital of Chengde Medical College, Chengde, Hebei Province, China
2 Postgraduate School, Chengde Medical College, Chengde, Hebei Province, China
3 Department of Neurosurgery, Af filiated Hospital of Chengde Medical College, Chengde, Hebei Province, China
4 Department of Hand and Foot Surgery, the First Hospital of Qinhuangdao, Qinhuangdao, Hebei Province, China
5 Binzhou Central Hospital, Binzhou, Shandong Province, China
Brain injury in combination with tacrolimus promotes the regeneration of injured peripheral nerves
Xin-ze He1,5, Jian-jun Ma2, Hao-qi Wang2, Tie-min Hu3, Bo Sun1, Yun-feng Gao1, Shi-bo Liu1, Wei Wang4, Pei Wang1,*
1 Department of Hand and Foot Surgery, Af filiated Hospital of Chengde Medical College, Chengde, Hebei Province, China
2 Postgraduate School, Chengde Medical College, Chengde, Hebei Province, China
3 Department of Neurosurgery, Af filiated Hospital of Chengde Medical College, Chengde, Hebei Province, China
4 Department of Hand and Foot Surgery, the First Hospital of Qinhuangdao, Qinhuangdao, Hebei Province, China
5 Binzhou Central Hospital, Binzhou, Shandong Province, China
Graphical Abstract
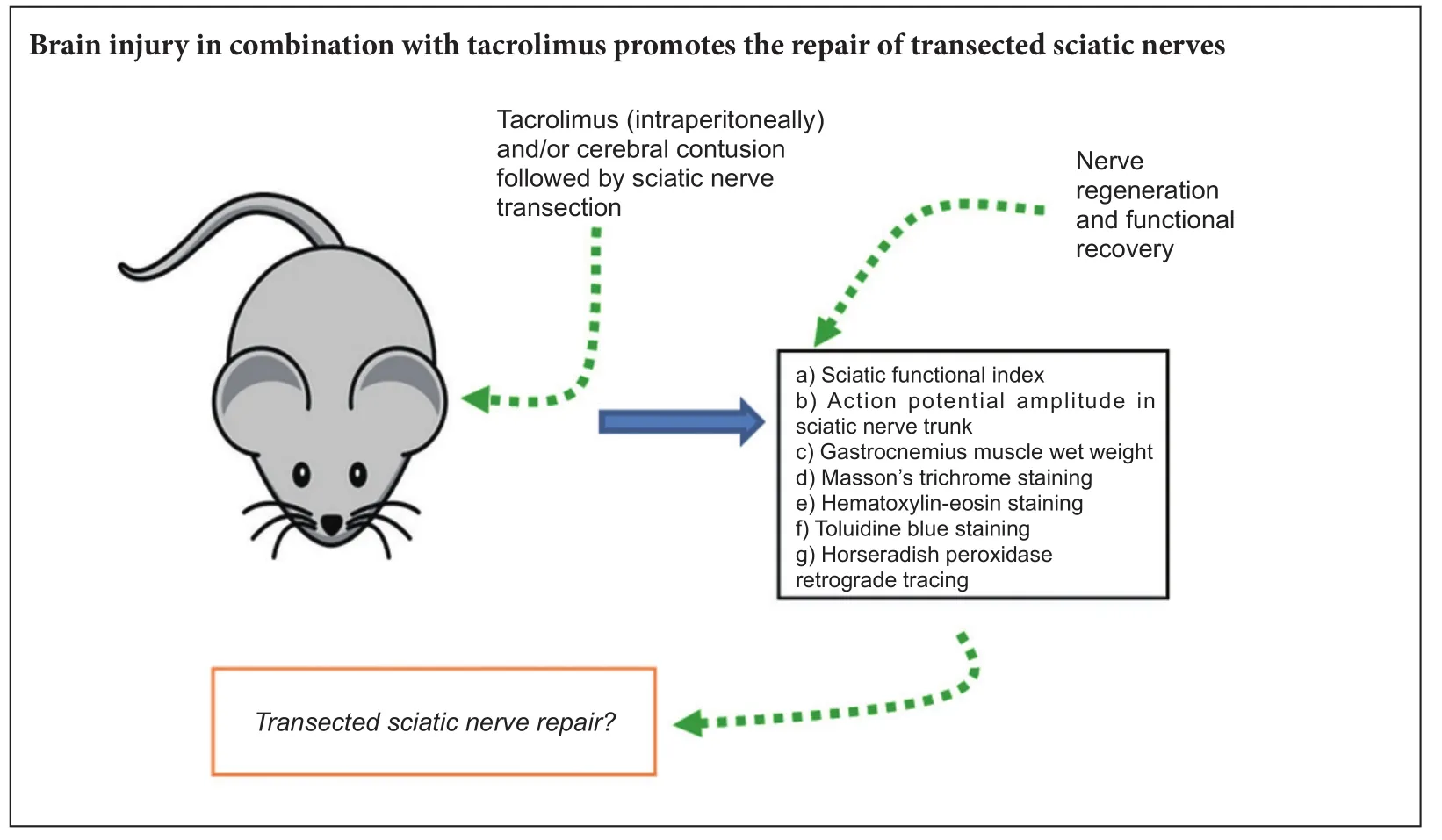
Both brain injury and tacrolimus have been reported to promote the regeneration of injured peripheral nerves. In this study, before transection of rat sciatic nerve, moderate brain contusion was (or was not) induced. Aer sciatic nerve injury, tacrolimus, an immunosuppressant, was (or was not) intraperitoneally administered. At 4, 8 and 12 weeks aer surgery, Masson’s trichrome, hematoxylin-eosin, and toluidine blue staining results revealed that brain injury or tacrolimus alone or their combination alleviated gastrocnemius muscle atrophy and sciatic nerve fiber impairment on the experimental side, simultaneously improved sciatic nerve function, and increased gastrocnemius muscle wet weight on the experimental side. At 8 and 12 weeks aer surgery, brain injury induction and/or tacrolimus treatment increased action potential amplitude in the sciatic nerve trunk. Horseradish peroxidase retrograde tracing revealed that the number of horseradish peroxidase-positive neurons in the anterior horn of the spinal cord was greatly increased. Brain injury in combination with tacrolimus exhibited better effects on repair of injured peripheral nerves than brain injury or tacrolimus alone.is result suggests that brain injury in combination with tacrolimus promotes repair of peripheral nerve injury.
nerve regeneration; brain injury; peripheral nerve; tacrolimus; toluidine blue staining; retrograde tracing; muscle atrophy; neural regeneration
Introduction
Brain injury is commonly associated with peripheral nerve damage (He et al., 2016). Brain injury has been shown to promote recovery of damaged peripheral nerves (Wang et al., 2014), however, the specific mechanism is not clear.
The immunosuppressant tacrolimus has been used to reduce immune rejection aer organ transplantation. Tacrolimus was first reported by Gold et al. (1994) to promote the regeneration of damaged peripheral nerves. Since then, several studies (Glaus et al., 2011; Mekaj et al., 2014, 2015a, b; Phillipset al., 2014; Zhao et al., 2014) have confirmed that tacrolimus contributes to peripheral nerve regenerationviaimmunosuppressive and neurotrophic pathways. It forms a complex with the binding region of tacrolimus binding protein (FKBP12), and then combines with the functional region of that protein, which increases the expression of growth associated protein-43 and contributes to the formation and extension of growth cones. Previous studies have shown that as damaged peripheral nerves recovered aer treatment with tacrolimus, action potentials began to reach target organs again, the inner diameter of the regenerated axon increased, the myelin sheath thickened, and the amplitude of action potentials increased (Mekaj et al., 2014; Carrasco et al., 2016).
In the present study, we investigated whether brain injury in combination with tacrolimus exhibits better effects on promoting regeneration of injured peripheral nerves than brain injury or tacrolimus alone.
Materials and Methods
Ethics statement
Animals
Establishment of rat models of brain injury combined with sciatic nerve injury
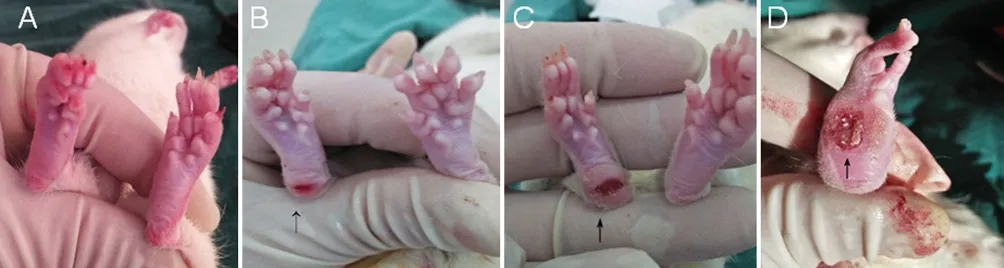
Figure 1 Hindpaw ulceration 12 weeks aer surgery.
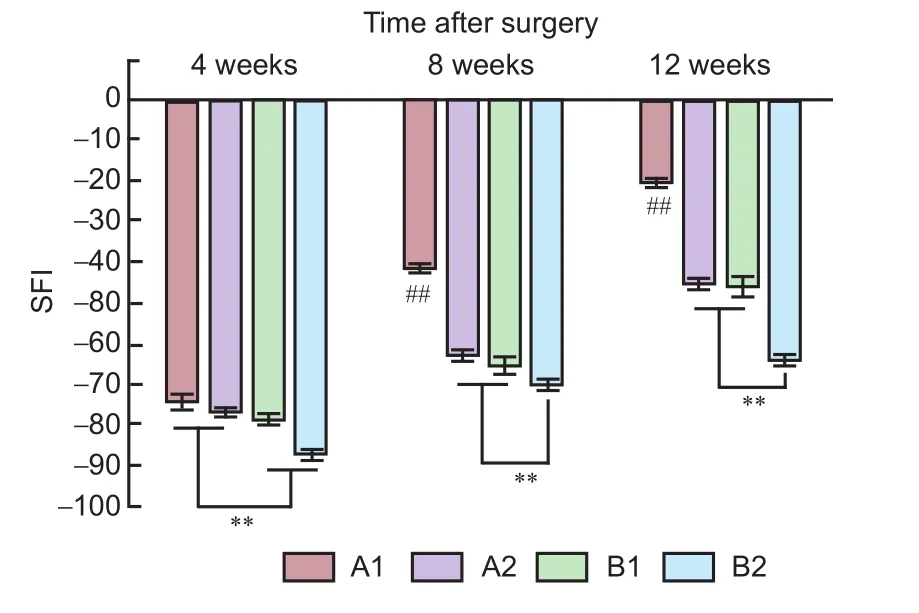
Figure 2 Sciatic function of rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment.
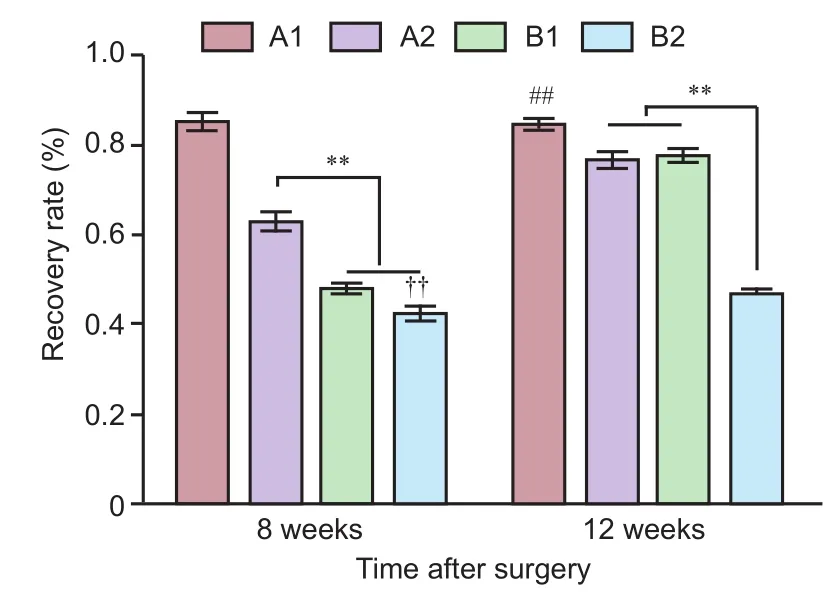
Figure 3 Recovery rate (%) of action potential amplitude in the sciatic nerve trunk in rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment.
Rats were injected with cefazolin sodium (100 mg/kg intramuscularly) 30 minutes before surgery. Surgical area was shaved. Rats were anesthetized with 10% chloral hydrate (3.5 mL/kg intraperitoneally) immediately before surgery.e sur-gical area was disinfected, and an aseptic towel was placed over the head with an aperture over the surgical site. A sagittal skin incision was made on the head, and a bone window (5 mm in diameter, 1.5 mm posterior to the coronal suture, and 5 mm to the leof the midline) was opened. A moderate contusion injury was induced using Feeney’s method (Feeney et al., 1981). The right sciatic nerve of rats was exposed, transected 1 cm below the lower hole of the piriformis of the rats, and then sutured with 9-0 nontraumatic thread using an epicardial suture technique under a surgical microscope (LZL-6A; Zhenjiang Zhongtian Optical instrument Co., Ltd., Zhenjiang, Jiangsu Province, China) at 12× magnification.e epineurium of the sciatic nerve was closed with 4—6 stitches using a 9/0 sterile medical non-absorbable sutures and non-destructive suture needle (Hangzhou Fu Yang Medical Suture Needle Factory, Hangzhou, Zhejiang Province, China) (Cheng et al., 2015).
Tacrolimus administration
Measurement of sciatic functional index (SFI)

Figure 4 Denervated (arrows) and contralateral (unlabeled) gastrocnemius muscles of rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment.
Recovery rate of action potential amplitude in sciatic nerve trunk
At 8 and 12 weeks after sciatic nerve surgery, five rats were randomly selected from each group. Stimulating electrode 1 was placed 0.5 cm distal to the anastomotic stoma, and stimulating electrode 2 was placed 3 mm proximal to it.e recording electrodes were placed near the nerve root. Electrical signal was input directly into the BL-420F bio-function experiment system (Chengdu Techman Soware Co., Ltd., Chengdu, Sichuan Province, China). The recovery rate of action potential amplitude in the sciatic nerve trunk was calculated to assess nerve regeneration (Schiaveto de Souza et al., 2004). Recovery rate of action potential amplitude = action potential amplitude recorded by stimulating electrode 1/action potential amplitude recorded by stimulating electrode 2 × 100%.
Recovery rate of gastrocnemius muscle wet weight
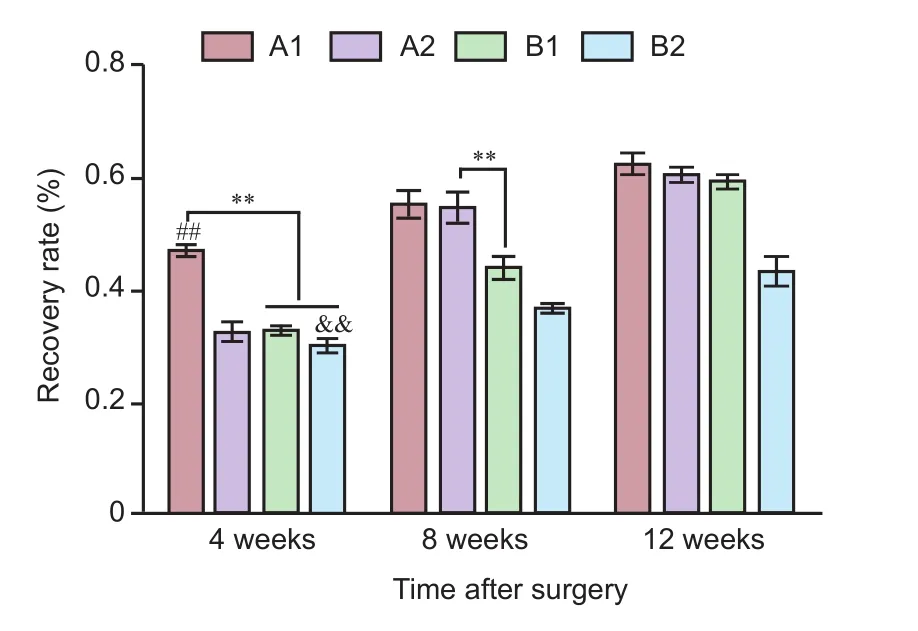
Figure 5 Recovery rate (%) of gastrocnemius muscle wet weight in rats with sciatic nerve injury following brain injury induction and/ or tacrolimus treatment.
Masson’s trichrome staining of gastrocnemius muscle
Hematoxylin-eosin staining of sciatic nerve
Masson’s trichrome staining of sciatic nerve
Toluidine blue staining of sciatic nerve
Horseradish peroxidase (HRP) tracing
At 8 and 12 weeks after surgery, 5 μL 30% HRP was infused into the tissue 0.5 cm distal to the sciatic nerve stump.oracotomy was conducted under deep anesthesia. Warm physiological saline (150 mL) was perfused rapidly through the leventricle to wash the blood vessels, and tissues were fixed with 500 mL 2% paraformaldehyde and 1.25% glutaraldehyde prepared in 0.2 M fresh phosphate buffer at 4°C.e corresponding spinal segment of the sciatic nerve was cut transversely into 30 μm-thick serial sections using a vibratome (VT 1000S, Solms, Germany). Sections were visualized with benzidine dihydrochloride (Kennelly, 2006). Cells containing blue-stained particles in motor neurons of the anterior horn of the spinal cord were counted under a light microscope (BH-3; Olympus).
Statistical analysis
Measurement data, expressed as the mean ± SD, were analyzed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA). Groups were compared using one-way analysis of variance followed by the Student-Newman-Keuls test.P< 0.05 was considered statistically significant.
Results
General observation of rats aer model establishment
All animals survived, and no wound infections were noted. Recovery time after anesthesia was 85 ± 2 minutes in groups A1 and A2, and 64 ± 2 minutes in groups B1 and B2. In both groups A1 and A2, appetite and activity of the rats appeared reduced. The character of stool and urine changed. One week later, the rats’ food intake returned to normal, but the character of the stool and urine was not restored. Three days after surgery, redness and swelling of the feet were marked in group B2, but mild in other groups. Active flexion of the right lower limb was limited, and dorsiflexion deformity of the right foot was seen.e heel touched the ground during walking.ree weeks aer model induction, varying degrees of ulceration were visible in the rat heel in groups A2, B1 and B2. No visible ulceration was detected in group A1. At 12 weeks, ulcers were mostly healed in all groups, and autophagy was noted in the feet of some rats in group B2 (Figure 1).
SFI
Recovery rate of action potential amplitude in sciatic nerve trunk
At 8 weeks after surgery, recovery was the best in group A1 compared with all other groups. Recovery was better in group A2 than in groups B1 and B2 (P< 0.01), and better in group B1 than in group B2 (P< 0.01). At 12 weeks aer surgery, recovery was better in group A1 than in all other groups (P< 0.01). Groups A2 and B1 also showed better recovery than group B2 (P< 0.01) (Figure 3).
Recovery rate of gastrocnemius muscle wet weight
At 4 weeks after surgery, the denervated gastrocnemius muscle was paler in color than the contralateral gastrocnemius, and amyotrophy was evident in each group. At 8 and 12 weeks aer surgery, recovery of the muscle was markedlybetter in groups A1, A2 and B1 than in group B2 (Figure 4). At 4 weeks aer surgery, muscle wet weight recovery rate was greater in group A1 than in all other groups (P< 0.01). At 8 and 12 weeks aer surgery, recovery rate of gastrocnemius muscle wet weight was better in group A1 than in groups B1 and B2, and better in groups A2 and B1 than in group B2 (P< 0.01). At 8 weeks aer surgery, recovery was better in group A2 than in group B1 (P< 0.01), whereas by 12 weeks aer surgery, no significant difference was observed between groups A2 and B1 (P> 0.01) (Figure 5).
Masson’s trichrome staining of gastrocnemius muscle
Hematoxylin-eosin staining of sciatic nerves
At 4 weeks after surgery, endoneurial tube formation was induced by Schwann cell aggregation, and a few mononuclear macrophages were visible in group A1. In groups A2 and B1, aggregation of mononuclear macrophages was observed, and the endoneurial tube was not formed by distinct Schwann cells. Similarly, mononuclear macrophage aggregation was observed in group B2. At 8 weeks aer surgery, regenerated axonal sprouts were uniformly distributed, and mononuclear macrophages and fibroblasts were occasionally observed in group A1. Endoneurial tube formation was induced by Schwann cell aggregation. Mononuclear macrophages and a few fibroblasts were visible in groups A2 and B1, and marked macrophage aggregation was observed in group B2. At 12 weeks aer surgery, Schwann cells and regenerated axonal sprouts were uniformly distributed and a few fibroblasts were visible in group A1. Scattered fibroblasts, Schwann cells and regenerated axonal sprouts were seen in groups A2 and B1. Distinct macrophages were visible in group B2 (Figure 7).
Masson’s trichrome staining of sciatic nerves
Toluidine blue staining of sciatic nerves
HRP tracing of neurons in the spinal cord
Under the light microscope, dark blue HRP-positive neurons were found in the anterior horn of the spinal cord in each group (Figure 10A). At 8 weeks aer surgery, significant differences in the number of HRP-positive particles per high-power field were observed between the groups (P< 0.01). The rate (stained cells/total cells × 100 %) of HRP-positive neurons was higher in group A1 than in all other groups, higher in group A2 than in groups B1 and B2, and higher in group B1 than in group B2. At 12 weeks aer surgery, the rate of HRP-positive neurons was significantly higher in group A1 than in all other groups (P< 0.01).ere were more HRP-positive neurons in groups A2 and B1 than in group B2 (P< 0.01) (Figure 10B).
Discussion
Brain injury is known to promote the repair of peripheral nerve injury (Wang et al., 2014). Here, we investigated the mechanism underlying this effect by comparing it to the effects of tacrolimus, which is also known to improve nerve regeneration aer injury. We found that sciatic nerve recovery was better in animals that underwent brain injury and received tacrolimus than in those that received either of those interventions alone, or no intervention, aer injury.is indicates that brain injury promotes the recovery of peripheral nerve injuryviaa different mechanism of action to tacrolimus (Reddaway et al., 2012; Lu et al., 2015; Ackerman et al., 2016).
When a peripheral nerve receives a class V Sunderland lesion, the loss of innervation to the target organ results in an interruption of nutrient supply from the afferent neurons, leading to target organ atrophy, intractable ulcers in pressure areas (Wade et al., 2013; Shu et al., 2015), poorer Sfi(Farjah and Fazli, 2015; Dong et al., 2016), and a decrease in target muscle wet weight (Wang et al., 2014).
Tacrolimus promotes peripheral nerve repair by a relatively well understood mechanism (Glaus et al., 2011; Mekaj et al., 2014, 2015a, b; Phillips et al., 2014; Zhao et al., 2014). It exerts its neurotrophic effects in two ways. It forms a complex with the binding region of tacrolimus binding protein (FKBP12), and then combines with the functional region of that protein, which increases the expression of growth associated protein-43 and contributes to the formation and extension of growth cones. Previous studies showed that as damaged nerves recovered after treatment with tacrolimus, action potentials began to reach target organs again, the inner diameter of the regenerated axon increased, the myelin sheath thickened, and the amplitude of action potentials increased (Mekaj et al., 2014; Carrasco et al., 2016).e present results confirmed the action of tacrolimus in the promotion of peripheral nerve recovery aer injury. Rats treated with tacroli-mus showed milder ulceration and improvements in SFI, gastrocnemius muscle recovery, and action potential amplitude.e effects were better in the group that received tacrolimus and brain injury than in the group that received tacrolimus without brain injury, suggesting that the mechanism by which brain injury promotes peripheral nerve repair is different from that of the tacrolimus—FKBP12 complex, which involves an increase in growth associated protein-43 expression. Further studies are needed to determine the specific mechanism.
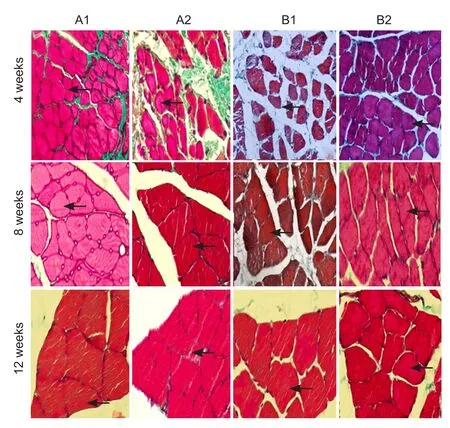
Figure 6 Masson’s trichrome staining of denervated skeletal muscle with sciatic nerve injury following brain injury induction and/or tacrolimus treatment (× 400).
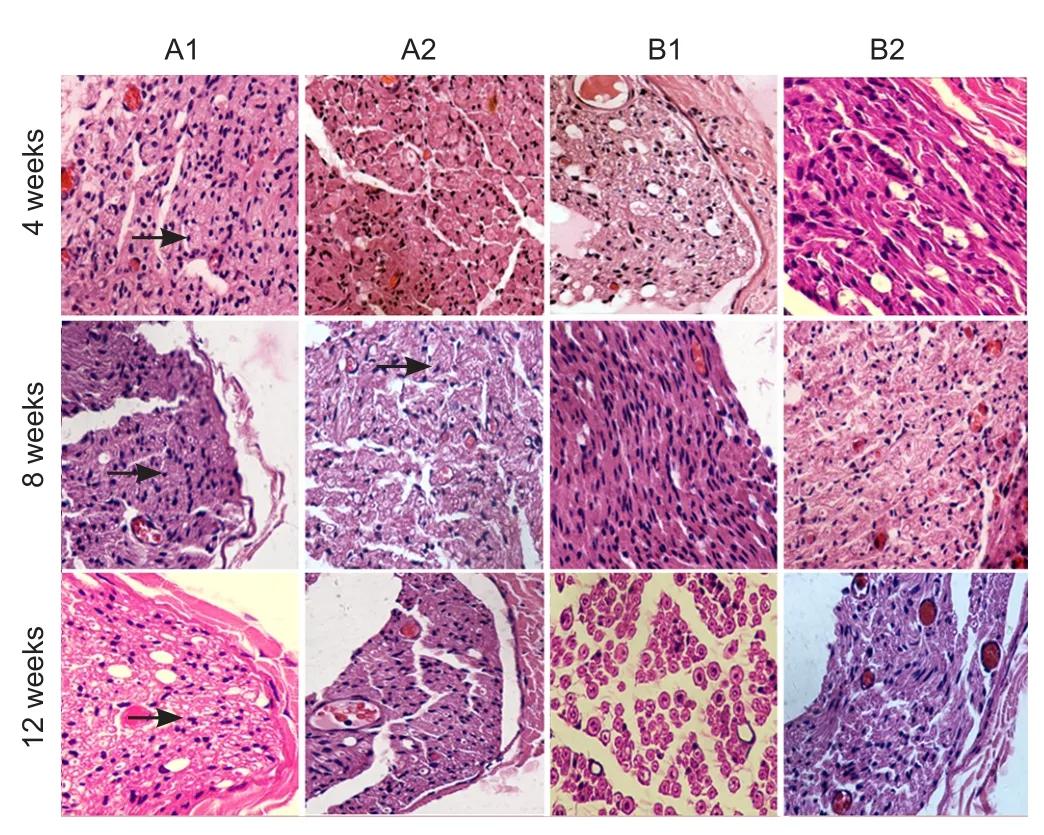
Figure 7 Hematoxylin-eosin staining of sciatic nerves of rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment (× 400).
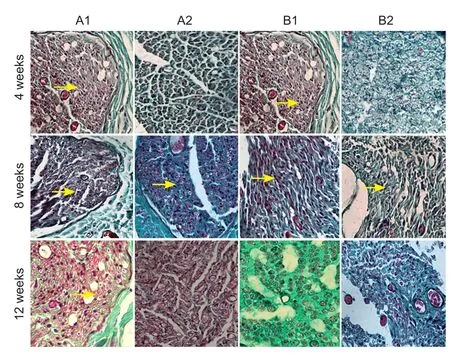
Figure 8 Masson’s trichrome staining of sciatic nerves of rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment (× 400).
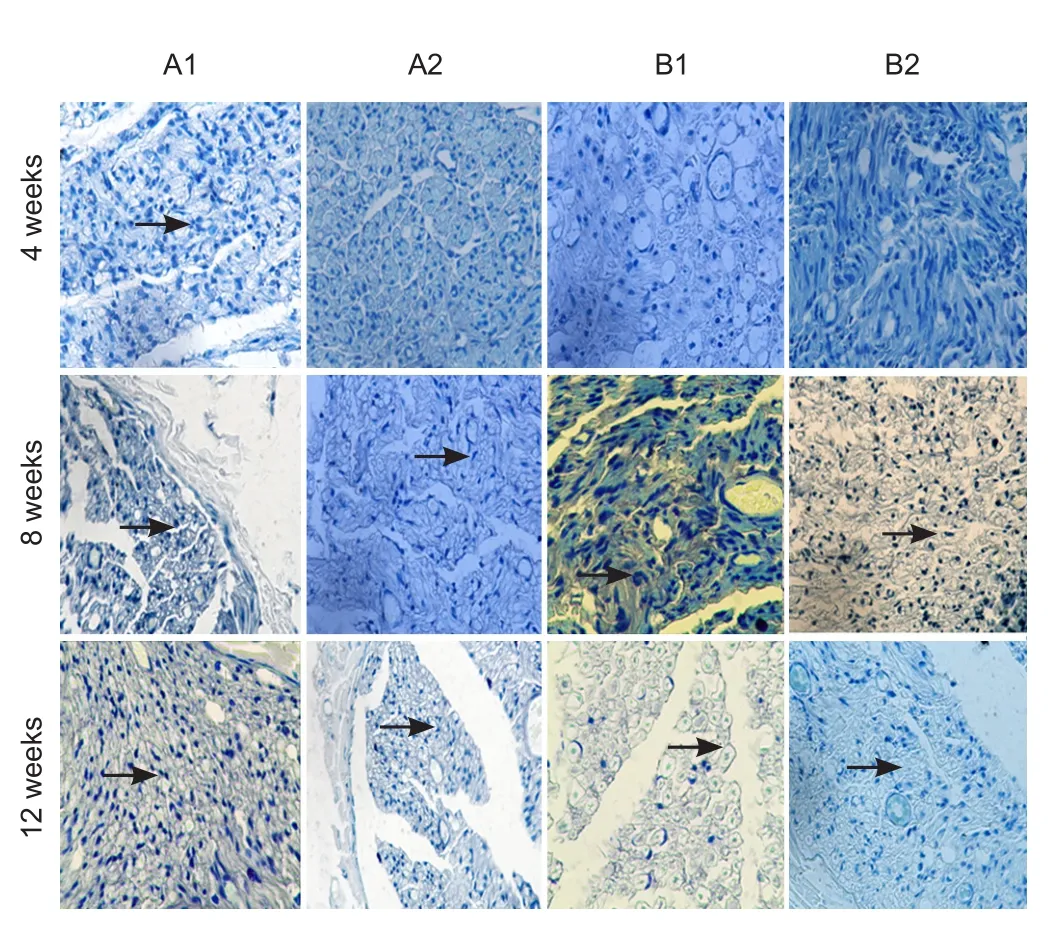
Figure 9 Toluidine blue staining of sciatic nerves of rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment (× 400).
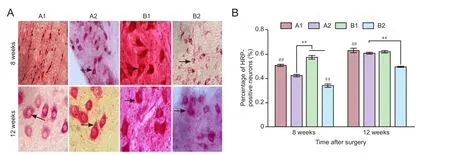
Figure 10 Horseradish peroxidase (HRP) tracing and benzidine staining of the spinal cord in rats with sciatic nerve injury following brain injury induction and/or tacrolimus treatment.
Wallerian degeneration appeared immediately after peripheral nerve injury. Axonal fragments were engulfed by macrophages, and surviving neurons formed new axonal sprouts and extended distally. Contact with the target organ is gradually established through the endoneurial tube, which is formed by Schwann cells (Carrasco et al., 2016), and the target organ is reinnervated and nourished. Here, Masson’s trichrome staining of target muscles (Mekaj et al., 2014) and sciatic nerves, toluidine blue staining, and HRP tracing all showed stronger results in group A1 than in all other groups.e likely reason for this is the immunosuppressive action of tacrolimus.e drug forms a complex by binding to FKBP12 in the cytoplasm, inhibiting CaN activity, reducing dephosphorylation of nuclear factor of activated T-cells (NFAT), and downregulating the expression of interleukins 2 and 3 and recombinant interferon, thus promoting neuronal regeneration (Glaus et al., 2011; Mekaj et al., 2015b).
Peripheral nerve injury damages the blood—nerve barrier. Macrophages and fibroblasts arrive at the site of injury, participate in the inflammatory reaction, and form a collagen scar, thus inhibiting neuronal regeneration (Konofaos and Ver Halen, 2013; Ribeiro et al., 2015). Tacrolimus reduces the local inflammatory reaction and blocks the transformation of fibroblasts, suppressing their proliferation, migration and collagen deposition, thus promoting the repair and regeneration of damaged neurons (Gao et al., 2013). Masson’s trichrome staining of nerves demonstrated that the collagen scar was smaller in group A1 than in all other groups. Regenerated axonal sprouts were connected to the endoneurial tube through the anastomotic stoma.
Schwann cells produce the myelin sheath aer regulation by endogenous signals and transcription factors (Xu et al., 2016). Schwann cells also remove fragments of degenerated myelin sheath, secrete nutritional factors, create a microenvironment conducive to axonal regeneration, and promote axonal regeneration. The toluidine blue staining results showed that the myelin sheath was thicker and more regular in the brain injury + tacrolimus group than in the other groups. Neuronal cell bodies achieve the material exchange of target organs through axoplasmic transport (Lundborg et al., 1973). Regenerated axonal sprouts connect to target organs through the anastomotic stoma.e better the axonal regeneration effect, the better the functional recovery of axoplasmic transport and target organ recovery (Wang et al., 2014). In the present study, HRP tracing and Masson’s trichrome staining of muscles revealed better recovery in group A1 than in all other groups.
The nervous, immune and endocrine systems are closely related (Sheth et al., 2016). When the central structure of the autonomic nervous system is damaged after brain injury, it induces alterations in the immune response and mitochondrial function in brain cells, and activates the caspase cascade, leading to neuronal apoptosis, an increase in neutrophils surrounding the site of injury, and an increase in vascular endothelial cell permeability.erefore, immune defense and repair are stimulated through body fluids.e activated astrocytes, oligodendrocytes and neurons produce interleukin-6, causing various physiological effects and pathologic responses (Glaus et al., 2011; Mekaj et al., 2014, 2015a, b; Phillips et al., 2014; Zhao et al., 2014). These cells secrete a variety of an-ti-inflammatory factors and neurotrophic factors to promote nerve repair (Glaus et al., 2011; Mekaj et al., 2014, 2015a, b; Phillips et al., 2014; Zhao et al., 2014). However, the mechanism for the promoting effect of brain injury on the repair of nerve injury warrants further study.
The regeneration of injured peripheral nerves remains a topic of great interest in neuroscience research. We have shown that brain injury and tacrolimus both contribute to the repair of peripheral nerve injury, but show better effects when combined.eir mechanisms of action are not identical.e precise mechanism by which brain injury promotes the repair of peripheral nerves aer injury requires further exploration.
Author contributions:PW and XZH participated in study concept and design. HQW, JJM, TMH, BS, YFG, SBL, and WW provided technical and material support. XZH and PW collected and analyzed data, ensured the integrity of the data, and wrote the paper. PW and YFG served as principle investigators, were in charge of paper authorization, and obtained the funding. All authors approved the final version of the paper.
Conflicts of interest:None declared.
Research ethics:
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Ackerman S, Smith LM, Gomes PJ (2016) Ocular itch associated with allergic conjunctivitis: latest evidence and clinical management.er Adv Chronic Dis 7:52-67.
Carrasco DI, Seburn KL, Pinter MJ (2016) Altered terminal Schwann cell morphology precedes denervation in SOD1 mice. Exp Neurol 275 Pt 1:172-181.
Cheng XL, Wang P, Sun B, Liu SB, Gao YF, He XZ, Yu CY (2015)e longitudinal epineural incision and complete nerve transection method for modeling sciatic nerve injury. Neural Regen Res 10:1663-1668.
Dong HY, Jiang XM, Niu CB, Du L, Feng JY, Jia FY (2016) Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. Neural Regen Res 11:156-162.
Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211:67-77.
Gao Z, Zhu Q, Zhang Y, Zhao Y, Cai L, Shields CB, Cai J (2013) Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury. Mol Neurobiol 48:690-701.
Glaus SW, Johnson PJ, Mackinnon SE (2011) Clinical strategies to enhance nerve regeneration in composite tissue allotransplantation. Hand Clin 27:495-509.
Gold BG, Storm-Dickerson T, Austin DR (1994)e immunosuppressant FK506 increases functional recovery and nerve regeneration following peripheral nerve injury. Restor Neurol Neurosci 6:287-296.
He XZ, Wang W, Hu TiM, Ma JJ, Yu CY, Gao YF, Cheng XL, Wang P (2016) Peripheral nerve repair: theory and technology application. Zhongguo Zuzhi Gongcheng Yanjiu 20:1044-1050.
Kennelly KD (2006) Electrophysiological evaluation of cranial neuropathies. Neurologist 12:188-203.
Konofaos P, Ver Halen JP (2013) Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg 29:149-164.
Lu YX, Su QH, Wu KH, Ren YP, Li L, Zhou TY, Lu W (2015) A population pharmacokinetic study of tacrolimus in healthy Chinese volunteers and liver transplant patients. Acta Pharmacol Sin 36:281-288.
Lundborg G, Nordborg C, Rydevik B, Olsson Y (1973) The effect of ischemia on the permeability of the perineurium to protein tracers in rabbit tibial nerve. Acta Neurol Scand 49:287-294.
Mekaj AY, Morina AA, Bytyqi CI, Mekaj YH, Duci SB (2014) Application of topical pharmacological agents at the site of peripheral nerve injury and methods used for evaluating the success of the regenerative process. J Orthop Surg Res 9:94.
Mekaj AY, Morina AA, Lajqi S, Manxhuka-Kerliu S, Kelmendi FM, Duci SB (2015a) Biomechanical properties of the sciatic nerve following repair: effects of topical application of hyaluronic acid or tacrolimus. Int J Clin Exp Med 8:20218-20226.
Mekaj AY, Morina AA, Manxhuka-Kerliu S, Neziri B, Duci SB, Kukaj V, Miarii(2015b) Electrophysiological and functional evaluation of peroneal nerve regeneration in rabbit following topical hyaluronic acid or tacrolimus application aer nerve repair. Niger Postgrad Med J 22:179-184.
Phillips BZ, Franco MJ, Yee A, Tung TH, Mackinnon SE, Fox IK (2014) Direct radial to ulnar nerve transfer to restore intrinsic muscle function in combined proximal median and ulnar nerve injury: case report and surgical technique. J Hand Surg Am 39:1358-1362.
Reddaway RB, Davidow AW, Deal SL, Hill DL (2012) Impact of chorda tympani nerve injury on cell survival, axon maintenance, and morphology of the chorda tympani nerve terminal field in the nucleus of the solitary tract. J Comp Neurol 520:2395-2413.
Ribeiro J, Pereira T, Caseiro AR, Armada-da-Silva P, Pires I, Prada J, Amorim I, Amado S, Franca M, Goncalves C, Lopes MA, Santos JD, Silva DM, Geuna S, Luis AL, Mauricio AC (2015) Evaluation of biodegradable electric conductive tube-guides and mesenchymal stem cells. World J Stem Cells 7:956-975.
Schiaveto de Souza A, da Silva CA, Del Bel EA (2004) Methodological evaluation to analyze functional recovery aer sciatic nerve injury. J Neurotrauma 21:627-635.
Sheth RU, Cabral V, Chen SP, Wang HH (2016) Manipulating bacterial communities by in situ microbiome engineering. Trends Genet 32:189-200.
Shu B, Xie JL, Xu YB, Lai W, Huang Y, Mao RX, Liu XS, Qi SH (2015) Effects of skin-derived precursors on wound healing of denervated skin in a nude mouse model. Int J Clin Exp Pathol 8:2660-2669.
Wade CE, Baer LA, Wu X, Silliman DT, Walters TJ, Wolf SE (2013) Severe burn and disuse in the rat independently adversely impact body composition and adipokines. Critical Care 17:R225.
Wang W, Gao J, Na L, Jiang H, Xue J, Yang Z, Wang P (2014) Craniocerebral injury promotes the repair of peripheral nerve injury. Neural Regen Res 9:1703-1708.
Xu Z, Yang B, Zhang J, Zheng J (2016)e regulation of sGC on the rat model of neuropathic pain is mediated by 5-HT1ARs and NO/cGMP pathway. Am J Transl Res 8:1027-1036.
Zhao J, Zheng X, Fu C, Qu W, Wei G, Zhang W (2014) FK506-loaded chitosan conduit promotes the regeneration of injured sciatic nerves in the rat through the upregulation of brain-derived neurotrophic factor and TrkB. J Neurol Sci 344:20-26.
Copyedited by Li CH, Song LP, Zhao M
How to cite this article: He XZ, Ma JJ, Wang HQ, Hu TM, Sun B, Gao YF, Liu SB, Wang W, Wang P (2017) Brain injury in combination with tacrolimus promotes the regeneration of injured peripheral nerves. Neural Regen Res 12(6):987-994.
*Correspondence to:
Pei Wang, cdgkwp@sina.com.
orcid:
0000-0002-0597-546X
(Pei Wang)
10.4103/1673-5374.208595
Accepted: 2017-04-14
 中國(guó)神經(jīng)再生研究(英文版)2017年6期
中國(guó)神經(jīng)再生研究(英文版)2017年6期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease
