Biofertilizing efficiency of Sargassum polycystum extract on growth and biochemical composition of Vigna radiata and Vigna mungo
Bharath B, Nirmalraj S, Mahendrakumar M, Perinbam K
PG and Research Department of Botany, Government Arts College for Men (Autonomus), Nandanam, Chennai, Affiliated to University of Madras,Tamil Nadu, India
Biofertilizing efficiency of Sargassum polycystum extract on growth and biochemical composition of Vigna radiata and Vigna mungo
Bharath B, Nirmalraj S, Mahendrakumar M, Perinbam K?
PG and Research Department of Botany, Government Arts College for Men (Autonomus), Nandanam, Chennai, Affiliated to University of Madras,Tamil Nadu, India
Sargassum polycystum Vigna radiate Vigna mungo Physiochemical Growth Biochemical
Objective:To evaluate the effect of marine brown alga Sargassum polycystum extract on growth and biochemical parameters of Vigna radiata and Vigna mungo.Methods:Different concentrations of algal extracts (0.5%, 1.0%, 2.0%, 3.0%, 4.0%, and 5.0%) were prepared and applied to the crops at every 10-day intervals under natural conditions. After 30 d, the plants were harvested to evaluate the growth and biochemical parameters.Results:Seaweed liquid fertilizers treated seedlings showed maximum growth in 3.0% concentration when compared to the untreated seedlings. Similarly, biochemical parameters such as photosynthetic pigments, protein, reducing sugar, total sugar and amino acids exhibited increases in 3.0%concentration seaweed extract. Decreases in growth and biochemical parameters were noticed in concentrations higher than 3.0%.Conclusions:Presence of micronutrients and growth regulating substances in the liquid extract help healthier and faster productivity of the crop.
1. Introduction
In the current global scenario, increasing pollution and contamination of farm lands is a growing concern of the farmers engaged in increasing food production. Indian economy is agriculture based particularly in the rural areas where 70% of the population live with agriculture and allied activities as its main occupation[1]. Currently, most agricultural lands are polluted and degraded to varying extents by persistent use of chemical fertilizers and pesticides, and alternative approaches are needed to safeguard the situation. Use of environment friendly biofertilizers as a sustainable resolution for eco-friendly agricultural practices is gaining momentum in many countries. India also intends toachieve second green revolution based on biodegradable, nontoxic, non-polluting and environmentally safe technology[2]. Natural seaweeds, the macrophytes are emerging as one of the sought-after biofertilizers amenable for fractional replacement of predictable chemical fertilizer[3-5]. At present, seaweed extract products are used in agriculture practice and are commercialized. Seaweed liquid fertilizers (SLF) are available as manure, foliar spray, granular powder for soil conditioners and soil drench[6].
SLF contains nutrients to promote the growth of the host plant,when applied to plant surfaces, seeds, soil and interior part of the plant. SLF has nutrients to enhance the plant growth through solubilising phosphorus, nitrogen fixation and the production of growth regulating substances. Moreover, plant growth nutrients suchas copper, molybdenum, potassium, selenium, magnesium, iodine,iron, nitrogen, cobalt, zinc, manganese and nickel are components of SLF[7]. Seaweed extracts improve seed germination, early seedling vigor, stimulate the extra buds, root growth and increase the validity of vegetables and fruits[8]. In addition to crop yield, others including nutrient uptake, protein content, resistance of pathogens and stress conditions, the quality of the crop improve[9-11]. Seaweed manure is common and a very ancient farming practice. SLF contains abundant presence of plant growth regulators such as auxin[12,13], cytokinin[14],indole-3-acetic acid[15] and gibberellins[16]. Hence, marine resources,particularly brown algae play a major role in agriculture. The present study was subjected to evaluate the growth efficiency of seaweed liquid fertilizer of brown macro algae Sargassum polycystum C.Agardh (S. polycystum C. Agardh) on legume plants of Vigna radiata(V. radiata) and Vigna mungo (V. mungo).
2. Materials and methods
2.1. Collection of seaweed
Fresh specimens of brown seaweed, S. polycystum C. Agardh collected from Gulf of Mannar region (09°19′N, 79°03′E),Rameswaram, Tamil Nadu, India. The specimens were thoroughly washed with seawater to remove epiphytes, then exhaustively washed with tap water twice, and followed by a wash with distilled water to remove salt content and other contaminants. The seaweed materials subsequently dried in shade for 7 d, then they were stored at 4 ℃ for future use after finely ground in an electric mixer.
2.2. Preparation of seaweed liquid fertilizer
A total of 500 g dried seaweed powder was mixed with 1 L of distilled water and then boiled for 1 h. After the hot water extraction,the extract was filtered through muslin cloth. The obtained filtrate was taken as 100% concentrated seaweed extract and it was made up to different concentrations (0.5%, 1.0%, 2.0%, 3.0%, 4.0%, 5.0%)using distilled water[17].
2.3. Physio-chemical and growth promoting substances of S.polycystum extract
Physical characteristics such as color and pH of S. polycystum extract were noticed. Chemical elements presented in the seaweed extract such as magnesium, iron, chloride, sulphate, copper, sodium,calcium, zinc, nitrate, cobalt, phosphate, potassium, and manganese were estimated using the procedures outlined by the American Public Health Association[18]. Furthermore, the liquid extract of S.polycystum was subjected for estimation of plant growth regulators such as auxin, gibberellin and cytokinin[19].
2.3.1. Selection of crop plant
Plants selected for the present study were of V. radiata and V.mungo seeds belonging to the family, Fabaceae. The seeds were purchased from a commercial market, Chennai, Tamil Nadu. Dry healthy seeds, with standardized size and weight were selected for the experimental study.
2.3.2. Preparation of pot study
Selected healthy seeds were surface sterilized with 0.1% mercury chloride for 1 min and followed by rinsing with distilled water for several times. Then the seeds were sown in pots (24 cm × 18 cm)with a mixture of red soil and sand. The pots were watered regularly.After every ten day interval 50 mL of different concentrations (0.5%,1.0%, 2.0%, 3.0%, 4.0%, 5.0%) of S. polycystum extract was treated for the tested plants V. radiata and V. mungo. Extract alone water irrigated plants were considered as control. All the experimental pots were repeated thrice.
2.3.3. Growth promoting efficiency of V. radiata and V.mungo
Plants were uprooted carefully from each treatment at the end of 30 d after seed sowing. All the uprooted plants from each triplicates were analyzed for growth parameters of shoot and root lengths (cm).The uprooted plants were washed and blotted on blotting paper before weighing fresh weight for calculation. Then the plants were dried in a hot air oven at 60 ℃ for 24 h for determining dry weight(mg/g fr.wt) by Infradigi digital weighing balance 1N201L, India.The number of root nodules was also calculated and expressed in nos. Leaf area (mm2) was calculated by using of systeronic Leaf Area Meter 211, India.
2.4. Biochemical profile of V. radiata and V. mungo
2.4.1. Estimation of photosynthetic pigments
The amount of photosynthetic pigments was determined by the standard method[20]. One hundred milligram of fresh leaves were homogenized with 10 mL of acetone using mortar and pestle. The filtrate was centrifuged at 10 000 r/min for 20 min, and the resultant supernatant was diluted with acetone. The absorbance was read at 663 nm for chl a and 645 nm for chl b and the total chlorophyll was calculated.
2.4.2. Estimation of protein
Fifty milligrams of fresh leaves were homogenized with 10% cold Trichloro acetic acid and centrifuged at 10 000 r/min for 15 min in 4 ℃. The reaction system contained 4.5 mL of 2% Na2CO3in 0.1 N NaOH, 0.5 mL of 2% CuSo4,2% KNaC4H4O6·4H2O and 0.1 mL of sample incubated at room temperature for 30 min. The absorbance was read at 660 nm using Elico double beam UV-VIS spectrophotometer SL 210. The calibration curve was prepared by using bovine serum albumin[21].
2.4.3. Estimation of total sugar
Total sugar was determined by phenol-sulfuric acid method[22]. A reaction mixture contained 1 mL sample, 1 mL of 5% phenol, 5 mL of concentrated sulphuric acid and incubated at room temperature for 30 min. The presence of total sugar was detected by recording the absorbance at 490 nm. Glucose was used as a standard.
2.4.4. Amino acid analysis
A test tube contains 1 mL sample neutralized with 0.1 N NaOH using methyl red indicator, and 1 mL ninhydrin reagent was boiled for 20 min. The test tube was added with 5 mL of dilution solution contains n-propanol and distilled water at 1:1 ratio. The absorbance was read at 570 nm and the concentration of amino acids (mg/g fr.wt) were calculated from the standard graph of leucine[23].
2.4.5. Determination of reducing sugar
Reducing sugar was determined according to the method described by Nelson[24]. Briefly, 1 mL sample was mixed with 1 mL of copper reagent (2.5 g of sodium potassium tartarate, 20 g of anhydrous sodium sulphate, 2 g of sodium bicarbonate), and 1 mL of arsenomolybdate reagent (2.5 g of ammonium molybdate, 0.3 g of sodium arsenate). The reaction mixture was incubated for 15 min at 30 ℃. Absorbance was read at 500 nm.
2.5. Statistical analysis
Statistical analysis was carried out by one way ANOVA followed by Duncan’s Multiple Range Test (P<0.05). Data were calculated using Software Package of Social Sciences (SPSS) version 18.0 for Windows[25].
3. Results
Physiochemical properties of the brown alga S. polycystum are presented in Table 1. The physical appearance of the extract was brown colored and the pH of the extract was 6.8. Similarly, in the micro elemental analysis sodium (85.30 mg/L), chloride (75.02 mg/L) and calcium (69.37 mg/L) accounted for large quantity and other elements were present in substantial level. In growth hormone analysis, cytokinin (7.35 mg/L) was present higher than gibberellin and auxin.
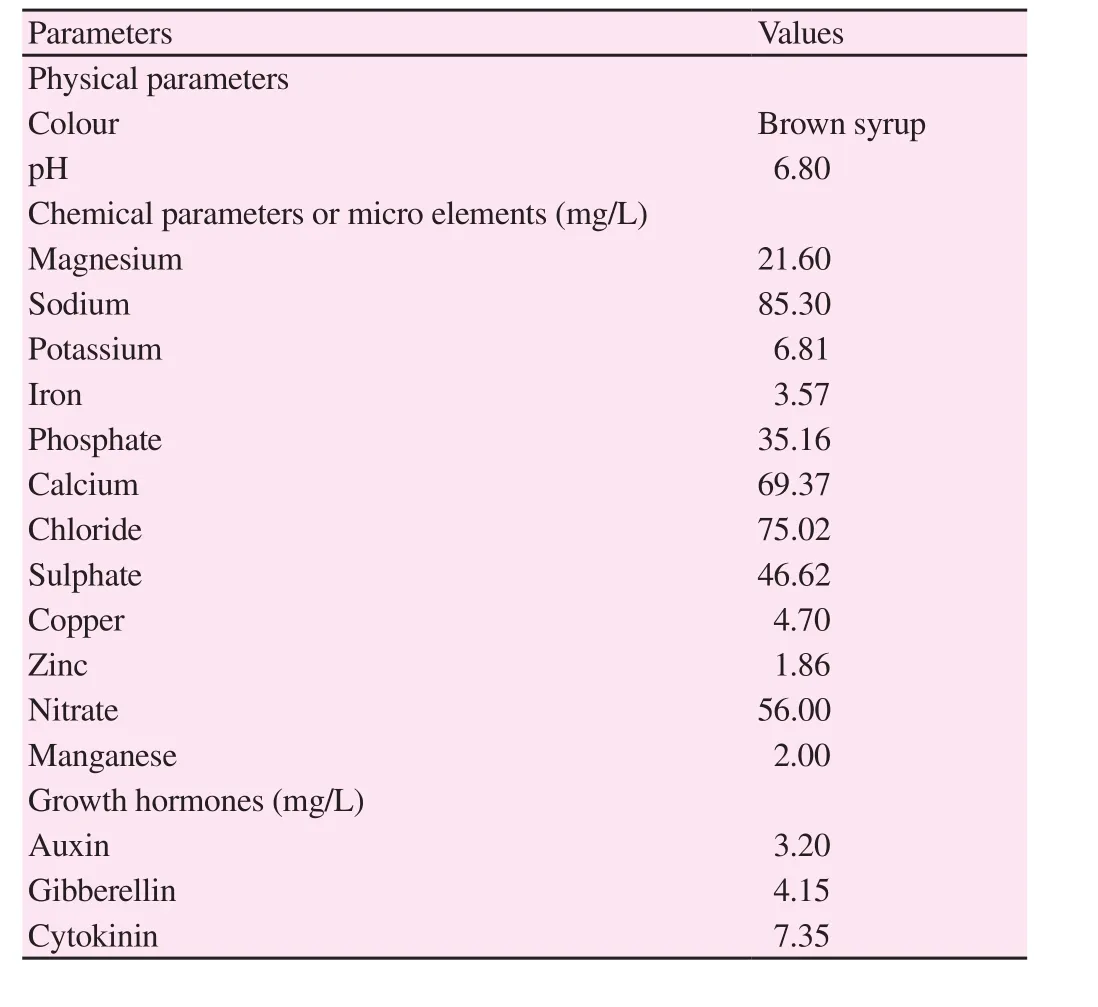
Table 1 Phsio-chemical parameters and growth hormones of S. polycystum liquid extract.
The effects of various concentrations of S. polycystum extracts on V.radiata and V. mungo were studied and their growth parameters and biochemical parameters were analyzed. The results showed in Table 2 and 3 indicated that seaweed extract enhanced the growth rate and various other physiological parameters of V. radiata and V. mungo.Growth parameters of V. radiata such as shoot length (142%), root length (149%), leaf area (219%), root nodules (249%), fresh weight(249%) and dry weight (250%); whereas in V. mungo, it showed that shoot length (170%), root length (139%), root nodules (172%),leaf area (170%), fresh weight (236%) and dry weight (271%) got a tremendous boost, respectively. All the above parameters of V.radiata and V. mungo were increased potential growth up to 3.0%and decreased up to 5.0%. The control plants showed lowest growth parameters when compared to the treated plants respectively.
Various biochemical ingredients such as photosynthetic pigments(chl a, chl b, and total chlorophyll), protein, amino acid, reducing sugar and total sugar were estimated in V. radiata and V. mungo(Table 4 and 5). It was evident that lower concentrations of 3% S.polycystum extract increased biochemical parameters in V. radiataplants including chl a (155%), chl b (196%), total chlorophyll(171%), protein (233%), amino acid (492%), reducing sugar (201%)and total sugar (130%). Similarly, the amount of biochemical parameters in treated plants of V. mungo including chl a (179%),chl b (202%), total chlorophyll (188%), protein (217%), amino acid(439%), reducing sugar (228%) and total sugar (135%) was found to be high at 3% concentration of the extract. The other treated concentrations (0.5%, 1%, 2%, 4% and 5%) showed reduced levels of biochemical contents in both treated plants.
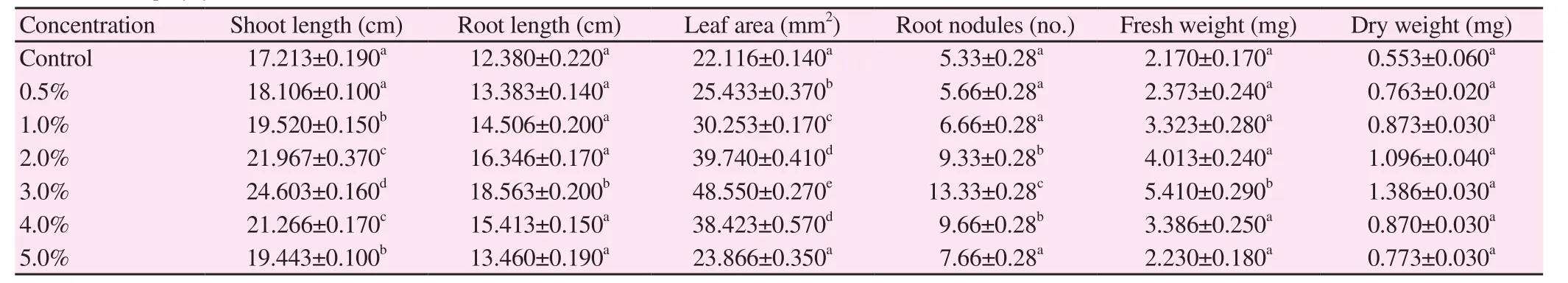
Table 2 Potential of S. polycystum liquid extract on growth parameters of V. radiata (n=6).
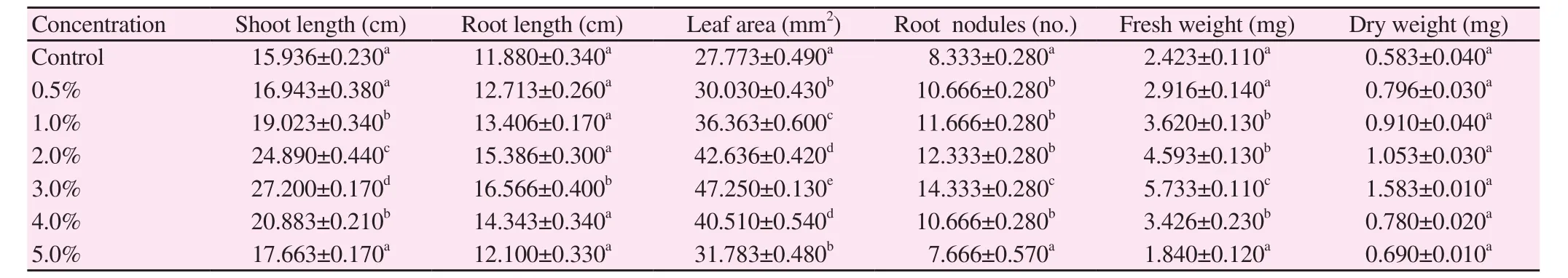
Table 3 Potential of S. polycystum liquid extract on growth parameters of V. mungo (n=6).
4. Discussion
Higher concentrations of cytokinin are also reported in earlier findings of brown algae Sargassum wightii (S. wightii)[26] and Stoechospermum marginatum[27] where cytokinin is reported in much higher concentration than auxin and gibberellins. The growth stimulation potential of SLF may be due to the presence of micro and macro elements, vitamins and more important plant growth hormones like the cytokinins[28,29]. In addition, chelating nutrients reportedly help the enhancement of nutrient uptake as in Ascophyllum nodosum because of the presence of some organic acids[11].
Previous investigations and reviews have documented the responses of the different concentrations of several seaweed extracts against many other plants. Surprisingly, the extract of same seaweed S.polycystum on seed germination and growth rate of Cajanus cajan(C. cajan) was studied and it promoted the seed germination and growth rate in lower concentrations of 1.5%[30]. Earlier studies of seaweed extracts were found to be effective in V. radiata[31,32], V.mungo[33], and Vigna ungiculata[34]. Ganapathy and Sivakumar[35]reported seaweed extract of Ulva reticulata (U. reticulata) to increase crop productivity of Arachis hypogea by spraying 2% concentration.In particular, V. mungo plant treated with seaweed extracts like Caulerpa scalpelliformis (C. scalpelliformis)[36] and U. reticulata[37]responded very favorably. Furthermore, data were obtained from the commercial seaweed extract and extract of Sargassum plagiophyllum treated V. radiata and V. mungo. Both the extracts enhanced seed germination and seedling growth up to 0.75% in V. mungo; whereas,1.0% commercial seaweed extract and 1.5 % SLF showed enhanced seedling growth in V. radiata[38].
In previous findings, 10% of SLF from brown seaweed Colpomenia sinuosa increased the chlorophyll content, protein, total sugars,lipids, phenols and carotenoids in V. radiata[2]. However, Blunden et al[39] have reported that the increase of photosynthetic pigments may be due to the presence of betaines. It helps a better grana development and increase the chloroplast number and size[40].Jebasingh et al[41] examined the seaweed liquid fertilizer from green seaweed C. scalpelliformis, brown seaweed Sargassum duplicatum and red seaweed Lamminaria pinnatifida treated against V. mungo. All the three extracts elevated the growth and chlorophyll content at lower concentrations of 10%. It also reported that lower concentration of U. reticulata (2%) treatmen on V. mungo resulted in increased photosynthetic pigments, protein, starch, reducing and non-reducing sugars[37]. The above results are comparable with the results of the present study. A support to this study comes from the observation that 20% of C. scalpelliformis SLF stimulates the photosynthetic pigments, amino acid, reducing sugar and total sugar content of V. mungo[36]. Moreover, when C. cajan was treated with SLF of Chaetomorpha linum, Grateloupia lithophila and S.wightii, the SLF of S. wightii enhanced the chlorophyll, carotenoid,amino acid, protein, lipid and total sugar of C. cajan at lower concentration of 20% when compared to Chaetomorpha linum and Grateloupia lithophila[42]. However, the effect S. polycystum on C.cajan increased the biochemical constituents that were chl a, chl b,ascorbic acid, sugars, starch, protein, nitrate reductase activity at lower concentrations of 1.5%[30]. The total sugar content was high in lower concentrations of all three extracts Laurencia obtusa, Corallina elongata and Jania rubens in Abelmoschus esculentu[43]. The presence of N, P, K in seaweed extract apparently helped to enhance the growth rate and increase the chlorophyll content as well as increase the protein and amino acid content of treated plants V. radiata and V.mungo. Lower concentration of 3% increases the crop productivity while decline is observed in higher concentrations may be due to the presence of high levels of nutrients like Mg, Ca, Cu, I, K, Zn, and Na that restrains the cell division[44]. Moreover, the presence of different levels of minerals and biostimulants fails to supply all the nutrients required by a plant in necessary quantities[45]. All these results make it clear that seaweed extracts have potential growth promoting substances which enhances the growth and biochemical constituents of the plants.
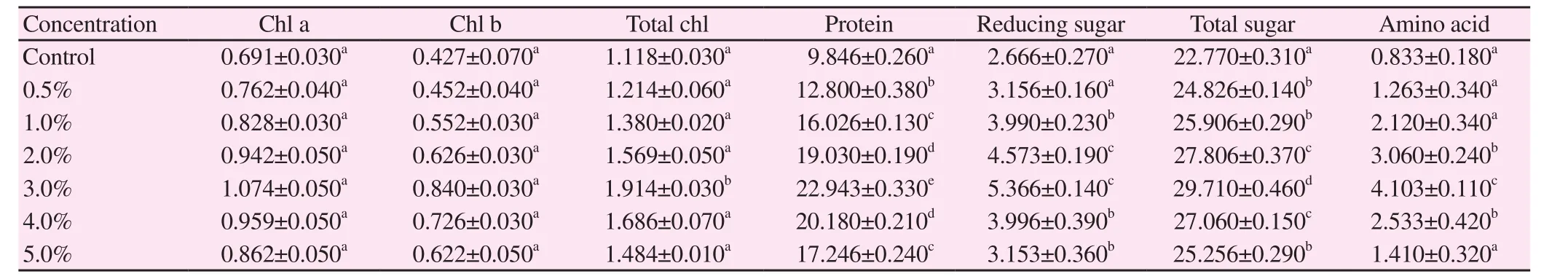
Table 4 Potential of S. polycystum liquid extract on biochemical parameters of V. radiata (mg/g fr.wt) (n=6).
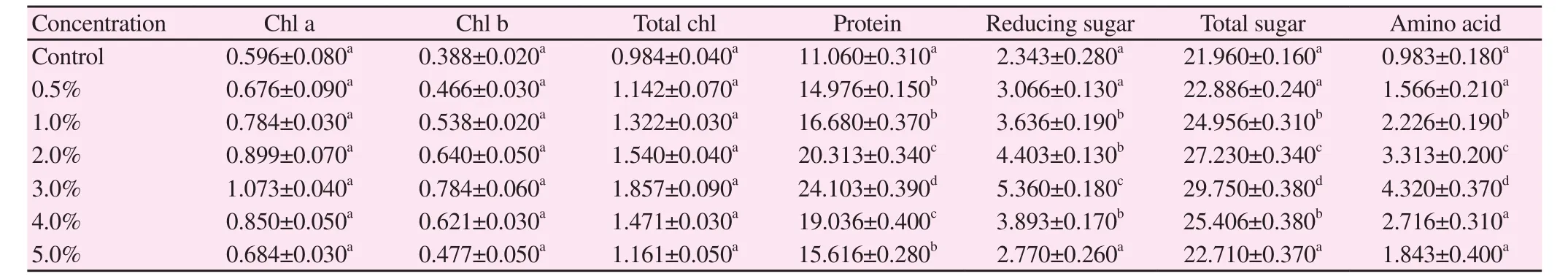
Table 5 Potential of S. polycystum liquid extract on biochemical parameters of V. mungo (mg/g fr.wt) (n=6).
In the current scenario of population increase, especially in the developing world, there is a need for increased food production.The farmers need to adapt to a fertilizer to improve the health of the crops free from chemical fertilizers and pesticides. Seaweed liquid fertilizer is the answer as it increases various crop productions as evidenced from this study. The extract of S. polycystum no doubt,improves the growth and biochemical parameters of V. radiata and V. mungo due to the presence of growth promoting hormones, micro and macro elements. Lower concentration of 3% increases the crop productivity while decline is observed in higher concentrations when compared to control respectively. The study also reveals that the S. polycystum extract is suitable to make a cost effective and ecofriendly organic farming for sustainable crop production.
Conflict of interest statement
The authors declare that there is no conflict of interest.
[1] Paul J, Mahadevi B. Effect of seaweed liquid fertilizer of Caulerpa peltata Lamour (green seaweed) on Vigna radiata (L.) R. Wilczek., in Idinthakarai, Tamil Nadu, India. World J Pharm Pharm Sci 2014; 3(6):1000-1007.
[2] Paul J, Yuvaraj P. Effect of seaweed liquid fertilizer of Colpomenia sinuosa(Mert. ex Roth) Derbes & Solier (Brown Seaweed) on Vigna radiata (L.)R. Wilczek. In Koothankuzhi, Tirunelveli district, Tamil Nadu, India. Int J Pure App Biosci 2014; 2(3): 177-184.
[3] Zodape ST, Mukhopadhyay S, Eswaran K, Reddy MP, Chikara J.Enhanced yield and nutritional quality in green gram (Phaseolus radiata L.) treated with seaweed (Kappaphycus alvarezii) extract. J Sci Ind Res 2010; 69: 468-471.
[4] Arun D, Gayathri PK. A review on seaweeds phytochemical analysis and utilisation of seaweeds as biofertilizer. Res J Eng Tech 2013; 4(4): 149-151.
[5] Chitra G, Sreeja PS. A comparative study on the effect of seaweed liquid fertilizers on the growth and yield of Vigna radiata (L.). Nat Environ Poll Tech 2013; 12(2): 359-362.
[6] Thirumaran G, Arumugam M, Arumugam R, Anantharaman P. Effect of seaweed liquid fertilizer on growth and pigment concentration of Cyamopsis tetrogonolaba (L) Taub. Amer Euras J Agron 2009; 2(2): 50-56.
[7] Khan W, Rayirath UP, Subramanian S, Jithesh MN, Rayorath P, Hodges DM, et al. Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 2009; 28: 386-399.
[8] Arioli T, Mattner SW, Winberg PC. Applications of seaweed extracts in Australian agriculture: Past, present and future. J Appl Phycol 2015; 27:2007-2015.
[9] Abhilash ES, Jacob J, Parayil SP. Effect of sea weed (Caulerpa racemosa)extract on biochemical variations, growth and yield of Vigna mungo. Asia Pac J Environ Ecol Sust Dev 2013; 1: 3-5.
[10] Spinelli F, Giovanni F, Massimo M, Mattia S, Guglielmo C. Perspectives on the use of a seaweed extract to moderate the negative effects of alternate bearing in apple trees. J Hortic Sci Biotech 2009; 17(1): 131-137.
[11] Jannin L, Arkoun M, Etienne P, La?ne P, Goux D, Garnica M, et al.Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol.Seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J Plant Growth Regul 2013; 32: 31-52.
[12] Divya K, Roja NM, Padal SB. Effect of seaweed liquid fertilizer of Sargassum wightii on germination, growth and productivity of brinjal. Int J Adv Res Sci 2015; 2(10): 868-871.
[13] Roja NM, Padal SB. Influence of seaweed liquid fertilizer of Ulva lactuca on the seed germination, growth, productivity of Abelmoschus esculentus(L.). Int J Pharm Res 2015; 5(12): 344-346.
[14] Dogra BS, Mandradia RK. Effect of seaweed extract on growth and yield of onion. Int J Farm Sci 2012; 2(1): 59-64.
[15] Ahmed YM, Shalaby EA. Effect of different seaweed extracts and compost on vegetative growth, yield and fruit quality of cucumber. J Hortic Sci Ornam Plants 2012; 4(3): 235-240.
[16] Flora G, Vishnupriya R. Effect of Ulva lactuca and Padina tetrastromatica concentrate on growth and yield of Momordica charantia. Seaweed Res Utiln 2016; 38(2): 117-123.
[17] Narasimha Rao GM, Chatterjee R. Effect of seaweed liquid fertilizer from Gracilaria textorii and Hypnea musciformis on seed germination and productivity of some vegetable crops. Univers J Plant Sci 2014; 2(7): 115-120.
[18] American Public Health Association. Standards methods for examination of water and waste water analysis. 19th ed. Washington: American Public Health Association, American Water Works Association, Water Environment Federation; 1995.
[19] ünyayar S, Topcuoglu SF, ünyayar A. A modified method for extraction and identification of indole-3-acetic acid (IAA), gibberellic acid (GA3),abscisic acid (ABA) and zeatin produced by Phanerochaete chrysosporium ME446. Bulg J Plant Physiol 1996; 22(3-4): 105-110.
[20] Arnon DI. Copper enzymes in isolated chloroplast, polyphenol oxidase in Beta vulgaris. Plant Physiol 1949; 24: 1-15.
[21] Lowry OH, Rosebrough H, Farr AL, Randall RJ. Protein measurement by folin-phenol reagent. J Biol Chem 1951; 193: 265-275.
[22] Dubios M, Gilles KA, Hamilton TK, Rebers PA, Smith F. Calorimetric method for determination of sugars and related substance. Anal Chem 1956; 28: 350-356.
[23] Moore S, Stein WH. Photometric method for use in the chromatography amino acids. J Biol Chem 1948; 176: 367-388.
[24] Nelson NA. Photometric adaptation of Somogyis method for the determination of reducing sugar. Anal Chem 1944; 31: 426-428.
[25] Duncan BD. Multiple range and multiple F-test. Biometric 1965; 11(1):1-42.
[26] Vijayanand N, Ramya SS, Rathinavel S. Potential of liquid extracts of Sargassum wightii on growth, biochemical and yield parameters of cluster bean plant. Asian Pac J Reprod 2014; 3(2): 150-155.
[27] Ramya SS, Vijayanand N, Rathinavel S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth,biochemical and yield of Solanum melongena. Int J Recycl Org Waste Agric 2015; 4(3): 167-173.
[28] Pramanick B, Brahmachari K, Ghosh A. Effect of seaweed saps on growth and yield improvement of green gram. Afr J Agric Res 2013;8(13): 1180-1186.
[29] Ahmed MY, Sehrawy EL. Effect of seaweed extract on fruiting of Hindy Bisinnara mango trees. J Am Sci 2013; 9(6): 539-544.
[30] Erulan V, Soundrapandian P, Thirumaran G, Ananthan G. Studies on the effect of Sargassum polycystum (C. Agardh.) extract on the growth and biochemical composition of Cajanus cajan (L.) Mill sp. Am Eur J Agric Environ Sci 2009; 6(4): 392-399.
[31] Bai NR, Christi RM, Kala TC. Growth and yield characteristics of Dolichos biflorus Linn. as influenced by seaweed liquid fertilizer. Plant Arch 2013; 13(1): 163-166.
[32] Parthiban C, Saranya C, Hemalatha A, Kavitha B, Anantharaman P.Effect of seaweed liquid fertilizer of Spatoglossum asperum on the growth and pigment content of Vigna radiata. Int J Recent Sci Res 2013; 4(9):1418-1421.
[33] Selvam GG, Sivakumar K. Micromorphological study of V. mungo L. using seaweed liquid fertilizer from Hypnea musciformis (wulf.)Lamouroux. Indian J Geo Mar Sci 2016; 46(9): 1199-1207.
[34] Ismail MM, El-Shafay SM. Variation in taxonomical position and biofertilizing efficiency of some seaweed on germination of Vigna unguiculata (L). Int J Environ Sci Eng 2015; 6: 47-57.
[35] Selvam GG, Sivakumar K. Influence of seaweed extract as an organic fertilizer on the growth and yield of Arachis hypogea L. and their elemental composition using SEM-energy dispersive spectroscopic analysis. Asian Pac J Reprod 2014; 3(1): 18-22.
[36] Kalaivanan C, Chandrasekaran M, Venkatesalu V. Effect of seaweed liquid extract of Caulerpa scalpelliformis on growth and biochemical constituents of black gram (Vigna mungo) (L.) Hepper. Phykos 2012;42(2): 46-53.
[37] Selvam GG, Sivakumar K. Effect of foliar spray form seaweed liquid fertilizer of Ulva reticulata (Forsk.) on Vigna mungo L. and their elemental compostition using SEM-energy dispersive spectroscopic analysis. Asian Pac J Reprod 2013; 2(2): 119-125.
[38] Kumar RV, Mohan VR, Murugeswari R, Muthusamy M. Effect of commercial seaweed extracts on seed germination and seedling growth in green gram and black gram. Seaweed Res Utiln 1993; 16(1-2): 23-27.
[39] Blunden G, Jenkins T, Wan LY. Enhanced leaf chlorophyll levels in plants treated with seaweed extracts. J Appl Phycol 1997; 8(6): 535-543.
[40] Atzmon N, Van Staden. The effect of seaweed concentrates on the growth of Pinus pinea seedlings. New For 1994; 8(3): 279-288.
[41] Jebasingh SEJ, Lakshmikandan M, Vasanthakumar P, Sivaraman K.Improved seedling growth and seed germination in legume crop Vigna mungo (L.) Hepper utilizing marine macro algal extracts. Proc Natl Acad Sci India Sect B Biol Sci 2014; 85(2); 643-651.
[42] Sathya B, Indu H, Seenivasan R, Geetha S. Influence of seaweed liquid fertilizer on the growth and biochemical composition of legume crop Cajanus cajan (L.) Mill sp. J Phytol 2010; 2(5): 50-63.
[43] Safinaz AF, Ragaa AH. Effect of some red marine algae as biofertilizers on growth of maize (Zea mayz L.) plants. Int Food Res J 2013; 20(4):1629-1632.
[44] MacArtain P, Gill CIR, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev 2007; 65(12): 535-543.
[45] Schmidt RE, Ervin EH, Zhang X. Questions and answers about biostimulants. Golf Course Manag 2003; 71(6): 91-94.
27 October 2017 Revision 8 November 2017 Accepted 28 November 2017 Available online 1 January 2018
10.4103/2305-0500.220982
?Corresponding author: K Perinbam, PhD., PG and Research Department of Botany,Government Arts College for Men (Autonomus), Nandanam, Chennai, Affiliated to University of Madras, Tamil Nadu, India.
Tel: +91- 9940867295
E-mail: drperinbam73@gmail.com
B Bharath, PG and Research Department of Botany, Government Arts College for Men (Autonomous), Nandanam, Chennai, Affiliated to University of Madras,Tamil Nadu, India.
E-mail: bharathbot@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix,tweak and buid upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
For reprints contact: reprints@medknow.com
?2018 Asian Pacific Journal of Reproduction Produced by Wolters Kluwer- Medknow
How to cite this article: Bharath B, Nirmalraj S, Mahendrakumar M, Perinbam K.Biofertilizing efficiency of Sargassum polycystum extract on growth and biochemical composition of Vigna radiata and Vigna mungo. Asian Pac J Reprod 2018; 7(1): 27-32.
 Asian Pacific Journal of Reproduction2018年1期
Asian Pacific Journal of Reproduction2018年1期
- Asian Pacific Journal of Reproduction的其它文章
- Non-ischemic priapism in dog: Case report
- Sperm dosage and site of insemination in relation to fertility in bovines
- Heritability and variance components estimates for growth traits in Saudi Ardi goat and Damascus goat and their crosses
- Effects of reduced glutathione on Boer goat semen freezability
- Effect of water extract of dates palm (Phoenix dactylifera) on semen characteristics and oxidative status in serum of male New Zealand rabbits under heat stress
- Genetic polymorphism and natural fertility in women
