Global significance of the carbon cycle in the karst dynamic system: evidence from geological and ecological processes
Jian-hua Cao, Xia Wu, Fn Huang, Bill Hu, Chris Grovs, Hui Yang, Chun-lai Zhang
a Institute of Karst Geology, CAGS, Karst Dynamics Laboratory, MNR, Guilin 541004, China
b International Research Center on Karst, UNESCO,Guilin 541004, China
c National Center for International Research of Karst Dynamic System and Global Change, Guilin 541004, China
d Florida State University, Tallahassee, FL 32395, USA
e Western Kentucky University, Bowling Green, KY 42101, USA
A B S T R A C T
On the basis of proposing the existence of a karst carbon cycle and carbon sink at a watershed scale, this paper provides four pieces of evidence for the integration of geology and ecology during the carbon cycle processes in the karst dynamic system, and estimated the karst carbon sink effect using the methods of comparative monitoring of paired watersheds and the carbon stable isotope tracer technique. The results of the soil carbon cycle in Maocun, Guilin, showed that the soil carbon cycle in the karst area, the weathering and dissolution of carbonate rocks under the soil, resulted in a lower soil respiration of 25% in the karst area than in a non-karst area (sandstone and shale), and the carbon isotope results indicated that 13.46% of the heavy carbon of the limestone is involved in the soil carbon cycle. The comparative monitoring results in paired watersheds, suggesting that the HCO3- concentration in a karst spring is 10 times that of a rivulet in a non-karst area, while the concentration of inorganic carbon flux is 23.8 times. With both chemical stoichiometry and carbon stable isotopes, the proportion of carbon in karst springs derived from carbonate rocks was found to be 58.52% and 37.65% respectively. The comparison on carbon exchange and isotopes at the water-gas interface between the granite and carbonate rock basins in the Li River showed that the CO2 emission of the karst water is 10.92 times that of the allogenic water from the non-karst area, while the carbon isotope of HCO3- in karst water is lighter by 8.62‰. However, this does not mean that the karst water body has a larger carbon source effect. On the contrary, it means the karst water body has a greater karst carbon sink effect. When the karst subterranean stream in Zhaidi, Guilin, is exposed at the surface, carbon-rich karst water stimulated the growth of aquatic plants. The values of carbon stable isotopes in the same species of submerged plants gradually becomes heavier and heavier, and the 512 m flow process has a maximum range of 15.46‰. The calculation results showed that 12.52% of inorganic carbon is converted into organic carbon. According to the data that has been published, the global karst carbon sink flux was estimated to be 0.53-0.58 PgC/a, equivalent to 31.18%-34.41% of the global forest carbon sink flux. In the meanwhile, the karst carbon sink flux in China was calculated to be 0.051 PgC/a, accounting for 68% of its forest carbon sink flux.
Keywords:
Karst carbon cycle
Carbon stable isotope
Carbon sink effect
Submerged plants
Guilin
1. Introduction
Carbon is a key element of life on Earth. The carbon stored in carbonate rocks reaches 61×1015t (Falkowski P et al., 2000), known as the largest carbon pool on the earth, with its carbon content representing 99.55% of the total carbon on Earth. As for fossil organic carbon alone, the proportion of dispersed particulate organic carbon present in shale and carbonate rocks, petroleum and coal is calculated to be 16000?32?1 (Golubic S et al., 1978). For this reason, the carbon cycle in the geological era or modern period of Earth has aroused considerable attention. At present, global warming is attributed to the burning of fossil fuels since the Industrial Revolution, and the consequent emission of large quantities of light carbon to the atmosphere (Ciais P et al., 1995a). In the latest IPCC-AR5 report, the technical methods for removing atmospheric CO2were summed up into four types: terrestrial ecological processes, marine carbon sink, direct air CO2capture,and pure limestone dissolution (Pu JB et al., 2015).
In the 1950s, Calvin M et al. (1952) proposed the CO2cycle pathway of plants’ photosynthesis and assimilation, following ten years’ systematic research with the methods of radio-isotope tracer and paper chromatography, etc. Geochemist Craig H (1953) found differences in carbon isotopic composition among different plant species. In the 1980s, Australian plant physiologist Farquhar and his colleagues conducted in-depth research on the mechanisms of carbon isotope effects in photosynthesis, which laid the foundation for the application of carbon isotopes in ecosystems and the global environment (Farquhar GD et al., 1989, 1984). In terms of global carbon cycle research, Ciais P et al. (1995a, b) used carbon isotope of atmospheric CO2to determine the distribution of global carbon sinks, and to quantify the relative contributions of marine and terrestrial plants to carbon transport in the atmosphere.
Globally, carbonate rocks are widely distributed on Earth,accounting for 10%-15% of land areas. Furthermore, the sensitivity to weathering and dissolution of carbonate rocks from climatic and environmental changes, makes the karst dynamic system an important part of Earth's surface system (Yuan DX, 1993, 2002). The process of the karst carbon cycle is not only closely associated with the global carbon cycle (Larson C, 2011), but is intimately linked to the ecosystem carbon cycle of Earth’s surface system (Golubic S et al., 1978). On the basis of proposing the karst carbon cycle process at a watershed scale, this paper demonstrates the integration of geology and ecology during the carbon cycle processes in the karst dynamic system, and finally estimates the karst carbon sink effects globally and in China.
2. The basic process of the carbon cycle in the karst dynamic system
Carbonate rocks, as soluble rocks, dissolve to removes atmospheric/soil CO2to water, under the action of rainwater,and this helps form carbon-rich karst water and results in the occurrence of the karst carbon cycle. While the chemical process of the karst carbon cycle is a reversible process of dissolution and deposition of carbonate rocks, the carbon-rich karst water stimulates the photosynthesis of aquatic plants. In aquatic ecosystems, aquatic plants contain aerenchyma, which can store O2released by photosynthesis for respiratory needs and store CO2released by respiration for photosynthesis needs (Wu ZB et al., 2011). In other words, in aquatic ecosystems, aquatic plants will not easily ‘release’ it back into the atmosphere once they have acquired a carbon source.
Taking limestone as an example, the (bio)chemical equation of the karst carbon cycle can be expressed as:
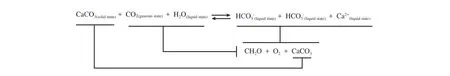
In terms of the equation, the result of the karst process, as is apparent, is to convert atmospheric CO2into organic carbon in water, although the actual process and results are much more sophisticated. In recent years, with more intense research, a karst carbon cycle process and carbon sink at watershed scale has been proposed (Cao JH et al., 2016). The karst carbon cycle process in a typical karst watershed can be shown as follows (Fig.1). In general, the carbon cycle process in a karst watershed mainly consists of three parts: carbonate rocks dissolving and transferring the atmospheric/soil CO2to water and to produce inorganic carbon; inorganic carbon transfer and conversion along with water flow; inorganic carbon converts to organic carbon with aquatic plant photosynthesis, part of the organic carbon deposits on the river / lake /reservoir beds mixing with the sediments.
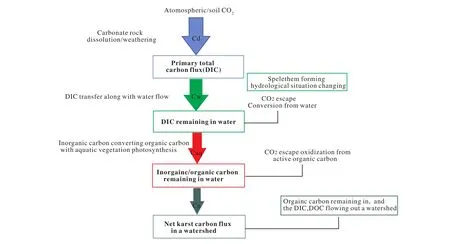
Fig. 1. Conceptual diagram of carbon cycle in karst watershed ( after Cao JH et al., 2016).
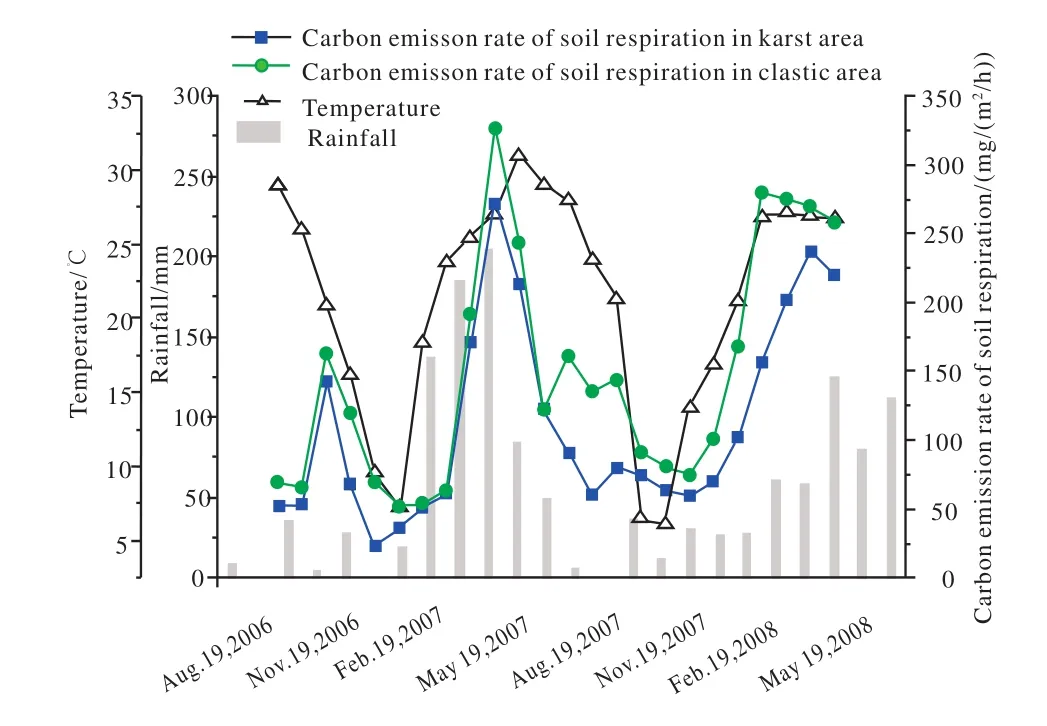
Fig. 2. Comparison of soil respiration change between limestone soil and red soil, Maocun, Guilin ( after Cao JH et al., 2011).
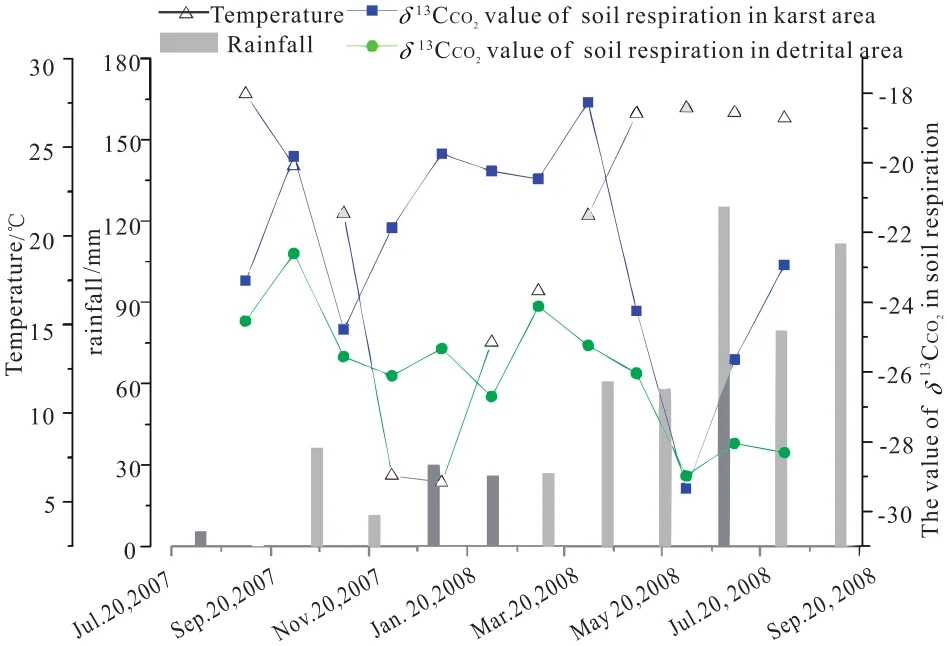
Fig. 3. Comparison of the δ13C value from soil respiration between the limestone soil and red loam, Maocun, Guilin (after Cao JH et al., 2011).
3. Evidence for the integration of geology and ecology in the karst carbon cycle process
The karst carbon cycle constitutes an important part of the process of Earth's surface system. It consumes atmospheric/soil CO2through the weathering and dissolution of carbonate rocks, and transport to the hydrosphere, and part of them convert into bio-organic carbon in the aquatic ecosystem. If we cannot fully understand the karst carbon cycle process, or only see part of the karst carbon cycle, or if we only see the ecological carbon cycle process whilst ignoring the karst geological process, we might consider or assume that the karst carbon cycle cannot produce carbon sink effects (Curl RL,2012), or even think it to be a carbon source (Zhang T et al.,2017; Butman D et al., 2011), as oversaturation of CO2in 57% of terrestrial freshwater lakes globally results from the dissolution and weathering of carbonate rocks in recharge areas (Rafael M et al., 2015).
To better understand the interaction between the karst carbon cycle (mainly the weathering and dissolution geological processes of carbonate rocks) and the ecological carbon cycle,as well as the ensuing carbon sink effect, this paper offers the following evidence using comparative study, carbon flux across the interfaces of terrestrial and aquatic ecosystems, and carbon stable isotope tracer technique, according to the results of projects funded by China Geological Survey (CGS)and the National Natural Science Foundation of China (NSFC) over recent years.
3.1. Evidence 1: Comparison of soil respiration and the release of CO2 isotopes between limestone soil and red soil
A comparison study on the soil carbon cycle, including CO2concentration in different soil layers and soil respiration between limestone soil and red soil was carried out in Maocun, Guilin, China (Cao JH et al., 2011). The recharge area of the underground river basin covers an area of 11.2 km2, including 7.6 km2of karst areas and 3.6 km2of nonkarst areas. The extent of the limestone soil formed by the weathering of carbonate rocks and the red soil formed by the weathering of sandstones and shale, a 1 m thick soil profile represents 1 km. The results of the monitoring were taken over two years, and showed that the rate of CO2emissions from soil respiration in limestone soils (ranging from 23.12?271.26 mg/(m2/h)), is significantly lower than that in red soils (ranging from 51.60?326.28 mg/(m2/h)). Taking the annual average values, the CO2emissions from soil respiration in karst areas are 25.12% lower than those in clastic rock areas; the CO2concentration in limestone soil profiles in karst areas exhibits a bi-directional gradient, which is more obvious during the rainy season with favorable water and heat conditions; while the CO2concentration in red soil profiles in clastic rock areas demonstrates a gradient in one direction,which increases with the depth of soil layers. Taking the average values of CO2concentration in soil profiles, the CO2concentration in limestone soils of karst areas ranges from 0.05%?0.60%, with an annual average value of 0.25%, while the CO2concentration in red soils of clastic rock areas ranges from 0.05%?1.09%, with an annual average value of 0.57%.This means that the dissolution of limestone at the soil-rock interface in karst areas will consume and transfer soil CO2to groundwater in the lower soil layers.
The range of δ13C values of CO2released from soil respiration in karst areas was heavier than that in clastic area, on average heavier by 3.53‰. While the δ13C value of CO2released from soil respiration in karst areas was calculated to be–29.35‰ to –18.26‰, with an average value of –22.68‰,that in the clastic area ranged from –29.21‰ to –22.60‰,with an average value of –26.21‰. A possible reason for this might be the involvement of the heavier carbon of CaCO3(+1‰ to –1‰) mixture in the limestone soil carbon cycle when carbonate rocks dissolve under the soil. If the values in the clastic areas are used as a background reference, when climate and ecological environmental conditions are assumed to be the same, the calculation shows 13.46% of the carbon from carbonate rocks was in the CO2emitted through soil respiration in the karst area.
As for the variation tendency of δ13C values of CO2emitted from soil respiration in karst and clastic areas, we can see that a greater differentiation occurs in the autumn and winter with lower temperatures and less rainfall, while the δ13C becomes lighter in the spring and summer with higher temperatures and humidity. The possible reason for this might be the high metabolic rate of vegetation and soil microorganisms under high temperature and humid climatic conditions. The carbon migration in the soil environment is dominated by the light carbon of biological origin, while the dissolution rate of carbonate rocks increasing the portion of the heavier carbon from the rock, lags somewhat behind the biological activities.On the other hand, the δ13C values of CO2emitted from limestone soil respiration might be impacted by intensification of biological activities and the rate of limestone dissolution.
3.2. Evidence 2: Comparison of HCO3- and carbon stable isotopes between karst water and allogenic water
The Maocun watershed area consists of two paired small watersheds of carbonate rocks and silicate rocks: the Xiaolongbei surface rivulet, which is composed of sandstone and shale from the Yingtang Formation of the Middle Devonian system, with a recharge area of 2 km2, adjacent to the Beidiping karst spring, which is made up of limestone-dolomites from the Donggangling Formation of the Middle Devonian system, with a recharge area of 1.5km2. The comparative monitoring results showed that:
(1) The concentration of HCO3-in the Beidiping karst spring is 4.2-5.5 mmol/L, while that in the Xiaolongbei surface rivulet is 0.2-0.4 mmol/L. The former value is ten times higher than the latter (Huang F et al., 2015). The fluxes of inorganic carbon generated by weathering and dissolution of rocks are 87.36 t HCO3-/km2·y and 3.67 t HCO3-/km2·y, respectively. The former value is 23.8 times that of the latter(Fig. 4). This proved the theory of Kump LR et al., (2000),who proposed that: over a relatively short time scale (103-104years), the dissolution rate of carbonate rocks will be much higher than that of silicate rocks, thus providing a large amount of inorganic carbon to the water.
(2) The δ13CDICvalue of karst spring water in Beidiping is–13.49‰ to –16.14‰, with the average value at –14.87‰.The δ13CDICvalue of allogenic rivulet water in Xiaolongbei is–4.01‰ to –11.67‰, with the average value at –8.68‰ (Fig. 5).The former is 6.19‰ lighter than that of the latter. We think this is because most inorganic carbon in the allogenic rivulet is from the atmosphere (the carbon stable isotope being –7‰to –8‰), and the CO2in rainwater becomes balanced, in other words, the CO2in rainwater is roughly at saturation, and when the rainwater falls, the light CO2in the soil is a little difficult to exchange with that in the rainwater, moreover, the silicate rocks are insoluble, therefore, the majority of inorganic carbon in the allogenic water comes from the atmospheric CO2; in contrast, the HCO3–of karst spring water is considered to be a mixture of both the soil CO2(with the δ13C value –23.94‰) and limestone heavy carbon (with the δ13C value 0.14‰).
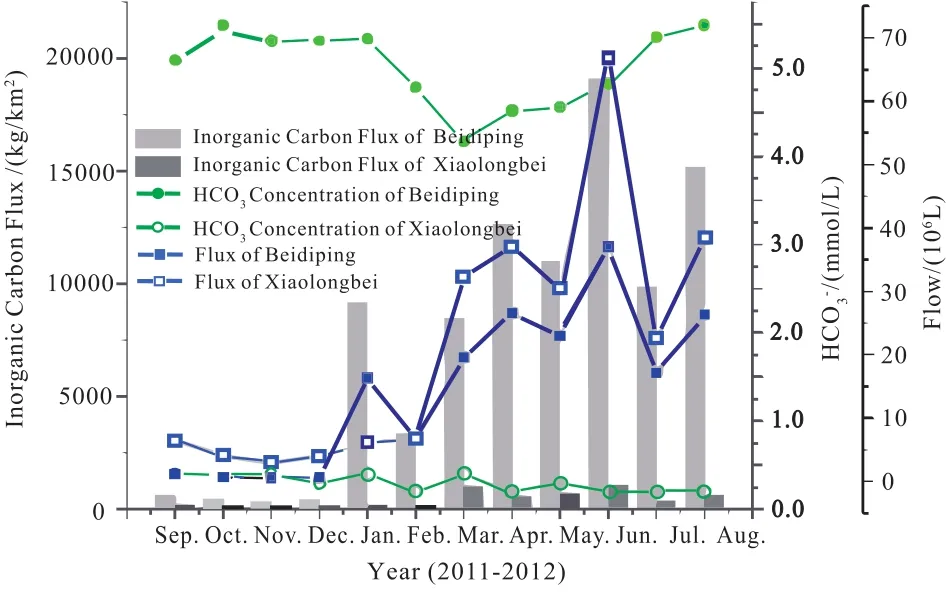
Fig. 4. Comparison of inorganic carbon concentration and flux between karst water in Beidiping and the allogenic water of the silicate rivulet in Xiaolongbei, Maocun, Guilin.
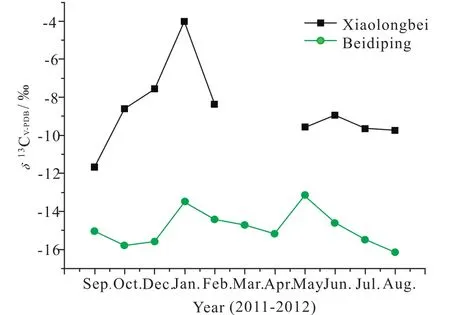
Fig. 5. Comparison of inorganic carbon isotopes between the karst spring water in Beidiping and the allogenic water in Xiaolongbei.
(3) According to chemical stoichiometry (Perrin AS et al.,2008), the flux of inorganic carbon in the karst spring water in Beidiping is 87.36 t /km2. y (monthly carbon flux at 315.19 to 18062.5 kg/ km2), with 54.83% to 62.95% (with an average of 58.52%) of them from carbonate rocks dissolving and weathering (monthly carbon flux at 167.22 to 11375.2 kg /km2). In comparison, the carbon from the atmosphere or the soil(monthly carbon flux at 141.92 to 7690.5 kg/ km2) accounts for 38.26% to 45.27%, with an average of 41.48% (Fig. 6a).
Based on the examination of the δ13C values of CO2in karst spring water, carbonate rocks and the soil, as well as the calculation of the two end-member mixing model (Goldscheider N et al., 2007), the contribution of carbon from atmosphere or the soil to karst spring water in Beidiping is 55.24% to 67.61%, with an average of 62.35% (Fig. 6b).
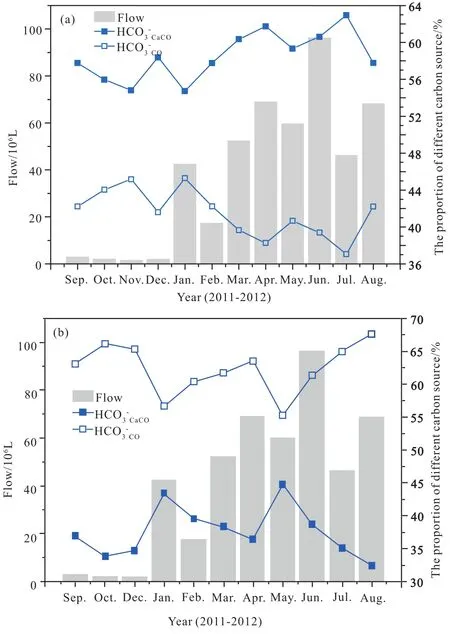
Fig. 6. Ratio of inorganic carbon sources in karst spring water. a:calculated from chemical stoichiometry; b: calculated from the two end-member mixing of carbon stable isotopes.
Some error exists between both results of chemical stoichiometry and the two end-member mixing of carbon stable isotopes, and intensive research of its mechanism is needed,however, it does confirm that the karst carbon cycle integrates the geological and ecological processes.
3.3. Evidence 3: CO2 exchange and carbon stable isotope variation at the water-air interface between karst water and allogenic water, Li River, Guilin
Li River with a recharge area of 5039 km2(from Yangshuo County to the Maoer Mountain), has an upstream covered with granites formed in the Caledonian Orogeny, and a downstream that reaches pure and thick limestone and dolomite of the Devonian and Carboniferous Systems, where the karst area covers 2392 km2, 51% of the total drainage basin(Fig. 7). We chose 4 typical allogenic sites of stream in the granite area and 4 typical karst water sites in the karst area. In rainy September and dry January, with the static floating boxes and vacuum bags, the exchanged flux of CO2at the water-air interface were examined and the samples for carbon stable isotopes were collected, and the samples of HCO3-carbon stable isotopes of water were also collected.
The results showed that:
(1) The CO2flux emitted from karst water to the air is much more than that from allogenic water in summer and winter. In September, the CO2flux of allogenic water in the granite area was 11.99 to 21.31 mg/m2.h, with an average of 16.38 mg/(m2/h); in comparison, that of karst water in the carbonate rock was 146.78 to 218.91 mg/(m2/h), with an average of 178.93 mg/(m2/h); the karst water was 10.92 times that of the allogenic water. In January, the amounts of the CO2fluxes were 9.00 to 34.01 mg/(m2/h) (with an average of 17.84 mg/(m2/h) and 23.04 to 136.13 mg/(m2/h) (with an average of 59.01 mg/(m2/h) respectively in the granite area and the carbonate rock area, the karst water was 3.31 times that of the allogenic water (Table 1, Fig. 8).

Table 1. Comparison of CO2 flux amount at the water-air interface, carbon stable isotopes of water, emitted CO2 between the allogenic and karst water in the Li River basin.

Fig. 7. Map of karst distribution, monitoring sites and sampling sites in the Li River basin.
In the summer, the monsoon climate produces extremely good conditions of heat and moisture for the karst carbon cycle, and more CO2was emitted from karst water to the air.With the karst carbon cycle at the watershed scale, much more atmospheric/soil CO2was removed to water with carbonate rock dissolution and weathering, and intensive karst carbon cycle and more carbon sequestration occurred. The monitoring data already showed that the CO2from the karst water to the air only took a small part of the total consumption of atmospheric/soil CO2by the carbonate rocks dissolution in the karst area (Mo X et al., 2014).
(2) Based on the results of emitted CO2and HCO3-carbon stable isotopes in the water, the intense karst carbon cycle and ecological carbon cycle in the summer is inferred to be the major reason for the carbon stable isotope difference of the water HCO3–and the emitted CO2. In January, the HCO3–carbon stable isotope in the allogenic water was –8.35‰ to–13.35‰, with an average of –10.26‰; the emitted CO2was– 11.87‰ to –12.66‰, with an average of –12.28‰; in comparison, the HCO3-carbon stable isotope in the karst water was –10.24‰ to –11.42‰, with an average of –10.92‰; the emitted CO2was –12.03‰ to –12.90‰, with an average of–12.28‰.
In September, the HCO3-carbon stable isotope of the allogenic water was – 6.67‰ to –8.45‰, with an average of–7.52‰; the emitted CO2was –9.87‰ to –13.15‰, with an average of –11.27‰; in comparison, the HCO3–carbon stable isotope of the karst water was –15.59‰ to –16.64‰, with an average of –16.14‰; the emitted CO2was – 10.51‰ to –12.24‰, with an average of –11.57‰.
One interesting observation was that in the granite area,the carbon stable isotope of emitted CO2is 2.02‰ to 3.75‰lighter than that of HCO3–, in January and in September;however, in the carbonate rock area, in September with more heat and moisture, the carbon stable isotope of HCO3–in karst water is 4.57‰ lighter than that of emitted CO2. The possible reason is closely related to the intensive karst carbon cycle and ecological cycle, the deep mechanisms of which will be researched in the future.
3.4. Evidence 4: The photosynthesis of aquatic plants to use the inorganic carbon and carbon stable isotope changes when the karst underground water becomes surface water
There is a stream named Zhaidi with length of 512 m, its upstream is the outlet of the Zhaidi karst underground river and the downstream is connected to the Chaotian River,flowering aquatic vegetation can be found in the stream, and 4 plants species namelyOttelia accuminata,Vallisneria natans,Potamogeton wrightii and Hydrilla verticillataare dominant submerged plants. The 4 species of plants were collected to examine their organic carbon isotope, and the results are shown (Table 2, Fig. 9):
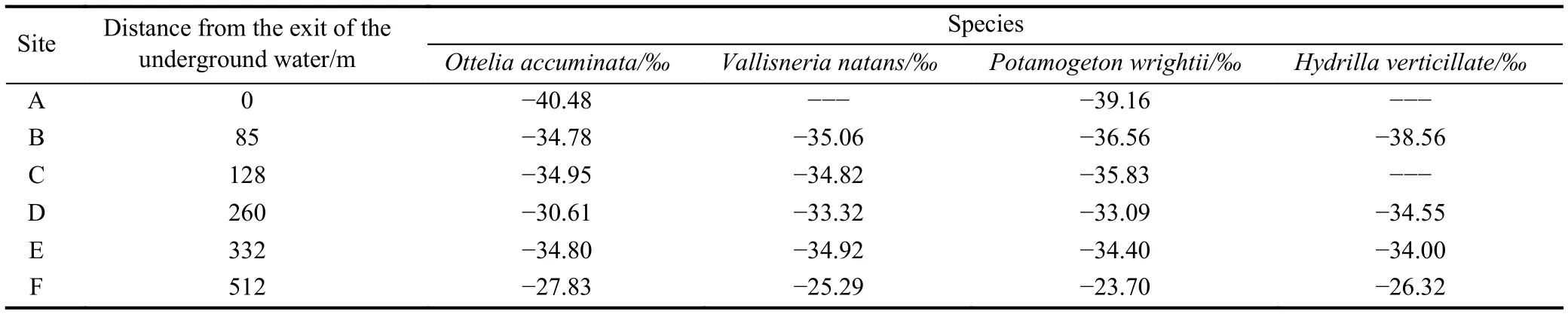
Table 2. Organic carbon isotope of 4 dominant submerged plants’ leaves at 6 monitoring sites in Zhaidi stream, Guilin, China.
(1) These aquatic plants’ organic carbon isotope became heavier and heavier moving from upstream to downstream,withOttelia accuminatain the range from –40.48‰ to–27.83‰, andPotamogeton wrightiifrom –39.16‰ to–23.70‰.
(2) At the B site, all 4 species of plants were collected,and their organic carbon isotope has the 3.78‰ maximum difference; at the F site, the maximum difference slightly increases to 4.31‰.
The A and F sites were chosen to monitor the diurnal variation of water chemistry, from Sept. 10 to Sept. 12, in 2014,with the results indicated (Fig. 10):

Fig. 8. Comparison of the CO2 flux at the water-air interface, carbon stable isotopes of water, emitted CO2 between the allogenic and karst water in the Li River basin. a: January; b: September.
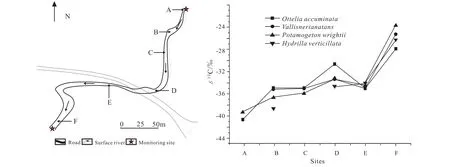
Fig. 9. Sketch of Zhaidi stream and changes of the stable carbon isotope of 4 dominant submerged plants’ leaves along the stream, Guilin,China (after Wang P et al., 2017).

Fig. 10. Diurnal variation of HCO3-, CO2, and δ13CDIC values in water at both monitoring sites (site A and site F. The shaded box shows the night time)(after Wang P et al., 2017).
(1) At site A, the karst underground water has just emerged, with the concentration of HCO3–at 3.9 to 4.1 mmol/L,and a high CO2partial pressure at 0.23 to 0.38 mmol/L; while,theδ13C value of HCO3-in the water was at –14.00 to–14.99‰, with an average of –14.5‰, the daily change is small.
(2) At site F, the karst water has flowed for 512m, theδ13C value of HCO3–was at 3.5 to 3.9 mmol/L, and the CO2partial pressure was of 0.11 to 0.22 mmol/L; while, theδ13C value of HCO3–in the water was at –14.25 to –13.07‰, with an average –13.5‰, the daily change is relatively large.
Compared to site A, at site F, the concentration of HCO3-and the CO2partial pressure decreased, theδ13C value of HCO3-in the water got heavier; the ratio of HCO3-to CO2turned from 16:1 to 32:1.
Therefore, it can be concluded that the aquatic plants absorb and use light carbon12C in preference to the heavier,moreover, the light carbon12C easily escapes from water to air, therefore, as more heavy carbon13C is left in the water,the aquatic plants are forced to absorb and use more heavy carbon13C.
Based on calculation of the dissolved oxygen, HCO3-concentration, and the inorganic carbon flux, 12.52% of inorganic carbon was converted into organic carbon along the 512m Zhai stream (Wang P et al., 2017).
Zhang C et al., (2015) found that in a surface stream 1.35 km long, the upstream is the outlet of the Guancun karst underground river, Liuzhou, the concentration of HCO3-and Ca2+of water were diminished along the stream way. The results of the monitoring data show that the aquatic vegetation photosynthesis consumed HCO3-at 1153mmol/d, and 88% of their biomass came from HCO3-in the water.
4. The contribution of the karst carbon cycle to the global carbon cycle
Most data shows that the CO2of carbonate rock weathering and dissolution is mainly derived from biological activities (soil CO2) (Li SL et al., 2004; Zhao M et al., 2009), and 10%–15% of the world’s karst terrain contributes 50% carbon flux in continental weathering (Gombert P et al., 2002; Suchet PA et al., 2003; Cao JH et al., 2012). Depending on the published data, the terrestrial rivers transport 0.26 to 0.30 PgC/a of DIC,0.20 to 0.22 PgC/a of DOC, and 0.18 to 0.23 PgC/a of POC to the oceans (Einsele et al. 2001); the underground water transports 0.2 PgC/a to the ocean; meanwhile, there are 0.23 to 0.60 PgC/a of carbon left in the inland waters (lakes and reservoirs) (Gombert P et al., 2002; Suchet PA et al., 2003;Cao JH et al., 2012), if the karst carbon sequestration effect is estimated by 1/2 whole continent weathering, the carbon flux derived from carbonate rock dissolution would be 0.53 to 0.58 PgC/a, which is higher than the 0.48 PgC/a estimated by Liu ZH et al., (2007). This value equals 31.18% to 34.41% of the global forest carbon sequestration and 66.25% to 73.12% of the soil carbon flux.
It is also equivalent to 22.08% to 24.17% of the missing carbon (2.5 PgC/a), as well as 7.5% to 8.3% of the emission of fossil fuels combusted globally (7.0PgC/a) (Lal R, 2008).
Wu ZB et al., (2011) estimated China’s karst carbon flux based on the chemical components and the hydrological situations of the nine major river drainages of China, and the results showed that the consumption of CO2through the dissolution and weathering of carbonate rocks was 0.016-0.024 PgC/a; Liu ZH et al., (2000) also estimated that China’s karst carbon flux was at 0.018 PgC/a; Jiang ZC et al., (2000) estimated to be 0.01 PgC/a. Qiu DS et al., (2004) estimated that China’s karst carbon sequestration was 0.014PgC/a. All estimates mentioned above were based on the runoff-hydrochemistry method, ignoring the inland aquatic eco-system and underground waters, and regardless of the large proportion of dams constructed in the karst areas. According to the global ratio of carbon flux among surface river and underground water flowing to the ocean and left in inland water of 1:1:1,China’s karst carbon flux would be at 0.03 to 0.072 PgC/a, the medium value of 0.051 PgC/a, equal to the estimation of forest carbon sequestration of 68% by Fang JY et al., (2007).
5. Conclusions
(1) The karst dynamic system is an important part of the Earth Surface System, and the karst carbon cycle is coupled with the geological process of carbonate rock weathering and the carbon cycle of the terrestrial ecosystem. The karst carbon cycle process on a watershed scale includes: (a). carbonate rock dissolution removing the atmospheric/soil CO2to water and to produce inorganic carbon; (b). inorganic carbon transfer and conversion along with water flow; (c). inorganic carbon converting to organic carbon via the aquatic plants photosynthesis, part of the organic carbon deposit on the river/lake/reservoir beds mixing with sediments. The conceptual model of the karst carbon cycle on a watershed scale bears some resemblances to the carbon cycle in the terrestrial ecosystem.
(2) The heavy-stable carbon isotope (–1‰ to 1‰) of carbonate rocks and the intensive biological fractionation lead to light stable carbon isotopes moving in the terrestrial ecosystem, providing tracer and indicator to prove the geologicalecological integration in the karst carbon cycle. Another way of demonstrating this is through dynamic monitoring and comparison of paired small karst watershed and non-karst watershed.
(3) The field experimental site of the karst carbon cycle,Maocun Village, Guilin, offers paired limestone soil and red soil profiles, which with the forest vegetation cover of 1m in depth, and the distance between both soil profiles being 1km,locates one in the karst area and the other one in the non-karst area, the data of the soil carbon cycle for two years showed that the limestone soil respiration in the karst area was 25% lower than that in non-karst, indicating the carbonate rock dissolution under the soil consumed large amounts of soil CO2and removed it to water, demonstrating the process of the karst carbon cycle . Depending on the carbon isotope of emitted CO2of soil respiration, the average δ13C value of emitted CO2in the karst area was –22.68‰, that in the non-karst area was –26.21‰; this suggests that 13.46% of the emitted CO2from the limestone soil respiration was derived from carbonate rock dissolution.
(4) The comparative results of paired small watershed monitoring showed that the HCO3–concentration in the karst water was 10 times that of in allogenic water in a non-karst rivulet, and the inorganic carbon flux was even 23.8 times higher. With both chemical stoichiometry and carbon stable isotopes, the proportion of carbon in karst springs derived from carbonate rocks was found to be 58.52% and 37.65% respectively.
(5) In September, the HCO3–carbon stable isotope of the allogenic water in the granite area, upstream of the Lijiang River, was an average –7.52‰, where the carbon mainly comes from the atmosphere; in comparison, the HCO3–carbon stable isotope of the karst water in the carbonate rock area was an average –16.14‰, where the carbon comes mainly from the soil and the carbonate rock.
With the intense karst carbon cycle process, the CO2in karst water is mostly either at saturation or super-saturation,and the amount of emitted CO2at the interface of karst waterair, was 10.92 times that of the allogenic water, which does not indicate a carbon source effect of karst water, but instead the great karst carbon sequestration effect.
(6) The underground karst water in Zhaidi in Guilin surfaced, and the carbon-rich karst water stimulated the growth of the aquatic plants; the photosynthesis of aquatic vegetation in the upstream absorbed light12C preferentially, leaving more13C in the water, and leading to submerged plants photosynthesis being forced to absorb and use more13C the further downstream that they were. Consequently, the organic carbon isotope of the same submerged plants became heavier from upstream to downstream, the maximum variation could be 15.46‰, in the meantime, the 12.52% inorganic carbon was converted into organic carbon over the course of the 512 m water flow.
(7) Depending on published data and following the conceptual model of the karst carbon cycle in a watershed, the global karst carbon sequestration effect estimated could be 0.53 to 0.58 PgC/a, equivalent to 31.18%-34.41% of the global forest carbon sequestration flux. In addition, the karst carbon sink flux in China was calculated to be 0.051 PgC/a, accounting for 68% of its forest carbon sink flux.
Acknowledgement
This paper is granted by the National Key Research and Development Program project (2016YFC0502500), a Key project of National Natural Science Foundation (41530316),the China Geological Survey Programs of the Geological Environmental Innovation, Comprehensive Survey of Hydrogeology and Environment Geology in Karst Area, IGCP-661 Processes, Cycle, and Sustainability of the Critical Zone in Karst Systems.
- China Geology的其它文章
- 2018 International Symposium on Deep Earth Exploration and Practices (DEEP-2018),Beijing, China, 24-26 October
- Thinking on improvement of natural resources management
- Foreword
- Introduction of the SinoProbe Center, China Geological Survey
- New prospecting progress using information and big data of coal and oil exploration holes on sandstone-type uranium deposit in North China
- A super-large graphite deposit discovered in granite rocks at Huangyangshan,Xinjiang, China

