The combined effects of lysozyme and ascorbic acid on microstructure and properties of zein-based films☆
Dongwei Wei,Weizhi Huo ,Guangmeng Li,Qiuling Xie ,3,Yanbin Jiang ,*
1 School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,China
2 College of Life Science and Technology,Jinan University,Guangzhou 510632,China
3 National Engineering Research Centre of Genetic Medicine,Guangzhou 510632,China
1.Introduction
Over recent decades,zein,the major co-product of the oil and rapidly growing bioethanol industries,has drawn dramatic attention in the applications of food and pharmaceuticals[1].Zein is a water-insoluble prolamin present in corn endosperm cells with a high content of hydrophobic amino acids(leucine,proline and alanine)[2].It is one of the rare proteins soluble in various organic solvents including ethanol,and can be cast into films by dissolving it in a hydrated organic solvent,such as ethanol or acetone,and then drying[3].Because of the good filming property[4],non-toxic[5],excellent biocompatibility[6],extensive sources[7]and low costs[8],dramatic investigation to zein utilized as a carrier in food and pharmaceuticals have been conducted[9].Meanwhile,considering the amphiphilic property of zein molecules,the main targets of the films used as packaging are the intermediate moisture foods,e.g.,cheese[10],natural apricots[11],tomato[12],walnut meat[13]and fruits[14].
Among zein-based films,development of the films containing active components with antimicrobial or antioxidant activity is one of the important aspects[15].For the active components,due to the health concerns of consumers and the environmental problems derived from packaging waste,various research groups are looking towards natural active materials,e.g.,essential oils[16],glucose oxidase[17],bacteriocins[18],sodium caseinate[19],and nisin[20].Among the natural active materials,lysozyme(LY)and ascorbic acid(AA)are the most promising active components.LY obtained from hen egg white is one of the most frequently used bio-preservatives in antimicrobial packaging[21,22].LYshows antimicrobial activity mainly on Gram-positive bacteria by splitting the bonds between N-acetylmuramic acid and N-acetylglucosamine of the peptidoglycan in their cell walls.Because of their protective outer membrane surrounding,the peptidoglycan layer,LY does not show antibacterial activity against gram-negative bacteria[23].AA is,apart from its role as a nutrient,an antioxidant commonly used for maintaining organoleptic quality in many food systems[24],and recently has been used to rapidly and continuously oxidize organic contaminants[25].The antioxidant effect is due to its ability to scavenge oxygen and protect double bonds[26].Therefore,AA has long been used in food,pharmaceuticals and cosmetic preparations[27].Another important and prevailing aspect of AA is the low cost,which makes it possible to be widely used in the applications of food and pharmaceuticals.Compared with the costs of other antioxidants frequently used as the model actives in other studies,e.g.,(+)?catechin hydrate(≥95%,HPLC),resveratrol(99%),(+)?γ?tocopherol(96%)and galic acid(99%),the cost of AA accounts for approximately 0.47%,1.57%,0.25‰and 20.87%,respectively(Aladdin?).The simultaneous addition of LY and AA not only protects the packed products due to the antimicrobial and oxidant activities,but also provides the nutrients which are beneficial to human health.However,few studies are conducted to combine LY and AA as antimicrobial and antioxidative actives to develop zein-based films.
The aim of this study is to develop zein-based films containing LY and AA,and investigate the combined effects of LY and AA on the films.The effects of LY and AA on the microstructure,mechanical and release properties of zein-based films were investigated in detail,and based on the physico-chemical characteristics,the mechanisms of mechanical properties and release behavior of zein-based films were discussed.This study is helpful for further developing zein films with well controlled release,antioxidant and antimicrobial properties in the applications of foods and pharmaceuticals.
2.Materials and Methods
2.1.Materials
Zein(food grade)was purchased from Sigma-Aldrich Shanghai Trading Co.Ltd.LY(BR,20000 U·mg?1),Listeria innocua(L.innocua,S25897)and Micrococcus lysodeikticus(BR)were purchased from the Shanghai Yuanye Bio-Technology Co.,Ltd.without further treatment.AA and 6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid(Trolox,99.99 wt%)were purchased from the Aladdin Ind.Corp.Potassium persulfate(K2S2O8),potassium bromide(KBr)and 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonic acid)diammonium salt(ABTS,99 wt%)were purchased from the Sun Chem.Tech.Co.Ltd.PEG 400,ethanol(Et)(AR)were purchased from the Guangdong Guanghua Sci.Tech.Co.,Ltd.Crystal Fuji apple was purchased from Longsheng Fruit Co.,Ltd.during the winter of 2016 and stored at 4°C until needed.Deionized water was used in the experiment.All other chemicals were reagent grade.
2.2.Preparation of zein-based films
Zein-based filmswere prepared as follows,with retro fitting from the method described by Soliman et al.[28].(1)Film-forming solutions were prepared by weighing with or without PEG 400(40%w/w of zein)and 30 ml of aqueous ethanol(80%v/v in water)into beakers,followed by adding 3.0 g of zein powder weighed using an analytical balance(AL104,Mettler Toledo,Switzerland).(2)The film-forming solutions were stirred by a stirrer(MS-M-S10,Dragon-Lab,China)at a speed of 500 r·min?1at ambient temperature for 20 min,then treated by an ultrasound device(KQ5200B,Kunshan Ultrasonic Instrument Co.Ltd.,China)for 40 s.(3)The beakers were heated at(70 ± 1)°C for 10 min and meanwhile stirred at a speed of 300 r·min?1,then cooled to room temperature.(4)AA(12.5 mg·g?1film-forming solution)and LY(16.5 mg·g?1film-forming solution)were added into filmforming solutions.(5)10 ml of the film-forming solutions were poured into rectangular polypropylene vessels(L×W×H:11.5×3.5×3 cm3),which were placed in a controlled test cabinet(SMC-22PF,Dongguan Haotian Test Equipment Co.Ltd.,China)at 30°C and 30%relative humidity(RH)for 36 h to evaporate the solvents and produce freestanding films.(6)After drying,the films were carefully peeled from the vessels and the average thicknesses of the films were obtained using a micrometer,and a minimum of7 measurements were conducted at different points of each film.(7)The films were cutinto 2×8 cm2wide strips and used in all other tests.
2.3.Film characterization
2.3.1.Scanning electron microscopy(SEM)of films
The cross-section morphology of typical zein-based films was observed using a SEM(Zeiss MERLIN Field Emission SEM,Carl Zeiss NTS GmbH,GER).Before observation,double-sided adhesive carbon tape was put on an aluminum stub and the films were also put on the tape.Then they were sputter-coated with a thin layer of gold under high vacuum conditions(0.05 mTorr).
2.3.2.Fourier-transform infrared(FT-IR)spectroscopy
The FT-IR spectra were obtained by using a FT-IR spectrophotometer(Nicolet Nexus 670,Thermo Electron Corporation,USA).Crystals of KBr were taken out and ground until they became fine powders,and then dried under an infrared lamp at least for 10 min.A tablet containing approximately 20 mg of KBr was prepared under pressure conditions of 6 MPa for 10 s.Samples were prepared by adding the film-forming solutions using a capillary to place it onto the surface of the tablet,then dried by an infrared lamp for 5 min.Interferogram was averaged for 32 scans at 4 cm?1resolution and the scanning range was 400–4000 cm?1.The background spectrum of KBr was automatically subtracted.Finally,the spectra were normalized in specific regions.
2.3.3.Mechanical properties of films
Tensile strength(TS),elongation atbreak(EB),and Young's modulus of zein-based films were measured using a universal testing machine(3367,INSTRON,USA)according to ASTM D882-02[29]and Arcan,et al.[30].Young's modulus of zein films is calculated based on the original linear part of the strain–stress curve.The films were conditioned for 48 h at25°C and(50±2)%RH.Films were cutinto 80 mm length and 20 mm wide strips,the initialgrip distance was 50 mm,and the crosshead speed was 50 mm·min?1.At least 6 replicates of each film were tested.
2.3.4.LY Release profiles and antimicrobial potential of films
LYrelease profiles of zein-based films were produced by the method described by Arcan et al.[30].Brie fly,the films were cut into 2×8 cm2wide strips and dried under the conditions of 30°C and 30%RH for 2 h before the tests.Each film was placed into a beaker containing 50 ml water,and the beaker was placed into a constant temperature shaker(ISHZ-300A,Suzhou Pei Experimental Equipment Co.Ltd.,CHN).The temperature and shaking speed were set at 4 °C and 80 r·min?1.The antimicrobial potential of zein-based films was tested by following the method described by Arcan et al.[30]and Moradi et al.[31]with minor modifications.The diameter of test films punched from the zein-based films by a cork borer was 11 mm.The concentration of bacterial suspensions was adjusted to approximately 1.0 optical density(OD)using a UV–vis spectrophotometer(Model 2450,Shimadzu,JP)at 600 nm.200 μl of the bacterial inoculum was added and spread evenly onto a Petri dish.Each three round films were placed into a dish,which was placed in an incubator at 37°C for 12 h,and the clear inhibition areas were formed and measured.The diameters of the areas were measured by a digital caliper in triplicate.The final antimicrobial activities of the tested films were obtained through calculating inhibition areas(mm2)by subtracting the areas ofthe applied film discs.
2.3.5.AA release profiles and antioxidant capacity of films
To determine AA release profiles of zein-based films,the release tests were conducted in water by referring to the section of LY release profiles[30].The soluble AA contents of the tested films were determined by a UV–vis spectrophotometer at(264± 1)nm according to the colorimetric method of Cupello et al.[32].The total soluble AA contents released from zein-based films were expressed as milligrams of AA per square centimeter of the films(mg·cm?2)using the AA calibration curve.All concentration measurements were conducted three times.The antioxidant capacities of the films were based on calculating the Trolox equivalentantioxidantcapacity(TEAC)of their total released AA content at the equilibrium.The percentages of free soluble AA in the films were determined by the formula of total released AA/initial AA added×100%.The TEAC of AA wasdet ermined by using the spectrophotometric ABTS radicalcation decolourization assay conducted at734 nm[33].The results were expressed as micromoles of Trolox equivalents released per square centimeter of films(μmol Trolox·cm?2).
2.3.6.Enzymatic browning at cut surfaces
To determine the ability of inhibiting enzymatic browning of the developed zein-based films,enzymatic browning tests at cut surfaces of the Crystal Fuji apple were conducted as described by Sapers et al.with minor modifications[34].Brie fly,an apple was cut in half along the stem axis,and the halves were positioned in a Petri dish with the cut side down.Then uniform plugs could be bored perpendicular to the cut surface using a cork borer with the diameter of 16 mm.At the beginning,a transverse cut was made in the plug and two little plugs were obtained which exposed fresh surface,one was used as test sample,and the other one was used as control sample.Therefore,many plugs with diameters about 14 mm were obtained in only one apple.The zein-based films were cut into pieces with the areas large enough to cover the fresh surface of the plugs,and placed onto the fresh surface of the plugs.
Colorimetry was performed with a Chroma Meter(CR-400,Konica Minolta Sensing,JP),operated with an illumination device and with a 13 mm opening aperture plate.Values of the tristimulus co-ordinates in the L,a,b and ΔL,Δa,Δb systems were recorded at 0,2,4,6,8,and 10 h.During measurements,plugs were held in covered petri dishes to minimize dehydration at the cut surface.
The extent to inhibit the enzymatic browning was expressed on a percentage basis,e.g.,the percentage difference between the control and treatment ΔL or Δ a values after a specified storage time t was calculated as follows:
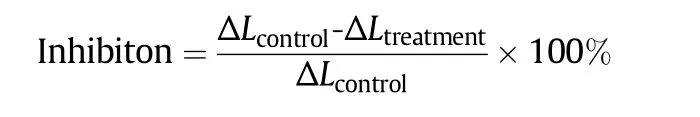
where ΔL(or Δa)is the difference between the L-(or a-value)at time t and the time at 1 min;“control”means that the plug is untreated with film and the “treatment”means that the plug is treated with zeinbased film.Positive values of the percentage inhibition between 0 and 100 would indicate that the treatment was effective as a browning inhibitor.Values greater than 100%,if significant,would indicate sample bleaching by the treatment,while negative values would indicate that the treatment promoted rather than inhibited browning.
2.3.7.Statistical analysis
To determine the effects of film compositions on mechanical properties,antimicrobial and antioxidant potentials of films,analysis of variance was conducted,means were subjected to Duncan's test and a P-value of<0.05 was considered statistically significant using software IBM SPSS Statistic 19.All experiments were carried out at least three times,and the results were reported as average and standard deviations of these measurements.
3.Results and Discussion
3.1.Microstructure
Photographs of typical zein-based films are shown in Fig.1,which demonstrates that the developed zein-based films are homogeneous and even though LY is hydrophilic,it is still evenly distributed in the zein-based films.
The cross-section morphologies of zein-based films(Fig.2)were obtained to investigate the morphological changes in films,which occurred when they underwent plasticization with PEG 400,AA and LY.Morphology of the film containing zein and PEG 400(Film 4)suggested that there were micro-bubbles embedded in the zein matrix or spaces occupied by PEG 400 previous to the drying process[35].When LY concentration was 4.1 mg·cm?2,with the increase of AA concentration(Films 5,7,9 and 11),it was clearly shown that the number of insoluble LY aggregates in the cross-section of the films increased gradually.Meanwhile,it was clear that cracks in the cross-section of the zein matrix disappeared,and the zein matrix became more compact and smooth.The reason for insoluble LY aggregates existing in zeinbased films could be attributed to the hydrophilicity of LY,which limits the solubilization and homogenous distribution of LY in hydrophobic zein matrix.Meanwhile,as a micro molecule,AA is dissolved more easily in zein-based films than LY,and dissolved AA increases the number of LY aggregates.The increased hydrophilic property of films caused by AA allows the LY aggregations to be well distributed in the zein matrix.Therefore,the number of LY aggregates increased dramatically with the increase in AA concentration.Actually,during the process of preparing zein-based films,it was observed that AA was completely dissolved in the film-forming solution,butLY dissolved in distilled water in advance was precipitated and heterogeneously distributed in the film forming solution.Furthermore,when the film-solutions were poured into the rectangular polypropylene vessels that were placed on the black table to see the solubility of LY in film solutions,it was clearly seen that the diameter of LY aggregates in film-solution containing AA was much smaller than that of in film solution without AA.With the increase of LY aggregates in zein-based films,this leads to the increase of initial LY release rate,total released LY and the anti-microbial activity.In contrast,the compact and smooth cross-section of the films decreases the initial LY release rate and total released LY.Therefore,it suggests that the LY release profile is jointly affected by LY aggregates and compact cross-section of zein-based films.
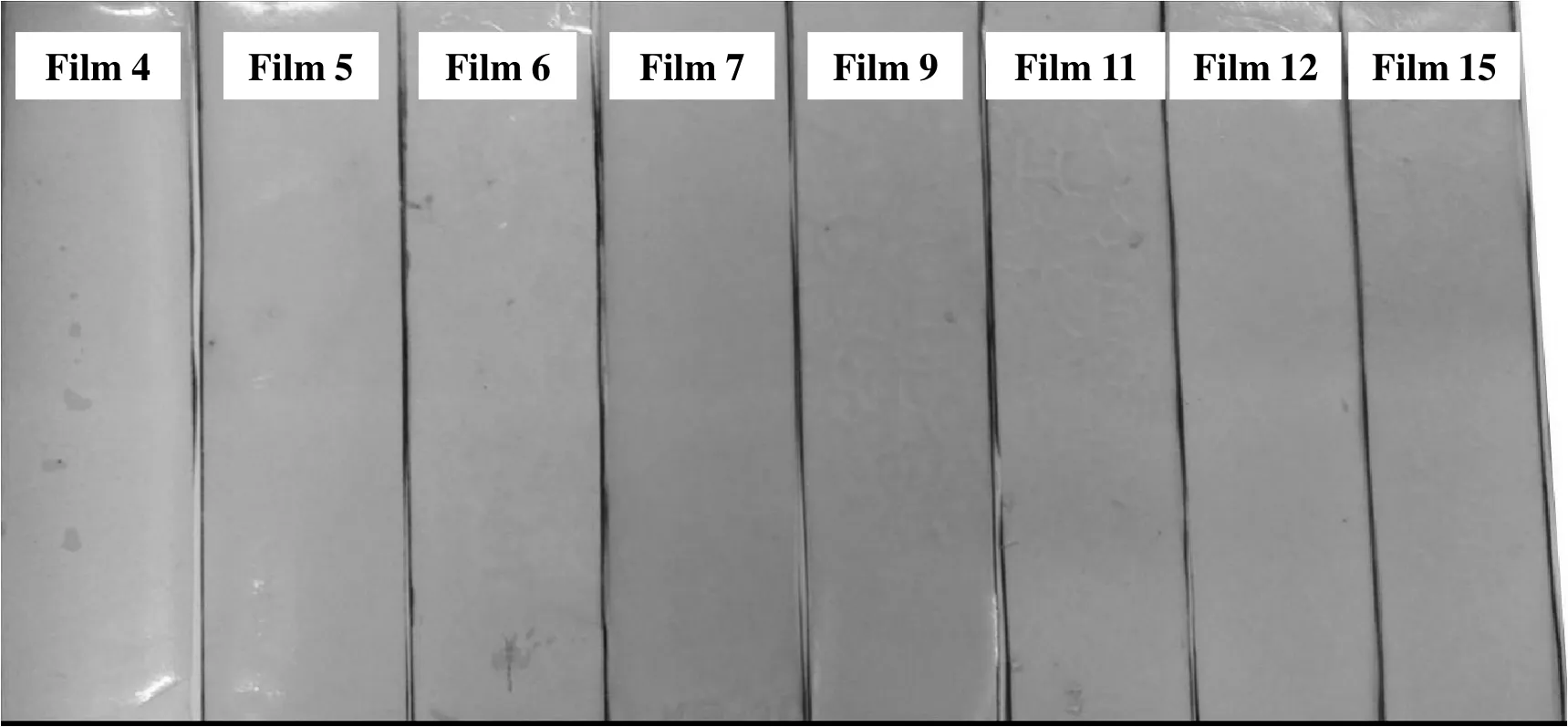
Fig.1.Photographs of typical zein-based films.
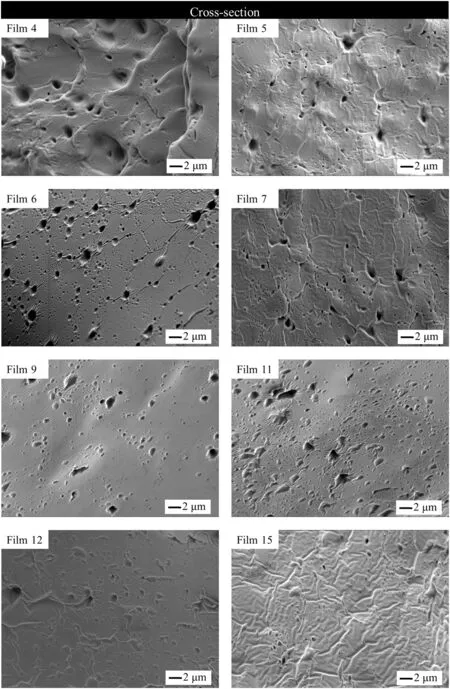
Fig.2.SEM images of cross-sections of typical zein-based films.
When AA concentration was 3.1 mg·cm?2,it was clear that with the increase ofLY concentration(Films 6,9,12 and 15),the number ofpores on the surface of zein-based films increased dramatically(not given),which could be due to the low concentration of zein and the fast rate of Et evaporation[36]as well as the immiscibility of zein and LY.Meanwhile,as shown in Film 15,a number of insoluble LY aggregates existed in the substrate of zein-based films and cracks appeared.This could be due to the weakened plasticization of LY on zein-based films,and will be further discussed in the section of testing mechanical properties of zein films.The morphology of the developed zein-based films shows that LY and AA jointly affect the microstructure of zein-based films,which would further affect the release profiles of the films.
3.2.FT-IR characterization
FT-IR is used to investigate the intermolecular/intramolecular interactions of different ingredients in zein-based films,and the FT-IRspectra of raw materials and typical samples are shown in Fig.3.As shown in Fig.3(A),for zein and LY,the main absorbance peaks are identified as C=O stretching at approximately 1647.1 cm?1(amide I),N--H bending vibrations at about 1529.4 cm?1(amide II),C--H deformation at 1444.6 cm?1and C--N stretching at 1236.3 cm?1(amide III).1647.1 cm?1(C=O stretching)and 1649.0 cm?1(C=O stretching)were selected as characteristic absorptions for zein and LY respectively.The peaks of characteristic absorption of AA are identified as C=O stretching at approximately 1762.8 cm?1which was bonded by intramolecular hydrogen bonds,and 1677.9 cm?1which was bonded by intermolecular hydrogen bonds.
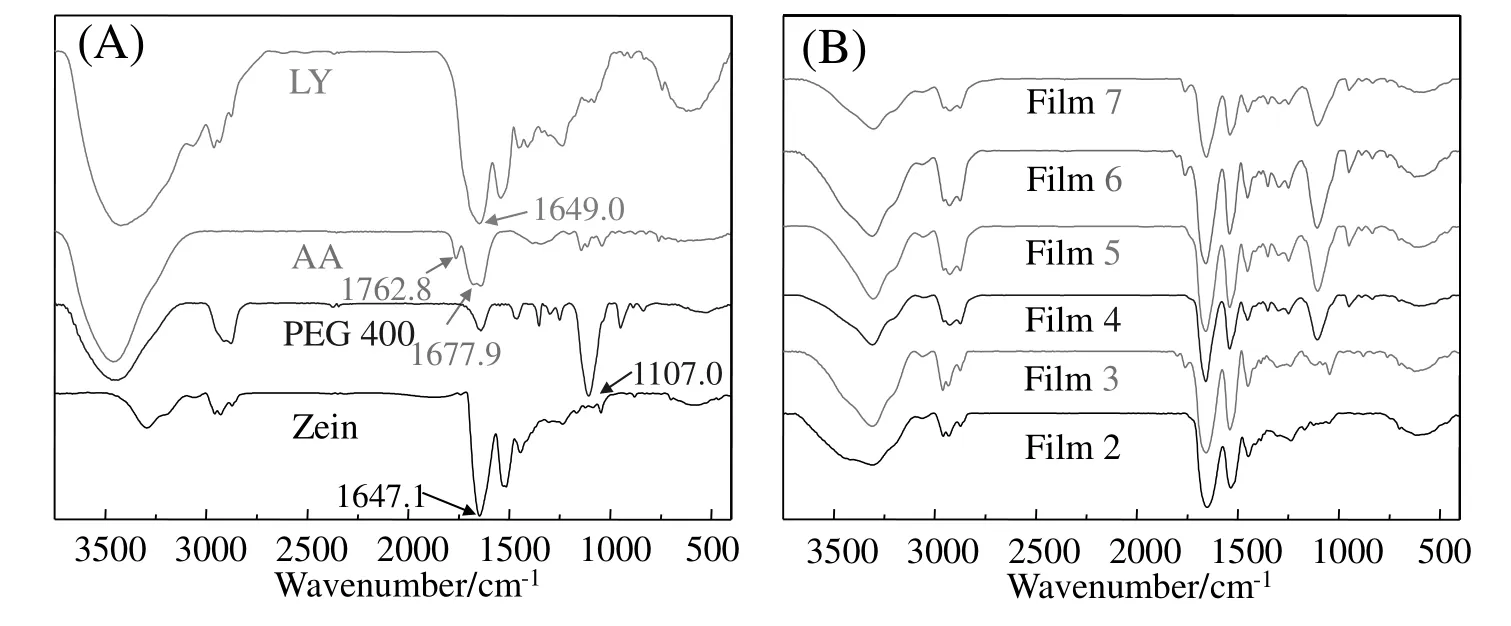
Fig.3.FT-IR of raw materials(A)and typical zein-based films(B).
The FT-IR spectra of typical samples were shown in Fig.3(B),which indicated that no new peak appeared in all of the spectra,which suggested that no chemical reaction occurred.However,it was clearly shown that molecular structure of zein,AA,PEG 400 and LY changed in zein-based films according to the shifts of the characteristic absorptions.For zein,1647.1 cm?1(C=O stretching)was selected as the characteristic absorption,and blueshifts at 1647.1 cm?1of Films 2,3,4,5,6 and 7 apparently occurred(Fig.4).The results demonstrated that the intermolecular and intramolecular hydrogen bonds of zein dramatically decreased,and zein film was clearly plasticized by LY,AA and PEG 400.As shown in Fig.4,the blueshifts in Films 2,3 and 4 significantly increased,which demonstrated that the plasticization effect of PEG 400 on zein-based film was the most significant.The results suggest that as a single additive,PEG 400 probably has the most effective plasticization for zein,which will be further proved by mechanical tests.Compared with Film 4,the blueshifts in Films 5 and 6 did not change,but Film 7 changed.The results suggested that PEG 400 was the main plasticizer among the additives,and the simultaneous addition of AA and LY probably weakened the plasticization of zein-based films,which would also be further proved by mechanical tests.
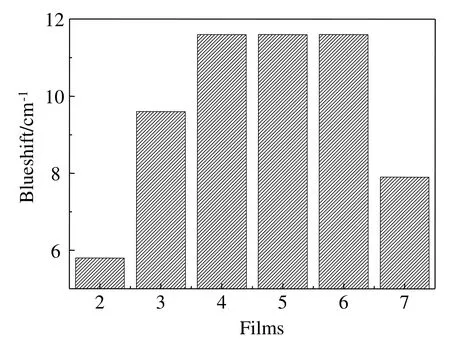
Fig.4.Blueshifts of zein at 1647.1 cm?1 for typical zein-based films.
For LY,1649.0 cm?1(C=O stretching)was selected as the characteristic absorption,which was shifted to 1652.9 cm?1(Film 2),1658.7 cm?1(Film 5)and 1654.8 cm?1(Film7)respectively.Little changes in the blueshifts indicated that no significantchange of molecularstructure of LYoccurred,which suggested that most of LY was still in the form of particles or aggregates in zein-based films,as shown in Fig.2.Furthermore,the blueshifts of Films 2 and 5 indicated that PEG 400 dominated the change of the LY molecular structure,and the addition of AA induced the shift from 1658.7 cm?1(Film 5)to 1654.8 cm?1(Film 7)(redshifts of 3.9 cm?1)of LY characteristic peak.The redshifts indicated that addition of AA increased the numbers of LY hydrogen bonds,which resulted in LY notbeing readily dissolved in zein-based films,and more aggregates were found in the Films 5,7,9 and 11(Fig.2).
For AA,1762.8 cm?1(C=O stretching)and 1677.9 cm?1bonded by intramolecular and intermolecular hydrogen bonds were selected as peaks of characteristic absorption.Fig.4 indicated that the peak at 1762.8 cm?1shifted to 1799.5 cm?1(Film 3),1803.3 cm?1(Film 6)and 1801.4 cm?1(Film7).The blueshifts(nearly 38 cm?1)indicated that the number of intramolecular hydrogen bonds of AA apparently decreased in Film 3,and the addition of PEG 400 further affected the molecular structure of AA(Films 6 and 7).Meanwhile,the peak at 1677.9 cm?1shifted to 1760.5 cm?1(Films 3,6 and 7),and the blueshifts(nearly 83 cm?1)indicated that intermolecular hydrogen bonds decreased more evidently than the intramolecular hydrogen bonds of AA.According to the theory of hydrogen-bonds,intermolecular hydrogen bonds are more easily affected by the change in its concentration in solvent than intramolecularhydrogen bonds.Thus,the interpretation for the blueshifts of the two peaks was well accorded with the theory of hydrogen bonding.For the dissolution of AA,it was clearly observed that during the preparation of zein films,AA crystals were completely dissolved in 3 min after being added into the film-forming solutions,which demonstrated that AA could be well dissolved in film-forming solutions.Furthermore,the extreme changes in blueshifts also indicated that the distance of AA molecules dramatically increased,which reflected that AA existed in the soluble form but not as crystals in zein-based films.
3.3.Mechanical properties of zein-based films
In order to analyze the mechanical properties,TS,EB,and Young's modulus values of zein-based films are measured and listed in Table 1.It was shown that zein control film(Film 1 of Table 1)was quite brittle with the lowest EB,but the average TS(17.67 MPa)was significantly higher than those of zein-PEG 400 films(0.51–2.6 MPa)(P< 0.05).Accordingly,the addition of PEG 400 effectively plastified zein film,and compared with the zein control film,EB increased by approximately 6–7 folds(Films 4–15).The addition of LY did not cause any significant change for mechanical properties(P>0.05)except for the film thickness(P<0.05)(Film 2).However,the addition of AA significantly reduced TS and Young's modulus of zein films(P<0.05)(Film 3),but it did not cause any significant change in EB(P>0.05).The result demonstrated that PEG 400 was the most effective plasticizer,which accorded with the results of Fig.4.For the zein-based films containing PEG 400-LY/AA,addition of LY increased TS and Young's modulus of zein films significantly(P<0.05),but no significant change occurred in EB(P>0.05)(Film 5).However,the addition of AA in the film(Film 6)significantly reduced TS(the minimum value of 0.51 MPa)and Young's modulus(P<0.05),and caused significant change in EB,which increased by approximately 22 times that of the zein control film(P<0.05).The results clearly demonstrated that PEG 400 could be utilized to plasticize zein films;the interaction between LY and zein occurred and strengthened the TS of zein-based films without any plasticizing effect,which apparently affected the thickness of zeinbased films(P<0.05);the synergistic effect of AA and PEG 400 on the flexibility of zein-based films was very significant(P<0.05).
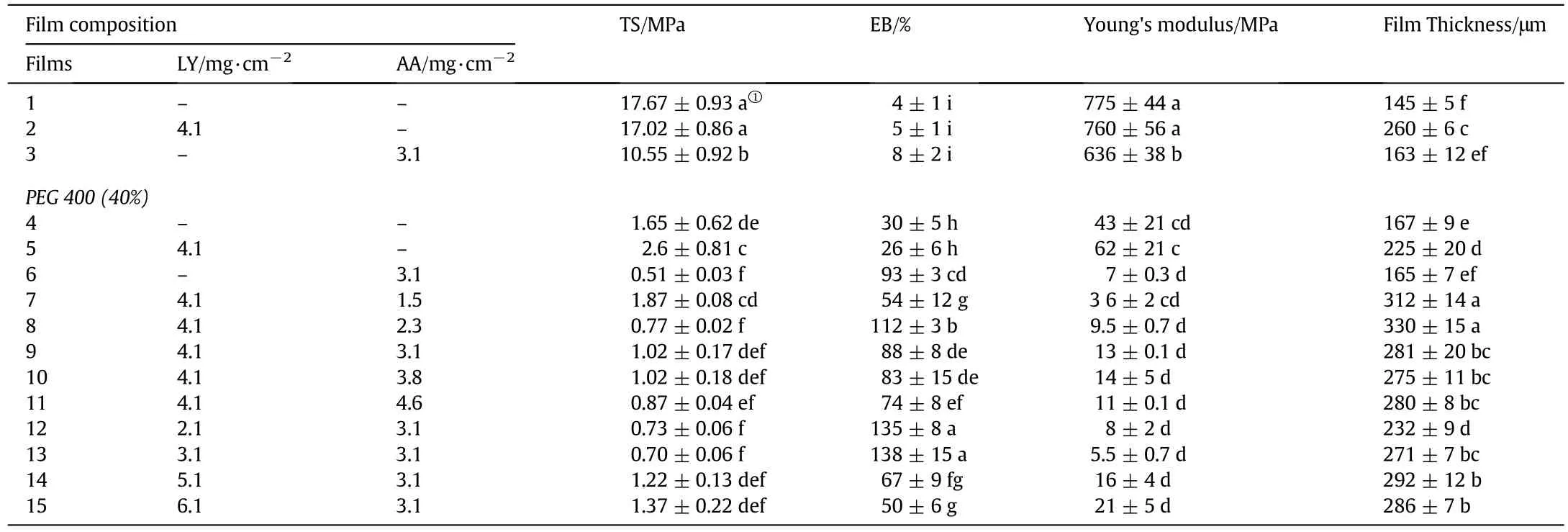
Table 1 Mechanical properties of zein-based films
For zein-based films containing PEG 400-LY-AA(Films 7–15),the addition of LY and AA did not cause any significant change in Young's modulus of zein films(P>0.05),but TS and EB did change(P<0.05).The effects of LY on TS and EB of zein-based films are shown in Table 1.As shown in Films 6,12,13,9,14 and 15,TS increased gradually with increasing the LY concentration at first with an insignificant change(P>0.05),but when the concentration of LY exceeded 10 mg·cm?2,TS significantly increased(P<0.05).However,EB of zein-based films significantly increased(P<0.05)to 138%and then decreased with increase of LY concentration(P<0.05).The results indicated that at low concentration of LY,the addition of LY significantly strengthened the plasticization of zein-based films,but high concentration of LY apparently weakened the synergistic effects of AA and PEG 400 on the flexibility of zein-based films.The effects of AA on TS and EB of zein-based films were also shown in Table 1(Films 5,7–11),which demonstrated that when the concentration of LY was constant,TS of zein-based films decreased significantly(P<0.05),and then tended to be stable(P>0.05)with increasing of AA concentration.EB of zein-based films increased significantly(P<0.05)and then apparently decreased(P<0.05)with the increase of AA concentration,which is in accord with the results of Fig.4.These results indicate that the synergism of LY and AA on the flexibility of zein-based films at low concentration of AA is significant(P<0.05).
The reasons for the brittleness of zein films have been investigated[32].It was found that at low concentration of zein,rod-like structures,with a deduced height of~8 nm and width of~25 nm,are observed.The rod-like structures have various shapes,including dumbbell,pole and branched structures,and some of the rods are aggregated into doughnut-like structures together with dissociated protein globules[37].When the concentration of zein reaches a certain value,the proteins will agglomerate,and the hydrogen bonding,disul fide bonding and hydrophobic interactions will occur,which maintain the mesh work and lead to the formation of the zein film[30,37].The bonds and interactions are the primary forces that maintain the film integrity.
The analysis of mechanical mechanism by adding PEG 400,LY and AA in zein-based films is based on the combined information of microstructure and FT-IR discussed above.From the results of mechanical test and FT-IR of Films 2 and 3 in Table 1 and Fig.4,it can be concluded that the addition of LY and AA slightly decreases the hydrogen bonds of zein molecules.It is due to the hydrophilic properties and the shortage of oxygen atoms of zein molecules,which repelled the molecules of LY and AA by the hydrophobic mesh work,and the flexibility of zein films cannot be influenced by the addition of LY and AA.Compared with the control zein film(Film 1),the addition of PEG 400 in the zein film(Film 4)dramatically increased the EB of zein films.It is probably due to the loosening of inter molecular forces between the chains of adjacent macromolecules of zein by breaking the hydrogen bonds of intermolecular and intramolecular structure,which can be proven by the increase in the intensity of β-sheets compared to α-helices in zein film[38,39]and the results of FT-IR(Film 4 of Fig.4)of zein film.In Film 5,the hydrophilicity of the film increases because of the addition of PEG 400,which makes LY completely dissolve in the film(Film 5 in Fig.2).Because of the hydrophilicity of LY molecules,they tend to interact with PEG 400,which weakens the plasticization of PEG 400 on zein film(Film 5 in Table 1).For Film 6,the mechanism of the synergistic effect of AA and PEG 400 on the mechanical properties of zein film could be attributed to the bonding of AA on zein protein's surface and resulting increase of free volume in film matrix[40].The hydrophilic groups of AA decrease the hydrophobic interaction among zein molecules,which increases the mobility and eliminates flexibility problems of zein films.From the mechanical results of Films 6,12,13,9,14 and 15,it can be concluded that a small quantity of LY can be completely dissolved in zein matrix.Because of the hydrophilic property of LY and AA,LY molecules interact with the AA bonded on the zein protein's surface,which further increases the free volume in film matrix and decreases the hydrophobic interaction among zein molecules.Furthermore,LY is a kind of macromolecular component which dramatically increases the free volume of zein molecules in film matrix.With the increase of LY concentration,LY cannot be well dissolved in the film matrix and LY aggregates break the microstructure of the film,which weakens the flexibility of developed zein-based film(Film 9 in Fig.2).
3.4.LY release profiles and antimicrobial potential of zein-based films
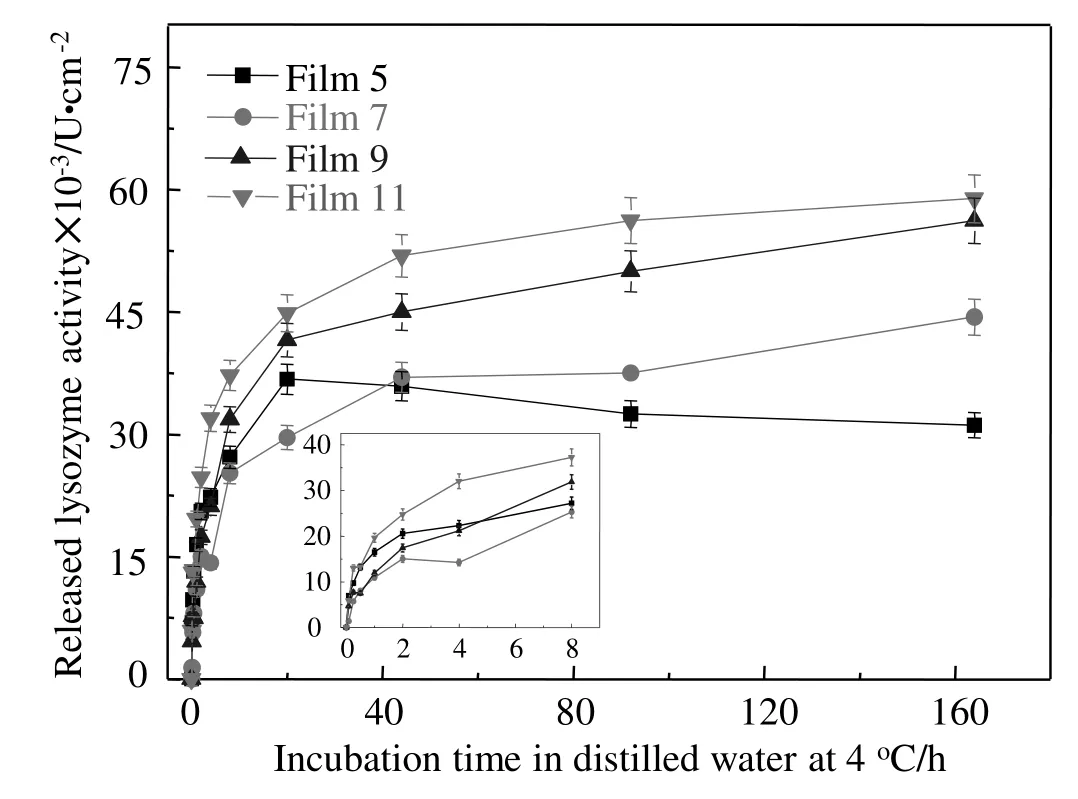
Fig.5.Effects of AA on release profiles of LY from typical zein-based films.
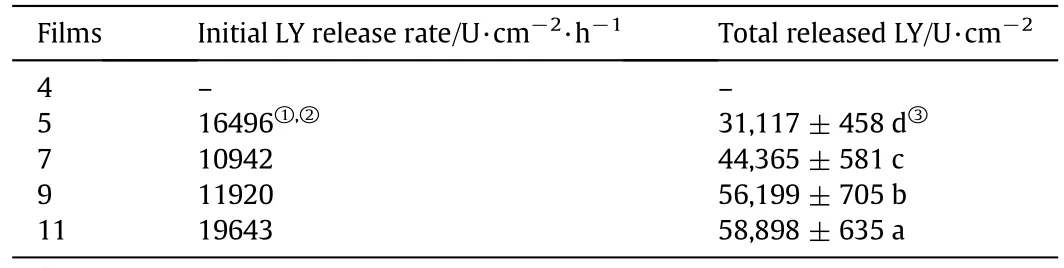
Table 2 The kinetic parameters determined from release curves of LY from developed films
The contribution of AA to LY release properties from zein-based films is shown in Fig.5,and the kinetic parameters,including initial LY release rate and total released LY,are listed in Table 2.Compared with the release curve of LY for Film 5 without AA,the addition of AA in Films 7 and 9 significantly decreased the initial LY release rate by approximately 33.7%(P<0.05),and increased the total released LY by approximately 29.8%–89.3%(P<0.05),it indicated that AA effectively sustained the release of LY.The results could be due to the smooth cross-section microstructure in the zein-based films and the shift from the surface to the inside of LY with the addition of AA,as shown in Fig.2.In zein-based films,AA not only served as an active component but also affected film morphology and conformation of LY,since it interacted with the zein matrix via hydrogen bonds bonding to available zein carbonyl groups[33].The morphological changes in zein-based films caused by AA can affect film porosity and the controlled release properties of films.
The results of antimicrobial tests on L.innocua are given in Fig.6.It was clearly shown that the zein control film(Film 4)did not form any inhibition zones,but all other films containing LYshowed an antimicrobial effect on L.innocua and formed clear zones.Compared with Film 5,although Film 7 with the addition of AA significantly decreased the initial LY release rate and increased the total released LY(P<0.05),only an insignificant change in antimicrobial activity was found(P>0.05).But with the increase ofAA concentration(Films 9 and 11),the antimicrobial activities of zein-based films significantly increased(P<0.05)and the antimicrobial zone was up to 334 mm2,which indicated that one of the ways to strengthen the antimicrobial activity was to increase the concentration of AA in zein-based films.According to the above results of mechanical properties and antimicrobial activity,it can be seen that when the concentration of AA is between 1.5 mg·cm?2and 2.3 mg·cm?2,zein films still have good flexibility(112%of EB),and AA has an obvious effect on the controlled release of LY in zein films.Therefore,the concentration of AA in zein films is appropriate between 1.5 mg·cm?2and 2.3 mg·cm?2.
3.5.AA release profiles and antioxidant potential of zein-based films
The effects of LY on release profiles of AA in zein-based films are shown in Fig.7,and kinetic parameters determined from release curves of AA and antioxidant activity of films are listed in Table 3.From the parameters of the release profiles,52.3%(Film 6 without LY)and 77.1%–84.5%of AA(Films 9,12 and 15 with the addition of LY)existed in soluble form in the zein-based films,while the remaining AA was bound in the film matrix by hydrogen bonds.Typically,it was found that Film 12 showed an approximately 1.7 times higher initial AA release rate and 1.6 times higher total released AA than Film 6 without LY.Because of the hydrophilic nature of LY,LY aggregates in the zein-based films could induce the rapid release of AA.It was found that with the increase in LY concentration,the total released AA was significantly decreased(P<0.05)due to the change in film microstructure(Fig.2).
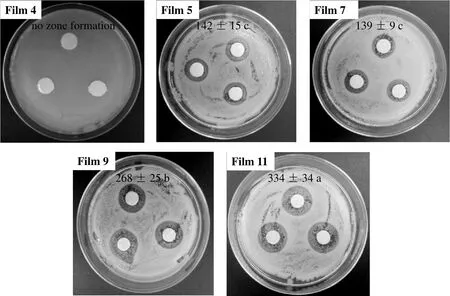
Fig.6.The results of antimicrobial tests on L.innocua.[Different letters(a,b,c)in the figure show significant difference at P<0.05.]
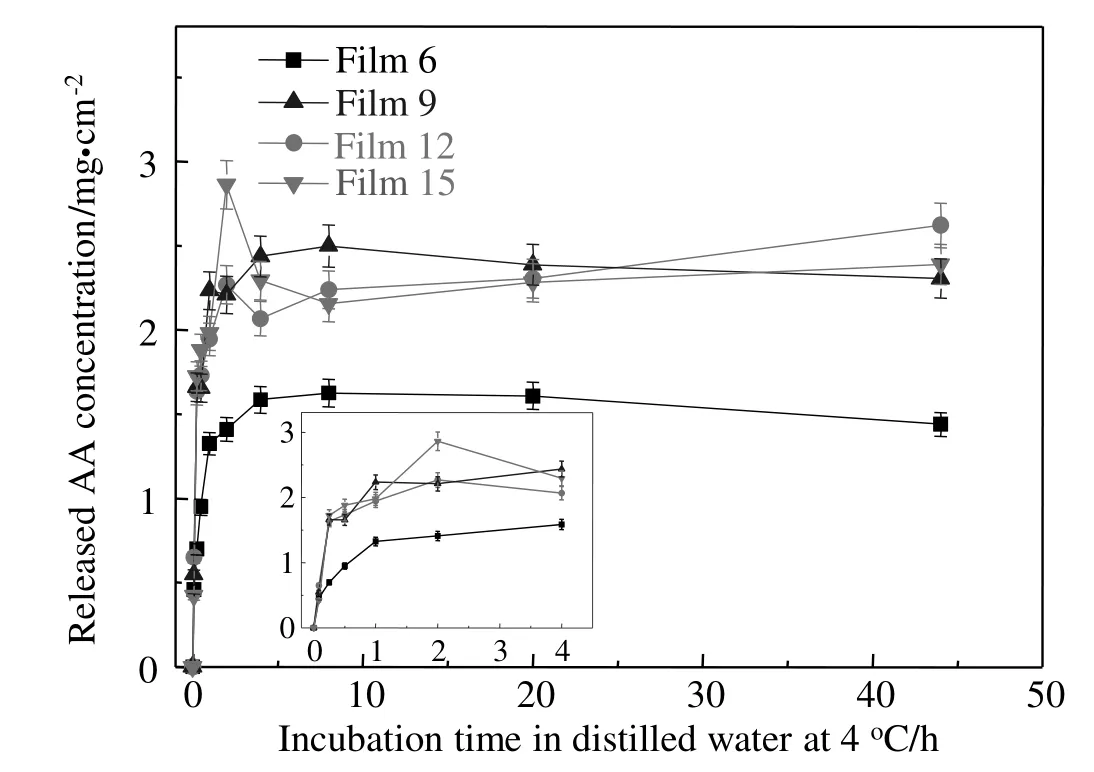
Fig.7.Effects of LY on release profiles of AA in zein-based films.
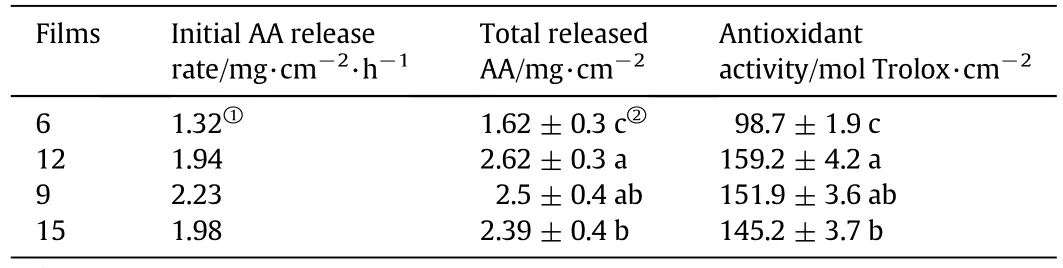
Table 3 The kinetic parameters determined from release curves of AA and antioxidant activity of the developed films
The antioxidant activity was up to 159μ mol Trolox·cm?2,and a positive correlation between the antioxidant activity and the total released AA was clearly found.The results suggest that the release mechanism of blending technology can be employed for delivery of multiple active compounds,and the released profiles of AA can be modulated by adjusting the concentration of LY in the zein-based films.
3.6.Inhibition of enzymatic browning at cut surfaces
The results of zein-based films to inhibit enzymatic browning at cut surfaces of Crystal Fuji apple plugs are listed in Table 4.The inhibition data clearly showed that the reflectance procedure could detect the different degrees of browning inhibition obtained in plugs that were treated with different films.It was found that the developed zeinbased films could significantly inhibit the enzymatic browning of apples.The inhibition data of Film 4 indicated that zein films withoutAA could also effectively inhibit the browning process of the apple,which was attributed to the good resistance to oxygen of zein films.The inhibition results of Films 6,12 and 9,i.e.,inhibition data significantly larger than 100%,indicated that the addition of AA could bleach apple plugs,and the bleaching extent became deeper with the increase ofinitial release rate of AA from zein-based films.Among the films,inhibition data of Film 12 suggested that the decrease of inhibition data was the most significant,which could be attributed to the good flexibility of the film.EB of Film 12 was the largest,which indicates that the free volume of zein molecules is much bigger than that of other films and it results in weakening the resistance to oxygen.From the inhibition of enzymatic browning at cut surfaces,it can be found that control zein film has good oxygen resistance,and the addition of AA significantly improves the inhibition effects.
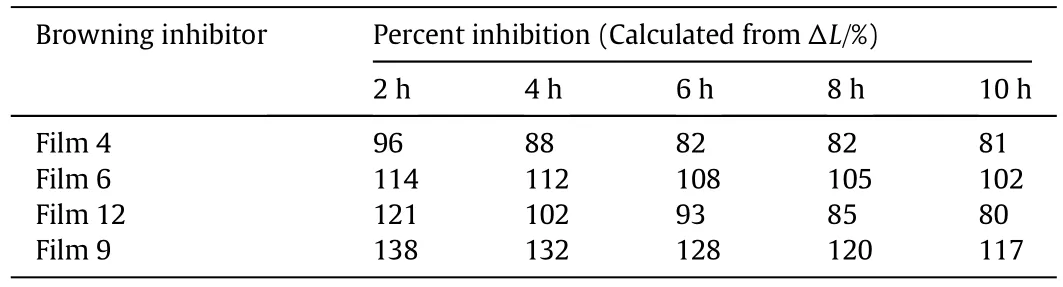
Table 4 Evaluation of zein-based films to inhibit enzymatic browning at cut surfaces ofCrystalFuji apple plugs
4.Conclusions
This study demonstrated the potential of developing zein-based films containing LY as an anti-listerial agent and AA as an antioxidant to improve the antimicrobial and antioxidant abilities of the films,which could be utilized in packaging applications to prolong the shelf time of foods and pharmaceuticals.The mechanical tests and FT-IR characterization indicated that PEG 400 dramatically plastified zein-based films,and the synergistic effect of LY and AA on the flexibility of zeinbased films was significant,which satisfied the requirements of mechanical properties in the packaging industry.LY release profiles and antimicrobial activities for the zein-based films demonstrated that AA effectively sustained the release of LY and antimicrobial activity increased with the increase of AA concentration,which suggested that the sustained release of LY could increase the antimicrobial period and further prolong the shelf time of goods.AA release profiles and antioxidant activities indicated that LY dramatically increased the release profiles of the zein-based films,and a positive correlation between antioxidant activity and total released AA was found.Further work is needed to improve the controlled release of LY and AA from zeinbased films for increasing the release period and then prolonging the shelf life of foods and pharmaceuticals.
[1]Y.Wang,H.Chen,J.Wang,L.Xing,Preparation of active corn peptides from zein through double enzymes immobilized with calcium alginate-chitosan beads,Process Biochem.49(2014)1682–1690.
[2]R.Shukla,M.Cheryan,Zein:the industrial protein from corn,Ind.Crop.Prod.13(2001)171–192.
[3]H.X.Guo,Y.P.Shi,A novel zein-based dry coating tablet design for zero-order release,Int.J.Pharm.370(2009)81–86.
[4]K.Shi,J.L.Kokini,Q.Huang,Engineering zein films with controlled surface morphology and hydrophilicity,J.Agric.Food Chem.57(2009)2186–2192.
[5]M.N.Emmambux,M.Stading,In situ tensile deformation of zein films with plasticizers and filler materials,Food Hydrocoll.21(2007)1245–1255.
[6]H.Xu,Y.Chai,G.Zhang,Synergistic effect of oleic acid and glycerol on zein film plasticization,J.Agric.Food Chem.60(2012)10075–10081.
[7]G.Liu,D.Wei,H.Wang,Y.Hu,Y.Jiang,Self-assembly of zein microspheres with controllable particle size and narrow distribution using a novel built-in ultrasonic dialysis process,Chem.Eng.J.284(2016)1094–1105.
[8]Q.S.Sun,J.Dong,Z.X.Lin,B.Yang,J.Y.Wang,Comparison of cytocompatibility of zein film with other biomaterials and its degradability in vitro,Biopolymers 78(2005)268–274.
[9]R.Paramawati,T.Yoshino,S.ISOBE,Properties of plasticized-zein film as affected by plasticizer treatments,Food Sci.Technol.Res.7(2001)191–194.
[10]S.Ghasemi,N.H.S.Javadi,M.Moradi,K.Khosravi-Darani,Application of zein antimicrobial edible film incorporating Zataria multi flora Boiss essential oilfor preservation of Iranian ultra filtered feta cheese,Afr.J.Biotechnol.14(2015)2014–2021.
[11]E.Apayd?n,The effects of corn zein film incorporated antimicrobial and antioxidant on the quality of intermediate moisture foods,J.Atmos.Sci.56(2007)3560–3572.
[12]P.J.Zapata,F.Guillén,D.Martínez-Romero,S.Castillo,D.Valero,M.Serrano,Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato(Solanum lycopersicon Mill)quality,J.Sci.Food Agric.88(2008)1287–1293.
[13]Z.Bailing,L.Lei,S.Qiuyan,D.Zemin,D.Xiaoyan,T.Zhifang,Inhibiting effect of zein film-coating to rancidification of walnut meat,TCSAE 3(2004)180–183.
[14]P.Zhang,H.Wu,Application of zein in strawberry preservation,Storage Pro.4(2005)023.
[15]M.Moradi,H.Tajik,S.M.R.Rohani,A.Mahmoudian,Antioxidant and antimicrobial effects of zein edible film impregnated with Zataria multi flora Boiss.Essential oil and monolaurin,LWT Food Sci.Technol.72(2016)37–43.
[16]M.Khajenoori,A.H.Asl,F.Hormozi,Proposed models for subcritical water extraction of essential oils,Chin.J.Chem.Eng.17(2009)359–365.
[17]Y.Zhang,D.Rochefort,Activity,conformation and thermal stability of laccase and glucose oxidase in poly(ethyleneimine)microcapsules for immobilization in paper,Process Biochem.46(2011)993–1000.
[18]T.Wang,Y.Liang,M.Wu,Z.Chen,J.Lin,L.Yang,Natural products from Bacillus subtilis with antimicrobial properties,Chin.J.Chem.Eng.23(2015)744–754.
[19]K.K.Li,S.W.Yin,Y.C.Yin,C.H.Tang,X.Q.Yang,S.H.Wen,Preparation of watersoluble antimicrobial zein nanoparticles by a modified antisolvent approach and their characterization,J.Food Eng.119(2013)343–352.
[20]J.Delves-Brougthon,Nisin and its uses as a food preservative,Food Technol.44(1990)100–117.
[21]Y.Lin,J.L.Sun,G.Jiang,J.Zan,F.X.Ding,In vitro evaluation of lysozyme-loaded microspheres in thermosensitive methylcellulose-based hydrogel,Chin.J.Chem.Eng.15(2007)566–572.
[22]L.D.Yan,S.C.Shen,J.X.Yun,K.J.Yao,Isolation of lysozyme from chicken egg white using polyacrylamide-based cation-exchange cryogel,Chin.J.Chem.Eng.19(2011)876–880.
[23]X.Diao,Y.J.Wang,J.Q.Zhao,S.L.Zhu,Effect of pore-size of mesoporous SBA-15 on adsorption of bovine serum albumin and lysozyme protein,Chin.J.Chem.Eng.18(2010)493–499.
[24]H.Chen,J.Wang,Crystallization thermodynamic and kinetic behaviors of vitamin C in batch crystallizer,Chin.J.Chem.Eng.8(2000)95–99.
[25]Y.Li,S.Kadam,T.Abee,T.M.Slaghek,J.W.Timmermans,M.A.C.Stuart,M.J.Kleijn,Antimicrobial lysozyme-containing starch microgel to target and inhibit amylaseproducing microorganisms,Food Hydrocoll.28(2012)28–35.
[26]A.Meister,Glutathione-ascorbic acid antioxidant system in animal,J.Biol.Chem.269(1994)9397–9400.
[27]P.D.Cotter,C.Hill,R.P.Ross,Bacteriocins:developing innate immunity for food,Nat.Rev.Microbiol.3(2005)777–788.
[28]E.A.Soliman,M.S.Mohy Eldin,M.Furuta,Biodegradable zein-based films:in fluence of γ-irradiation on structural and functional properties,J.Agric.Food Chem.57(2009)2529–2535.
[29]American Society for Testing and Materials(Ed.),Standard Test Method for Tensile Properties of Thin Plastic Sheeting,American Society for Testing and Materials,West Conshohcken 1991,pp.194–202.
[30]I.Arcan,A.Yemenicio?lu,Controlled release properties of zein–fatty acid blend films for multiple bioactive compounds,J.Agric.Food Chem.62(2014)8238–8246.
[31]M.Moradi,H.Tajik,S.M.Razavi Rohani,A.R.Oromiehie,Effectiveness of Zataria multi flora Boiss essential oil and grape seed extract impregnated chitosan film on ready-to-eat mortadella-type sausages during refrigerated storage,J.Sci.Food Agric.91(2011)2850–2857.
[32]A.Cupello,M.V.Rapallino,M.Tabaton,G.L.Lunardi,A simple,inexpensive,and precise spectrophotometric method for evaluating the concentration of ascorbic acid in CSF samples:data from different neurological pathologies,Int.J.Neurosci.112(2002)1337–1345.
[33]R.Re,N.Pellegrini,A.Proteggente,A.Pannala,M.Yang,C.Rice-Evans,Antioxidant activity applying an improved ABTS radical cation decolorization assay,Free Radical Bio.Med.26(1999)1231–1237.
[34]G.M.Sapers,F.W.Douglas,Measurement of enzymatic browning at cut surfaces and in juice of raw apple and pear fruits,J.Food Sci.52(1987)1258–1285.
[35]W.X.Ribeiro,J.F.Lopes Filho,M.S.Cortes,Characterization of biodegradable film based on zein and oleic acid added with nanocarbonate,Ciênc.Rural 45(2015)1890–1894.
[36]S.T.Arun,P.Binsy,E.Richard,B.J.Basu,Properties of phase separation method synthesized superhydrophobic polystyrene films,Appl.Surf.Sci.258(2012)3202–3207.
[37]Y.Guo,Z.Liu,H.An,M.Li,J.Hu,Nano-structure and properties of maize zein studied by atomic force microscopy,J.Cereal Sci.41(2005)277–281.
[38]K.E.Gebhardt,S.Ahn,G.Venkatachalam,D.A.Savin,Role of secondary structure changes on the morphology of polypeptide-based block copolymer vesicles,J.Colloid Interface Sci.317(2008)70–76.
[39]S.S.Lal,P.Tanna,S.Kale,S.T.Mhaske,Ka firin polymer film for enteric coating on HPMC and gelatin capsules,J.Mater.Sci.52(2017)3806–3820.
[40]I.Arcan,A.Yemenicio?lu,Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials,Food Res.Int.44(2011)550–556.
 Chinese Journal of Chemical Engineering2018年3期
Chinese Journal of Chemical Engineering2018年3期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of butter fly-like BiVO4/RGO nanocomposites and their photocatalytic activities☆
- Fabrication of chitosan microspheres for efficient adsorption of methyl orange☆
- Effects of nitrogen doping on surface-enhanced Raman scattering(SERS)performance of bicrystalline TiO2 nano fibres☆
- Parametric study and effect of calcination and carbonation conditions on the CO2 capture performance of lithium orthosilicate sorbent
- Effect of heat flux and inlet temperature on the fouling characteristics of nanoparticles☆
- Variation of toxic pollutants emission during a feeding cycle from an updraft fixed bed gasifier for disposing rural solid waste☆
