Physics,chemistry,and Hirshfeld surface analyses of gamma-irradiated thalidomide to evaluate behavior under sterilization doses
Vlner A.F.S.N.Mussel,Mx P.Ferreir,Mri B.F.Mrques,Mri I.Yoshid,Mrin R.Almeid,Bernrdo L.Rodrigues,Wgner N.Mussel,*
aDepartamento de Química,ICEx,Universidade Federal de Minas Gerais-UFMG,Av.Ant?nio Carlos 6627,31270-901 Belo Horizonte,MG,Brazil
bCNEN-CDTN,Comiss?o Nacional de Energia Nuclear-Centro de Desenvolvimento da Tecnologia Nuclear,Av.Ant?nio Carlos,6627 Belo Horizonte,MG,Brazil
1.Introduction
To ensure adequate conditions of use,sterility is a crucial attribute to any pharmaceutical material,main component,excipient,or formulation.In general,sterilized materials should have microbial survivor probability of<10-6.This criterion is the basis of the sterility assurance level.
There are several sterilization procedures,and each has advantages and disadvantages[1,2].There is no suitable procedure for general use.Physical removal of microorganisms by membrane filtration does not require heat.Dry heat or even moist heat promotes microbiological reduction at high temperature,but results in considerable degradation of temperature-sensitive materials or devices.Sterilization using ethylene oxide is highly effective but can leave a toxic residue in porous materials such as implants.Electron-beam radiation can be used to prevent temperature effects and toxic residues in the final material,but is limited by poor penetration in bulky materials.
Gamma irradiation has advantages over other conventional sterilization methods in solids∶high penetration,uniform efficacy,low isothermal stability,and absence of toxic residues.The main advantage is that irradiation can be used as the final sterilization procedure in starting materials and final products.In this way,the usual 25kGy dose can ensure sterilized pharmaceutical materials[2,3].Due to the potential sensitivity of pharmaceuticals,validation procedures with lower doses are usually accepted as long as reliable and adequate reduction of the biologic burden can be ensured.In this way,the risk of undesired effects over pharmaceuticals,formulations,or devices submitted to the sterilization process is minimized[4].
Thalidomide ((RS)-2-(2,6-dioxopiperidin-3-il)-1H-isoindol-1,3(2H)-dione)was synthesized by Chemie Grünenthal in West Germany in 1954.It was introduced to the West German market in 1956 as an antiemetic for pregnant women.In the 1960s,the teratogenic effects of this drug were recognized.Fetal malformation due to the S-isomer of thalidomide resulted in restricted use of thalidomide and increased surveillance by regulatory agencies[5].
Since then,thalidomide has been recognized as having antiangiogenic and anti-inflammatory properties.It has been used to treat leprosy and multiple myeloma.Hence,stability studies of thalidomide under radioactive stress aimed at sterilization of the drug are warranted[5].
2.Materials and methods
A sample of thalidomide from a validated production batch was obtained during the shelf-life of this pharmaceutical.All analyses were conducted within the validity period of the batch.
2.1.Powder X-ray diffraction(PXRD)
PXRD data were collected in an XRD-7000 diffractometer(Shimadzu,Kyoto,Japan)at room temperature under 40 kV,30 mA,using CuKα (λ =1.54056 ?)equipped with polycapillary focusing optics under parallel geometry coupled with a graphite monochromator.The sample was spun at 60 rpm,and scanned over an angular range of 4–60°(2θ)with a step size of 0.01°(2θ)and a time constant of 2s/step.All fitting procedures were obtained using FullProf Suite[6,7].Crystalexplorer v 17 was used to calculate the Hirshfeld surface[8].
2.2.Single-crystal X-ray diffraction(SCXRD)
SCXRD data were collected in a Gemini A Ultra X-ray Diffraction system(Agilent Technologies,Santa Clara,CA,USA)at room temperature using a MoKα (λ =0.71073 ?)tube as the X-ray source,equipped with a graphite monochromator and a charge-coupled device plate detector.Data collection and re finement details are given in Table 1.
2.3.Thermogravimetric analysis(TGA)and differential thermal analysis(DTA)
TGA and DTA experiments were carried out on a DTG60H system(Shimadzu)in a dynamic N2atmosphere(50 mL/min)using alumina pans containing≈2.0 mg of sample.Experiments were conducted at a heating rate of 10 °C/min from 25 °C to 400 °C.
2.4.Differential scanning calorimetry(DSC)
DSC experiments were undertaken on a DSC60 system(Shimadzu).The equipment cell was calibrated with indium(melting point,156.6 °C;heat of fusion,ΔHfus=28.54 J/g)and lead(melting point,327.5°C).Aluminum pans containing ≈1 mg of samplewere used under a dynamic N2atmosphere(50 mL/min)and a heating rate of 10 °C/min from 25 °C to 300 °C.Thalidomide can exist as two polymorphs,α and β,and the latter shows different thermal behavior.Therefore,an isothermal experiment was carried out at 270°C to obtain a pure material for comparison,as needed.
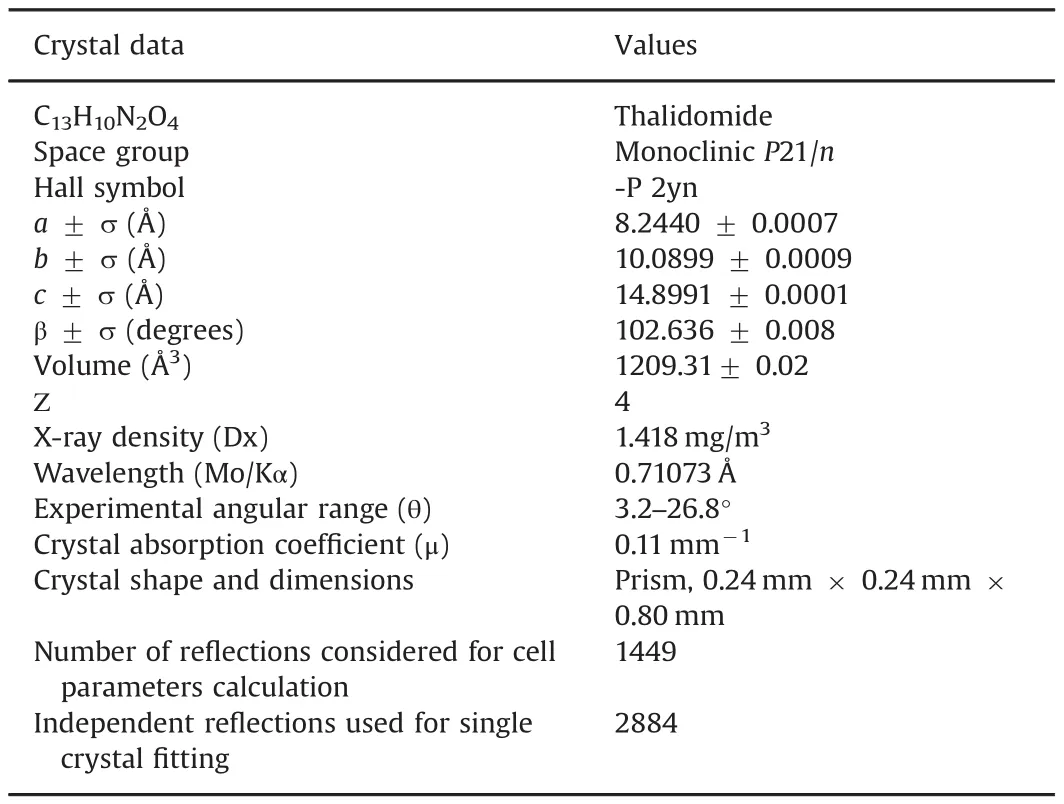
Table 1 Single crystal re finement data for polymorph α,space group,Hall symbol,lattice parameters a,b and c(?),? angle(θ),volume,number of formulae unit per unit cell,X-ray density,wavelength,experimental angular range(θ),crystal absorption coefficient,crystal shape and dimensions,number of reflections considered for cell parameters calculation,and independent reflections used for single crystal fitting.
2.5.Ultraviolet spectroscopy
Ultraviolet spectroscopy was undertaken at 200–400 nm for thalidomide at 10μg/mL in ethanol on a spectrophotometer(1800;Shimadzu).Origin v9.1 was used to adjust data.
2.6.Raman spectroscopy
Raman spectroscopy of solid thalidomide was done on a confocal micro-Raman spectrometer(Senterra;Bruker,Billerica,MA,USA)with an excitation laser set at 785 nm.The measurement conditions were as follows∶integration time of 5 s;spectral resolution of 3–5cm-1;and spectral range of 2000–100cm-1.The laser was focused with a 4×dry objective lens,with the laser power set to 25 mW.Origin v9.1 was used to adjust data.
2.7.Gamma irradiation
Experiments involving gamma irradiation were done at Comiss?o Nacional de Energia Nuclear-Centro de Desenvolvimento da Tecnologia Nuclear(Belo Horizonte,MG,Brasil).The radiation system(IR-214;MDS Nordion,Ottawa,Canada)was equipped with a dry cobalt-60 source.The source had a maximum activity of 2200 TBq(60,000 Ci).The specific irradiation times were calculated,and then all samples were exposed to doses of 2,5,10,15,25,30 or 100 kGy.
2.8.Attenuated total reflection Fourier transformed infrared spectroscopy(ATR-FTIR)
FTIR analysis was performed at room temperature on a Spectrum 1000 spectrophotometer(PerkinElmer,United States)equipped with an attenuated total reflectance(ATR)accessory.The sample was pressed into a zinc selenide crystal,and 32 scans were averaged.For single FTIR without ATR,the samples were measured in KBr pressed pellets in the wavenumber range between 400 and 3400cm-1at room temperature,with a resolution of 4cm-1.
2.9.Statistical analyses
Data are the mean±standard deviation.All fitting procedures took into account three independent measurements with statistical analyses conducted using Origin v9.1.
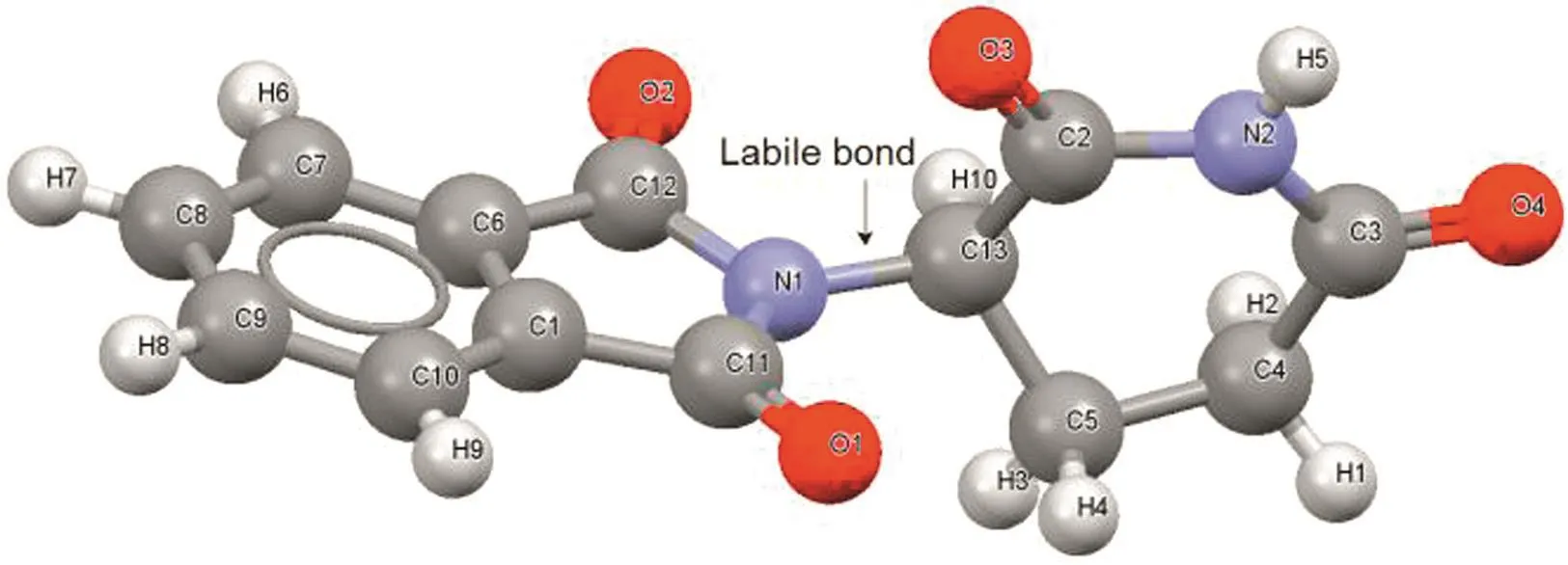
Fig.1.Thalidomide molecule showing the labile bond between phthalimide and glutarimide rings.
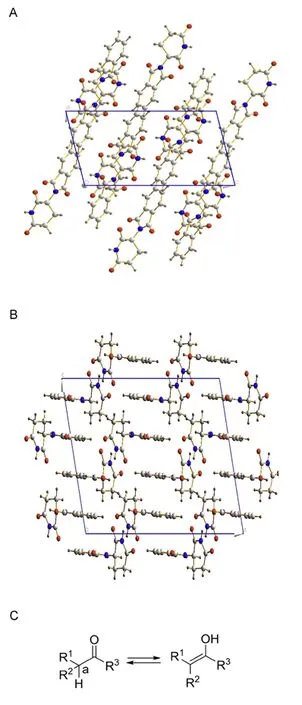
Fig.2.The crystal structure of the polymorphs α (A)(a=8.233(1)?,b=10.070(2)?,c=14.865(2)?,α = γ =90.0°and β =102.53(2)°,monoclinic,P 21/n,Z=4)and β (B)(a=20.679(5)?,b=8.042(2)?,c=14.162(5)?,α = γ =90.0°and β =102.86(3)°,monoclinic,C 2/c,Z=8),and(C)keto-enol tautomerization.
3.Results and discussion
The thalidomide molecule has a labile bond that can be turned around from phthalimide and glutarimide rings(Fig.1).
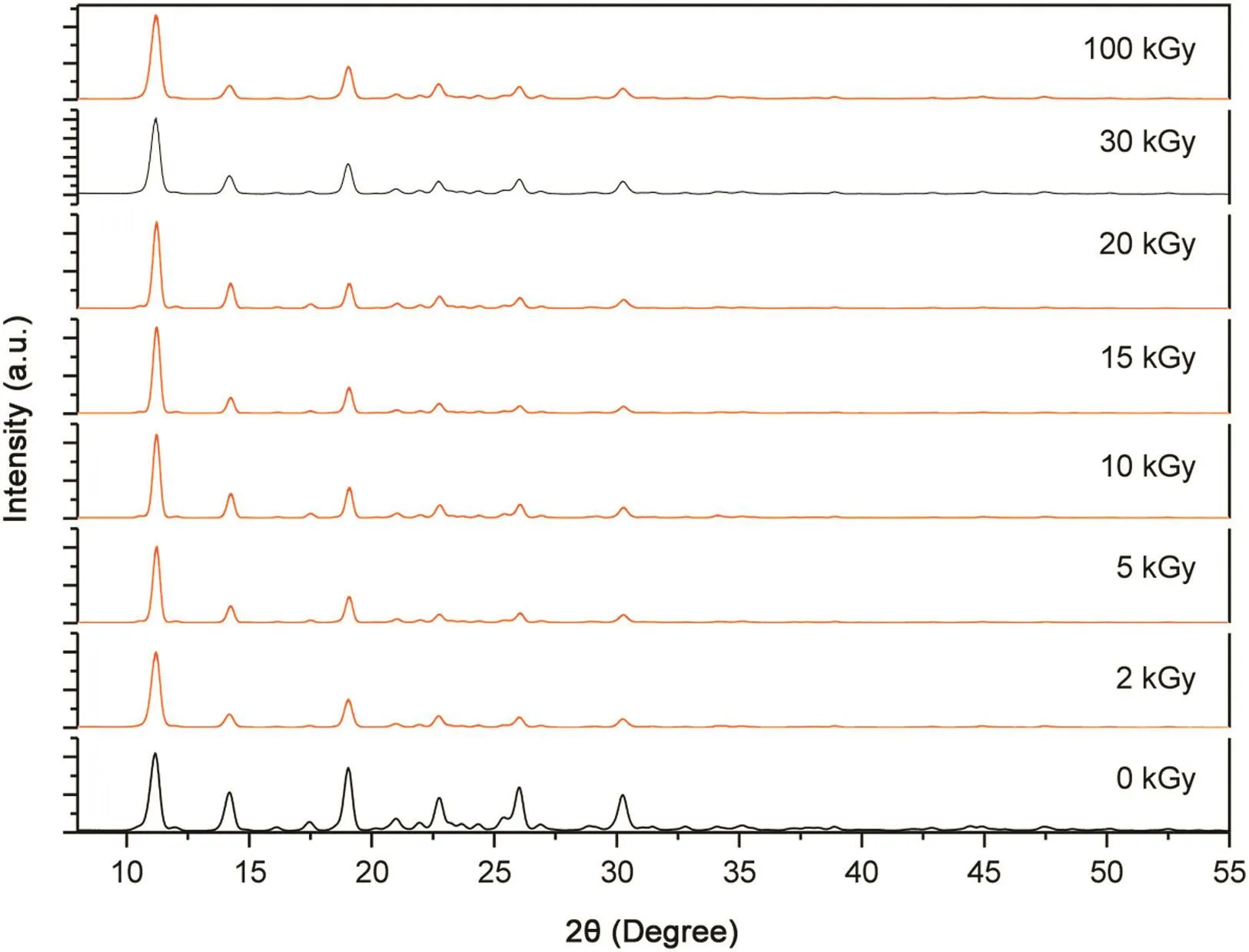
Fig.3.Powder X-ray diffraction experiments for irradiated thalidomide samples for 0,2,5,10,15,20,30 and 100 kGy.All samples were irradiated under the same conditions,only different times.

Fig.4.Crystal projection of the asymmetric unit.Carbon(grey),oxygen(red)and nitrogen(blue)atoms.ORTEP plotted ellipsoids with 50%probability.
In the thalidomide chemical structure,the chiral center has a neighboring ketone that may undergo to the enol form,then reforming it when switching back to the keto form.Even with uptaking of the correct R-thalidomide,a keto-enol tautomerization happened inside the human body,it would racemase into a mixture of R,S-thalidomide and the corresponding enol forms.The S-thalidomide causes the birth defects(Fig.2).
The intensity of a diffracted peak of a certain reflection(hkl)plane for a given chemical structure is a direct contribution of the structural factor,which in turn corresponds to the number of electrons diffracting the X-ray beam on that plane.If any plane in the structure reduces its number of electrons,a direct effect of that condition will be a decrease in the intensity of that specific plane,and the resulting system will be a plane with lower electron density.In the solid state,the atoms in a structure are much less labile than in solubilized material because of numerous mutual interactions(e.g.,Van der Waals forces and/or hydrogen bonding).The fitting procedure was designed to allow the torsion angle between phthalimide and glutarimide rings to vary freely within the extraction and adjustment of the intensities in the diffraction.
The thalidomide structure C13H10N2O4space groupP21/nhas a torsion angle of 57.28°(2θ).This structure was taken as a reference,with all procedures starting from the same template molecule, by varying the fitting sequence as follows∶(i)parameterization of the background with five polynomial terms;(ii)U,V and W(FWHM)of the pseudo-Voight function;(iii)pro file parameters NA and NB of the pseudo-Voight function;(iv)asymmetry factors P1,P2,P3 and P4 of the Berar–Baldinozzi asymmetric correction;(v)a and b beyond the beta angle of the crystal lattice;(vi)torsion angles N1-C11-C13-C2 with the initial value set to 57.28°(2θ);(vii)isotropic thermal parameter functions for all atoms.Fig.3 shows the experimental XRD pattern for all doses.

Fig.5.Hirshfeld surface analysis and corresponding overall fingerprints for polymorphs α and β (A and B,respectively),the torsion angles(C),the fingerprint O-O interactions(π-πrespectively)(D).The 2 kGy irradiated αpolymorph with respectively torsion angle and overall fingerprint(E).
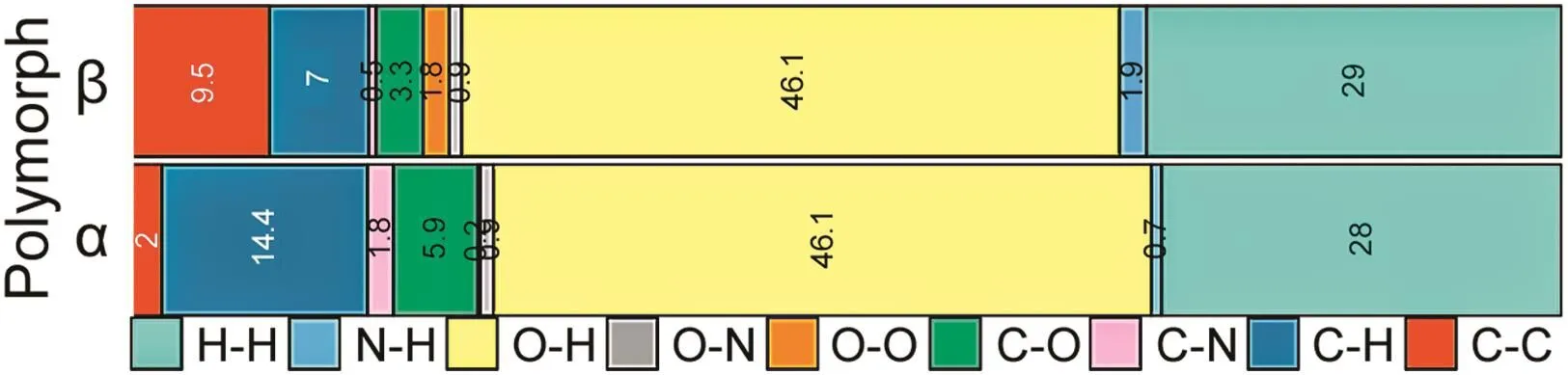
Fig.6.Hirshfeld surface analysis and overall contributions for all atoms pairs in polymorphs α and β.
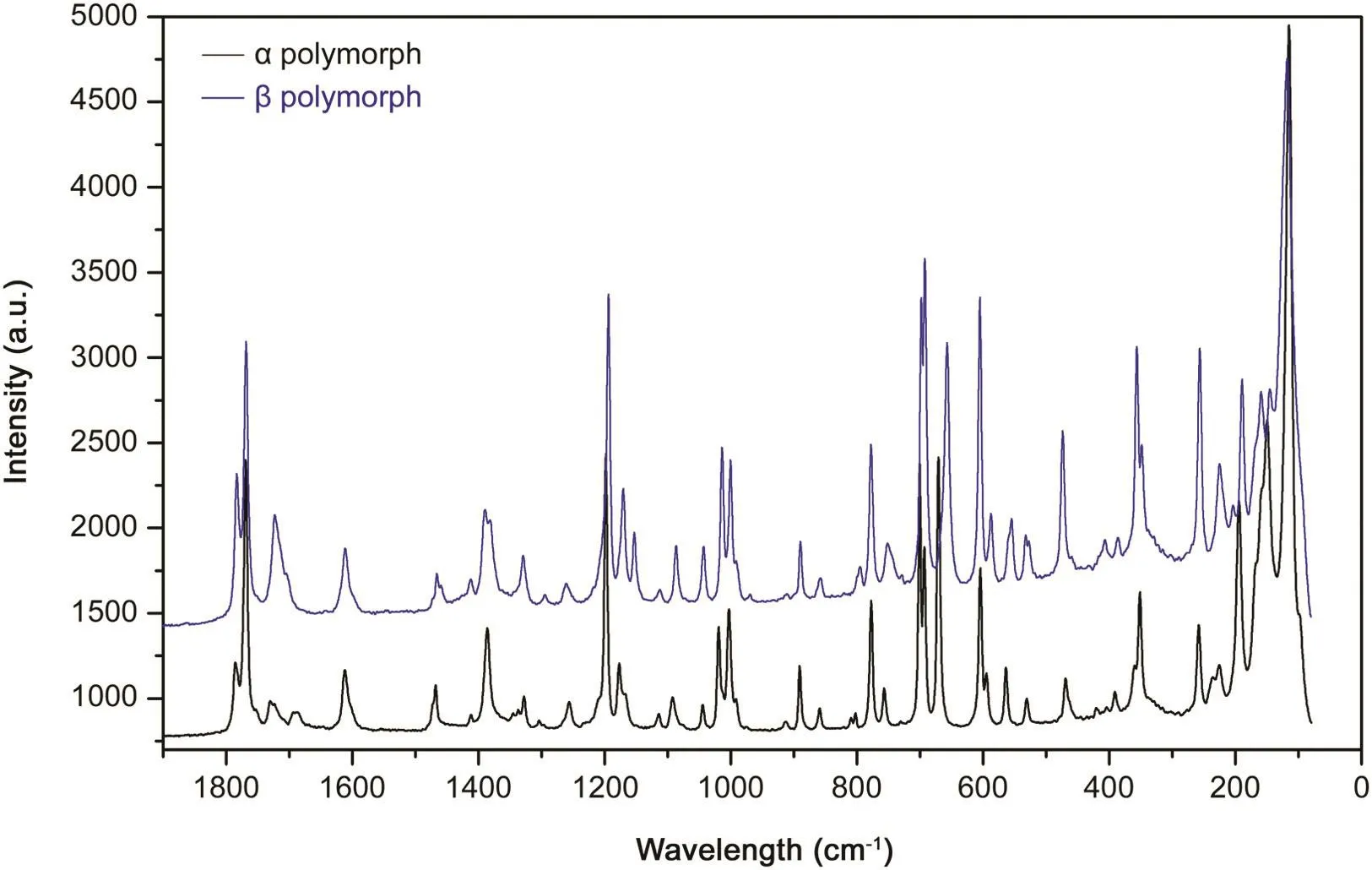
Fig.7.Raman experimental spectra of polymorphs α and β evidencing the spectra differences.
SCXRD was carried out on a recrystallized sample from an original polymorphαsample by solvent evaporation.To 20 mL of a methanol∶water(5∶3)solution was added 25 mg of polymorph α,which resulted in a supersaturated solution.Non-solubilized crystals were filtered out,and the solution was allowed to stand to recrystallize over 23 days.The crystal data,collection,and details of structure re finement of polymorphαare summarized in Table 1.Refinement was carried out in the absence of anomalous scattering.Changes in illuminated volume were kept to a minimum,and were taken into account[9–14]using multi-scan inter-frame scaling.Hydrogen atoms were geometrically fixed to their bonded atoms,with their thermal isotropic term,Uiso(H)in the range 1.2–1.5 times Ueqof the parent atom,after which the positions were re fined with adequate constraints.Fig.4 shows the asymmetric unit as an Ortep plot for the determination of crystal structure,as well as the unit cell ellipsoids with 50%probability.
Hirschfeld surface analyses can provide a deep understanding of certain characteristics based on electron distribution,πinteractions,and the contributions of pairs of atoms.Polymorphsαand βshowed substantial differences for each fingerprint(Figs.5A and B).Polymorphβshowed a relatively largeπinteraction on the phthalimide ring.This was a direct evaluation of close contact and the internal distribution of theβcell lattice(Fig.5B).A largedistance of approximately 1.1 ? from the inside surface(di)interaction on polymorphβwas related to the appearance of hydrogenatom interaction from the phthalimide–glutarimide transversional ring arrangement within the unit cell(Figs.5B and C).The two interactions at about 1.0 and 1.3 ?(Fig.5B)from the inside surface(di)were due to the glutarimide–glutarimide nitrogen-hydrogen and carbonyl group of two close molecules within the unit cell.The overall O-H interactions showed shorter distances from the inside surface(di)of about 1.0 and 1.3 ? forα and β,respectively,and showed a more compact unit cell for polymorphβ(Fig.5D).For polymorphαirradiated at 2 kGy,the two adjacent glutarimide rings within the unit cell were responsible for the mutual O-H interactions leading to hydrogen-bond formation and/or the possibility of a tautomeric pair structure(Fig.5E).Fig.6 shows the individual contribution from each atom pair to the overall probability of interaction over the thalidomide molecule[15–17].

Table 2 Torsion angle(degrees θ),lattice parameters(?),β (degrees θ)and Rp goodness of fitting parameter(%).
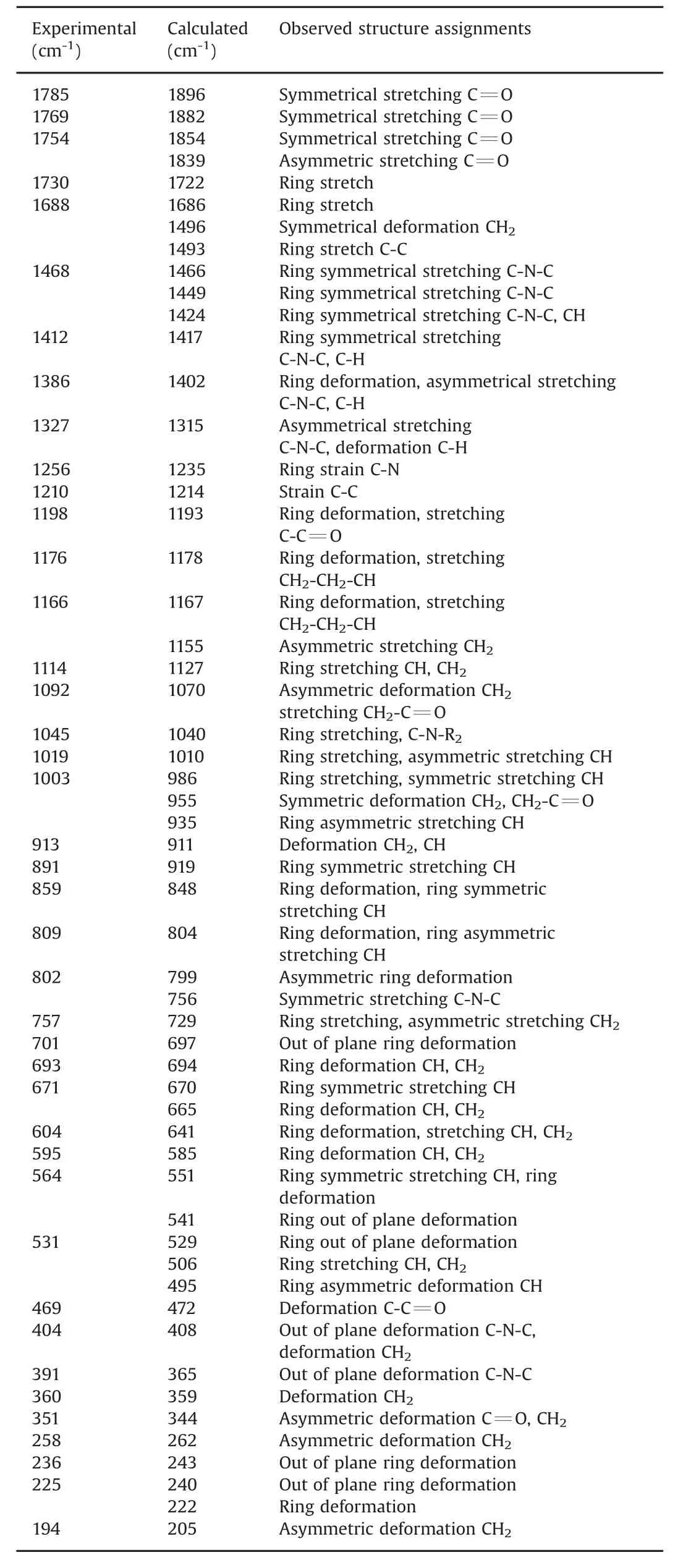
Table 3 Experimentaland calculated Raman'sobserved peak,fully assigned forα polymorph.
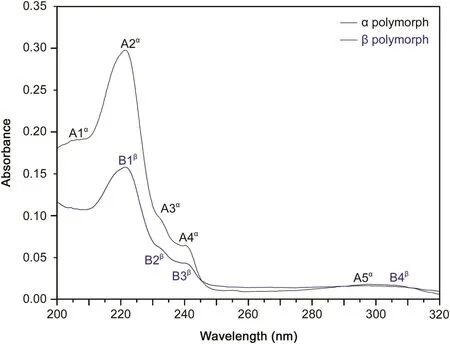
Fig.8.UV experimental spectra for α and β polymorphs.
Raman spectroscopy was undertaken for both polymorphic forms of thalidomide.Theoretical calculations were carried out to increase understanding of the observed vibrational modes.Theoretical calculations were done using the structures of each polymorph published by the Cambridge Crystallographic Data Center(Cambridge,UK)using Spartan v14.Fig.7 shows the experimental Raman spectra for polymorphsα andβ.
Table 2 shows the experimental and theoretical bands(as assigned)for each mode of polymorphα.For symmetric stretching of the carbonyl group,centered at 1785 and 1769 cm–1,no equivalent vibrational modes,when compared with polymorphβ,were identified.
Asymmetric stretching of the carbonyl group was identified at 1754 cm–1.Vibrational modes appeared at two carbonyl groups for polymorphαwhereas,in polymorphβ,such modes were related primarily only to one carbonyl group.The stretching region of the CH2-CH bond in the glutarimide ring showed peaks at 1166 and 1176 cm-1,and showed a substantial difference for the ratio and axial offset for the two polymorphs.Peaks on the spectrum for polymorphαat 701 and 693 cm-1were assigned to the vibrational modes corresponding to ring deformations outside the plane.Peaks at 604 and 595 cm-1were assigned to the ring deformation and stretching of the CH group and CH bonds.For deformation out of the plane,peaks at 404,391,236 and 225 cm-1were observed.For crystalline structures in different polymorphs,the vibrational modes in the low vibrational frequency region(<200 cm-1)are attributed to vibrations of the crystal lattice,and that region can be regarded as a “second fingerprint”of the Raman spectrum for each substance(Table 3)[18,19].Comparison of these data suggested that differences in the spectra of polymorphsαand βwere due to compression of their molecules and the way they were interacting in their crystal lattices;these effects influenced their vibrational modes directly.Transformation between thalidomide polymorphs was achieved by providing adequate energy for the crystalline lattice with the aim of reorganization.This procedure was accompanied by TGA,DTA and DSC.
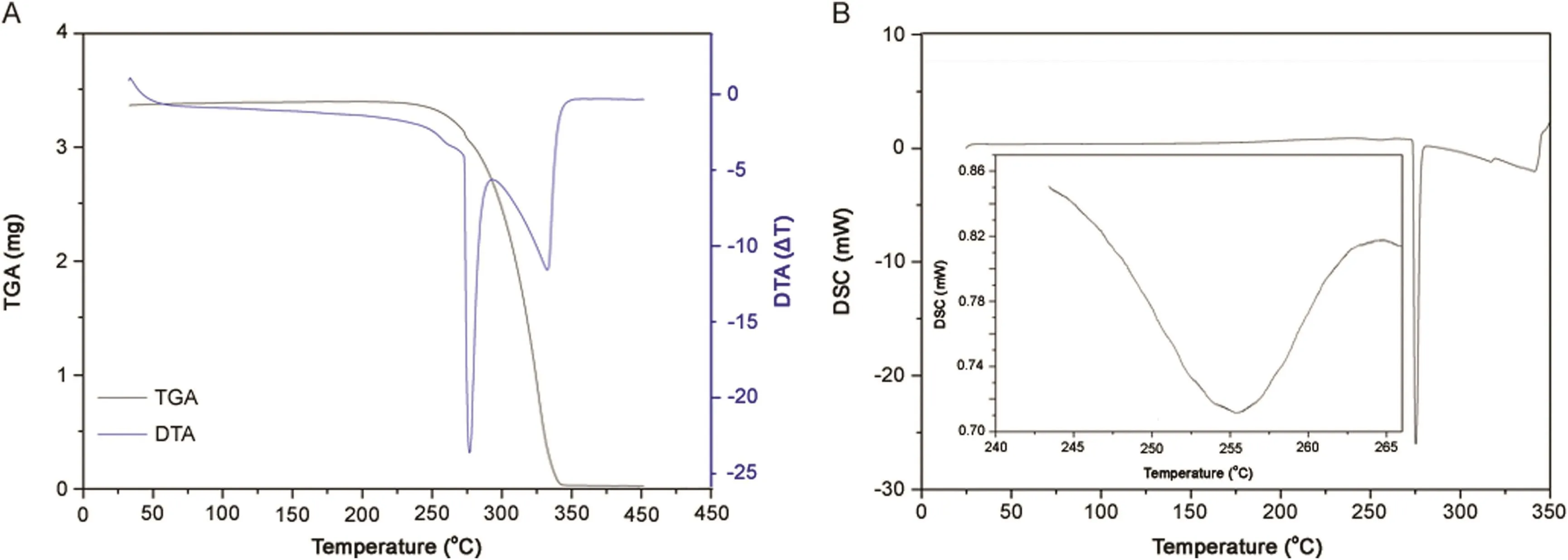
Fig.9.(A)TGA/DTA simultaneous curve of α polymorph form;(B)DSC curve of α polymorph form,with inset zoom of the endothermic peak.
In simultaneous TGA/DTA,mass loss was observed only once at an onset temperature of 264°C,suggesting that the material was anhydrous and pure.The DTA curve revealed two endothermic peaks corresponding to fusion of polymorphsα andβ,respectively.The DSC curve showed two endothermic events at onset temperatures of 245 °C and 274 °C.Fig.8 shows the UV spectra for polymorphsαandβ.We observed five bands for polymorphα(A1,A2,A3,A4 and A5)and four bands for polymorphβ(B1,B2,B3 and B4).The A1 band at 207nm is related to the n→π*transitions in aromatic compounds.The A2 and B1 bands at 221nm and 222 nm,respectively,are related toπ*conjugated systems,showing aromatic compounds to have chromophore substitution.The A3 and B2 bands at 232 nm and 233nm,respectively,are related to tautomers generated by the working pH of the solution.The A4 and B3 bands at 240nm and 241 nm,respectively,are the characteristic bands of thalidomide.The A5 and B4 bands both at 300 nm are related to groups with a low-energy configuration state,just like the carbonyl groups in thalidomide.For better visualization of the first endothermic peak,enlargement of this region in the curve is shown(Fig.9).This event was identified as a crystalline transition between the two polymorphs of thalidomide.
The second endothermic event corresponded to decomposition of the formed material,with this being only theβform in the case of total conversion and a mixture ofαandβin the case of partial conversion[19].To confirm these occurrences,an isotherm at 270°C using the material for further powder XRD was undertaken(Fig.10).Comparison of the diffractograms and interplanar distances enabled us to confirm and identify the material as polymorphβ.
We wished to visualize possible changes in thermal behavior of the material after irradiation.Hence,DSC was done with samples receiving doses of 2,5,30 or 100 kGy.In the DSC curve of the samples irradiated with 2 and 5kGy,a single endothermic peak with an onset temperature of 275°C was noted for both samples.This finding suggested total conversion of theαform into theβ form during heating,so this peak was designated as the fusion follow by decomposition of polymorphβ(Fig.11).The DSC curves of samples irradiated with 30 and 100 kGy revealed two endothermic peaks with onset temperatures of 272 °C and 275 °C for samples irradiated with 30 kGy and at 272 °C and 274 °C for samples irradiated with 100 kGy(Fig.12).
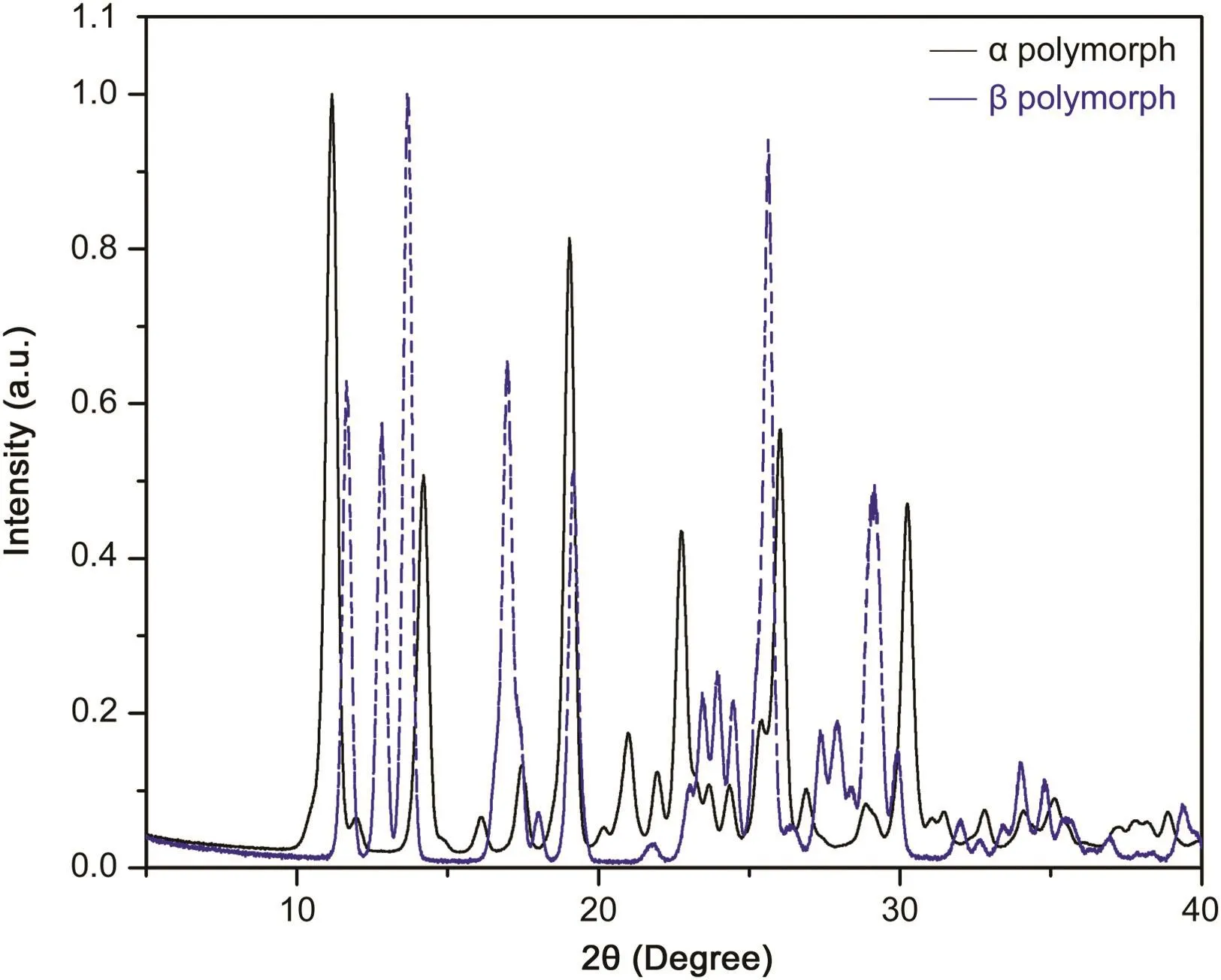
Fig.10.Comparative diffractogram between α and β polymorphs.
We designated the first peak as the fusion of polymorphαand the second peak as the fusion of polymorphβfor both curves.Different from the report by Reepmeyer and colleagues[14],the DSC curve in our study was carried out at a heating rate of 10°C/min,but we observed values very close to those reported by Reepmeyer and colleagues.We propose that after irradiation with doses of 30 and 100 kGy,polymorphαacquired higher thermal stability in relation to polymorphic transformation.Therefore,the fusion and decomposition temperature ofαform was visualized in DSC curves instead of its crystalline transformation,as shown in the physicochemical characterization of the material.The irradiated sample had a more compact unit cell,so there was an increase in hydrogen-atom interactions within the unit cell,resulting in an increase in thermal stability of polymorphα.
4.Conclusion

Fig.11.DSC curves of α polymorph form after(A)2 kGy dose and(B)5 kGy dose.
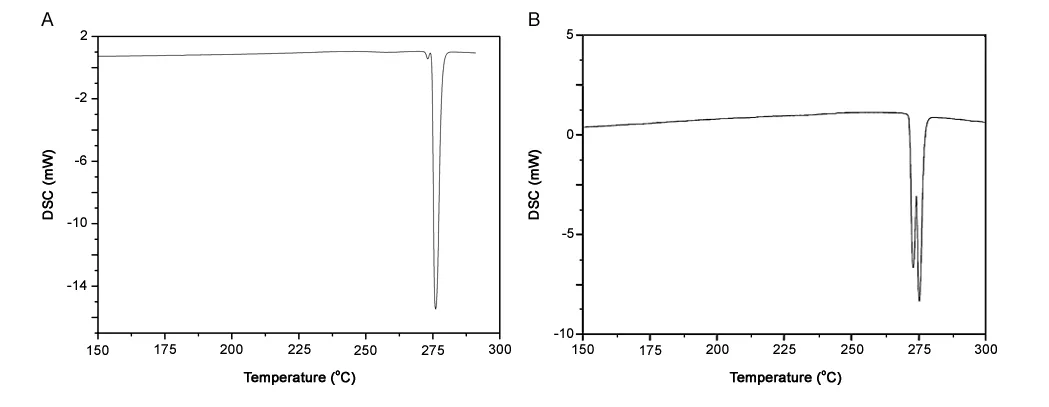
Fig.12.DSC curves of α polymorph form after(A)30 kGy dose and(B)100 kGy dose.
The observed turning around phthalimide and glutarimide rings already occurs at low radiation values(e.g.,2kGy).Eventually,the absorbed energy will overcome the repulsive force due to the proximity of the carboxyl group and produce a full turn.With a continuous supply of energy,the system rotates completely at higher doses of 15,20,30 and 100kGy.With higher doses,the full turning effect is reached,allowing the network to relax its tension.The thalidomide molecule has a center of symmetry.Therefore,one full turn of phthalimide and glutarimide rings between each other,starting from 57.3°,will lead to the same molecule,with stabilization of the final angle based on the total amount of absorbed energy.After a full turn,the process starts again.Irradiated samples at 30 and 100kGy had more compact unit cells and a lower volume,so there was an increase in the intermolecular interaction between hydrogen atoms within the unit cell,which resulted in higher thermal stability for polymorphα.At 30 and 100kGy,each melting point could be seen separately,which was a different situation compared with that of the non-irradiated sample.A fourfold increase in the usual dose used in pharmaceuticals is employed for gamma-ray sterilization.Thalidomide molecules can release excess energy by turning the bond between phthalimide and glutarimide rings.Hence,gamma-ray sterilization of pure thalidomide before use in fixed-dose pharmaceutical formulations is possible.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors would like to thank the CNEN-CDTN(Comiss?o Nacional de Energia Nuclear–Centro de Desenvolvimento da Tecnologia Nuclear)LIG(Laboratório de Irradia??o Gama)facility for the assistance,and Funda??o de Amparo à Pesquisa do Estado de Minas Gerais(FAPEMIG)(APQ-02087-14),Conselho Nacional de Desenvolvimento Científico e Tecnológico(CNPq)(245914/2012),Coordena??o de Aperfei?oamento de Pessoal de Nível Superior(CAPES)(PNPD-N-1648694-scholarship No2016752283)and Pró-Reitoria de Pesquisa/UFMG for financial support.
[1]Health Products and Food Branch Inspectorate∶Process Validation∶Terminal Sterilization Processes for Pharmaceutical Products,Supersedes∶GUI-0007,GUI-0009 and GUI-0010,(GUIDE-0074),OTTAWA,Ontario,Canada,2006.
[2]EN ISO 13408-1∶2008(en),Aseptic processing of health care products—Part 1∶General requirements,compilation prepared by Online Browsing Platform(OBP),2008,〈https∶//www.iso.org/obp/ui/#iso∶std∶iso∶13408∶-1∶ed-2∶v1∶en〉.
[3]AAMI/ISO 11137-2∶2013,Sterilization of health care products-Radiation-Part 2∶Establishing the sterilization dose(revision of 11137-2∶2012),ISBN(s)∶1570205027,2013.
[4]J.Agalloco,USP Microbiology&Sterility Assurance,Expert Committee USP∶Activities Impacting Sterilization&Sterility Assurance,USP 35 1211,revision 2008.
[5]F.Hasanain,K.Guenther,W.M.Mullett,et al.,Gamma sterilization of pharmaceuticals-a review of the irradiation of excipients,active pharmaceutical ingredients and final drug product formulations,J.Pharm.Sci.Technol.68(2014)113–137.
[6]T.Roisnel,J.Rodriguez-Carvajal,WinPLOTR∶a Windows tool for powder diffraction patterns analysis Materials Science Forum,Proceedings of the Seventh European Powder Diffraction Conference,EPDIC 7,2000∶118–123.
[7]J.Rodriguez-Carvajal,T.Roisnel,FullProf.98 and WinPLOTR∶new windows 95/NT applications for diffraction.Commission for powder,Int.Union Crystallogr.Newsl.20(1998)35.
[8]M.J.Turner,J.J.McKinnon,S.K.Wolff,et al.,CrystalExplorer17,University of Western,Australia,2017 〈http∶//crystalexplorer.scb.uwa.edu.au〉.
[9]C.H.G?rbitz,What is the best crystal size for collection of X-ray data?Reif nement of the structure of glycyl-L-serine based on data from a very large crystal,Acta Cryst.B55(1999)1090–1098.
[10]R.I.Cooper,A.L.Thompson,D.J.Watkin,CRYSTALS enhancements∶dealing with hydrogen atoms in re finement,J.Appl.Cryst.43(2010)1100–1107.
[11]G.M.Sheldrick,A short history of SHELX,Acta Cryst.A64(2008)112–122.
[12]P.W.Betteridge,J.R.Carruthers,R.I.Cooper,et al.,CRYSTALS Version 12∶software for guided crystal structure analysis,J.Appl.Cryst.36(2003)1487.
[13]D.J.Watkin,C.K.Prout,J.R.Carruthers,et al.,Crystals Issue 10,Chemical CrystallographyLaboratory,Oxford,UK,1996.
[14]J.C.Reepmeyer,M.O.Rhodes,D.C.Cox,et al.,Characterization and crystal structure of two polymorphic forms of racemic thalidomide,J.Chem.Soc.Perkin Trans.2(9)(1994)2063–2067.
[15]M.A.Spackman,D.Jayatilaka,Hirshfeld surface analysis,CrystEngComm 11(2009)19–32.
[16]M.A.Spackman,J.J.McKinnon,Fingerprinting intermolecular interactions in molecular crystals,CrystEngComm 4(2002)378–392.
[17]M.J.Turner,J.J.McKinnon,D.Jayatilaka,et al.,Visualisation and characterisation of voids in crystalline materials,CrystEngComm 13(2011)1804–1813.
[18]P.J.Larking,M.Dabros,B.Sars field,et al.,Polymorph characterization of active pharmaceutical ingredients(APIs)using low-frequency Raman spectroscopy,Appl.Spectrosc.68(7)(2014)758–776.
[19]E.P.J.Parrot,B.M.Fischer,L.F.Fladden,et al.,Terahertz spectroscopy of crystalline and non-crystalline solids,Terahertz spectroscopy and imaging Springer Series in Optical Sciences book series(SSOS),171,2012∶191–227.
 Journal of Pharmaceutical Analysis2018年3期
Journal of Pharmaceutical Analysis2018年3期
- Journal of Pharmaceutical Analysis的其它文章
- JPA Prize in 2016
- Sensitive and rapid determination of amantadine without derivatization in human plasma by LC–MS/MS for a bioequivalence study
- Enrichment and immobilization of macromolecular analytes on a porous membrane utilizing permeation drag
- Enhancing the dissolution of phenylbutazone using Syloid?based mesoporous silicas for oral equine applications
- Identi fication of three kinds of Plumeria flowers by DNA barcoding and HPLC specific chromatogram
- Evaluation of physicochemical properties as supporting information on quality control of raw materials and veterinary pharmaceutical formulations
