A comprehensive review on clinical outcome of probiotic and synbiotic therapy for inflammatory bowel diseases
Bhagavathi Sundaram Sivamaruthi
Innovation Center for Holistic Health, Nutraceuticals, and Cosmeceuticals, Faculty of Pharmacy, Chiang Mai University, Chiang Mai 50200,Thailand
1. Introduction
Gastrointestinal (GI) diseases are one of the severe hindrances of human health. The most reported GI diseases are inflammatory bowel disease (IBD) and intestinal neoplasia. Ulcerative colitis (UC)and Crohn’s disease (CD) are the frequently described IBDs. The incidence of UC is more commonly reported than that of CD, and the frequency of UC cases are higher in developed countries. UK,Northern European and North American countries are reported for the high incidence of UC and CD[1-4]. Intestinal neoplasia is responsible for several deaths, particularly colorectal cancer, which is the third and fourth most common cause of morbidity and mortality,respectively[5-8].
The composition of gut microflora and its metabolic activity are closely correlated with the host immune system, and the changes in the biometric of the microbiome lead to inflammatory diseases like IBD.The functional food supplements are believed to sustain the health and to diminish the risk of developing diseases in human. Probiotics are live bacteria that have been revealed to exhibit beneficial effects on human health. The use of raw probiotic supplements and probiotic based foods like yogurt, fermented beverages and meats are gradually increased over the years. Several scientific reports revealed that the consumption of probiotic-based diet improved the health status of several diseases[9-11].This paper mainly focuses on the beneficial clinical outcomes of probiotic supplements against IBD.
2. Probiotics
Probiotics are well-defined as live microbes that exhibit health benefits on the host when administered in sufficient amounts[12].The definition is also appropriate for fermented dairy products[12],dietary supplements, conventional foods, probiotic-containing drugs,probiotic mediated fermented foods, infant formula, medical foods,animal feed, non-oral probiotics and designer probiotics[13]. Probiotics are used from the ancient times and believed to have a history of usage for the past 10 000 years[14].
A microorganism is categorized as a probiotic based on the several regulations. The safety regulations for the use of probiotic are different among the countries. The probiotic based products are marketed as dietary supplements to healthy people in the USA[15]. The regulatory requirements for a probiotic-based drug and dietary supplement are different. A probiotic strain or combination of several probiotic strains that are proposed to treat any diseases or any ill health must undergo the regulatory process as a drug as per the Food and Drug Administration (FDA) regulations. Whereas regulatory process which is necessary for the drug is not required for the probiotic based products that are intended for use as food supplements, but it must stick with the guidelines of FDA’s Center for Food Safety and Applied Nutrition[15].Standard regulations have been developed to claim a microbial strain as probiotic by the experts from Food and Agriculture Organization of the United Nations and World Health Organization Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics.The guidelines endorse the following: (1) Strain identification using biochemical, and molecular methods to confirm the health benefits; (2)In vitrostudies on evaluation of the possible mechanism of the probiotic effect; (3) The clinical trials to assess the health benefits on humans; (4)The biochemical and genetic safety assessments such as determination of drug resistance pattern, metabolic activities, side effects, production of any toxin, and no reports of pathogenicity[15-17].
The council also suggests third-party evaluation of the strain for its safety and stability. Some of the producers have evidence for probiotic effects and safety through small, randomized, controlled studies in human volunteers. Moreover, clinicians and medical prescriber must consider the scientific reports on the specified product and its beneficial effects before prescribing to people[15,17].
The strains of the generaBifidobacterium,Lactobacillus,Enterococcus,Streptococcus,Saccharomyces,Leuconostoc,Pediococcus, andBacillusare claimed as probiotics with proven health benefits. The bifidobacteria and lactic acid bacteria are commonly used probiotics while other bacteria and yeasts strains are also used as probiotics.
The probiotics were proved for several health benefits against antibiotic-associated diarrhea[18-20], travelers’ diarrhea[21], functional constipation[22], urinary tract infections[23], infantile colics[24,25],ulcerative colitis[26], necrotizing enterocolitis[25], radiation-induced diarrhea[27], allergies[25,28], hypercholesterolemia[29]. It has also been proved that the administration ofSaccharomyces boulardii(S.boulardii) along with antibiotics reduced the severity ofClostridium difficileinfection[30,31], and the spores ofBacillus subtilishave been considered as probiotics to treatHelicobacter pyloriinfection and nosocomial bacteremia[32,33].
3. IBD
UC and CD are the most frequent types of IBD characterized by chronic intestinal inflammation. UC and CD differ in their histopathological signatures. An inflammatory reaction can be observed in UC with several blisters in the crypts and infiltration of eosinophils, plasma cells, and neutrophils that continuously affects the lining of the rectum and colon. In general, inflammation occurs in the same area in the case of UC with reduced disease period, and symptoms including abdominal pain, mucus discharge, rectal bleeding, diarrhea,and tenesmus. In the case of CD, the entire intestine may have affected by chronic inflammation with the physiognomies of scattered healthy tissues in between the affected area. The symptoms of CD are regular abdominal pain, fever, diarrhea, and weight loss. Most frequently, CD affects the colon and ileum[34-36].
The etiology of IBD is not understood completely. But, unhygienic lifestyle, pathogenic exposure, genetics, environmental factors like exposure to nonsteroidal anti-inflammatory drugs, tobacco, unhealthy diet (e.g., fatty foods), and intestinal microbiota are the possible cause of IBD[36,37]. The composition of gut microbiota, which can alter the host immune response, is closely associated with the development of IBD[38-42]. The complete pathogenesis and molecular mechanism of IBD are not yet studied. The studies suggested that the IBD is the result of complex immune response against the intestinal microbiota[43]. The known mechanism of development of IBD has illustrated in Figure 1.
4. Probiotics and IBD
The incidence of IBD in a person may depend on the genetic aberrations, which stimulates the abnormal inflammatory response against intestinal microbiota. The intestinal microbiome is also responsible for the continuation of the inflammatory response, and it is proven that the intestinal bacteria can penetrate the mucosa and strengthen the intestinal epithelial inflammation[44-47].
The probiotics can change the microbial composition of the intestine,and it is believed that the probiotics support the growth of beneficial microbes. Thus, several probiotic based products and treatments are employed around the world. Nevertheless, the prescribed amount of probiotics and intervention strategies are varying based on the strain and the person who is consuming the product. It is necessary to have a minimal number of live bacterium, or combinations of bacteria with proven efficacy[48]. Manyin vivostudies revealed the protective effects of several probiotic strains against IBD[49-52]. The present paper complied the results of a probiotic-based intervention to treat or abate the sternness of IBD (CD and UC) in human subjects.
The probiotic preparation, VSL#3, composed of viable cells ofLactobacillus casei(L. casei),Streptococcus salivariussubspthermophilus,Lactobacillus delbrueckiisubspeciesbulgaricus,Lactobacillus plantarum,Lactobacillus acidophilus(L. acidophilus),Bifidobacterium breve(B. breve),Bifidobacterium infantis, andBifidobacterium longum(B. longum). Several clinical studies were conducted in UC and CD patients to assess the protective nature of VSL#3. The studies proved that the supplementation of VSL#3 increased the remission rate and reduced the rate of relapse in UC patients, and also reduced the symptoms of the CD. TLR-2 expression,inflammatory cytokines, and IL-12p40 production were reduced,and IL-10 production was increased during VSL#3 intervention in UC patients. About 60% of UC disease activity index was reduced,and the endoscopic and histological scores were reduced by probiotic treatment. Moreover, VSL#3 supplementation increased the load of bifidobacteria, lactobacilli, andStreptococcus salivariusssp.thermophilus. But, there were no changes in Bacteroides, clostridia,coliforms, total aerobic and anaerobic bacteria load. Over all, all the studies suggested that VSL#3 supplementation was safe, which not only reduced the expression level of inflammatory cytokines and the severity of both adult and pediatric active UC but also maintained the remission of UC[53-58].
UC patients were treated with sulphasalazine and glucocorticoid; then they were supplemented daily with 1.26 g of Bifico, a bifid triple viable capsule, for eight weeks. About 20% of patients exhibited the relapses after two months of study. The fecal lactobacilli and bifidobacteria load were increased. DNA binding property of NF-κB and NF-κB p65 expression was reduced. The expression level of anti-inflammatory cytokines was improved. The authors claimed that the Bifico was effective in prevention of the relapse in chronic UC[59]. UC patients were treated with salazosulphapyridine (3-4 g/d), or mesalazine (2 250-3 000 mg/d) for one month prior to the supplementation of bifidobacteria-fermented milk(BFM) (10 billion cells ofL. acidophilusstrain Yakult,Bifidobacterium bifidumstrain Yakult, andB. brevestrain Yakult per 100 mL) per day for 12 weeks. After 12 weeks of treatment, the clinical activity index was more reduced in BFM treated group than that of the placebo.BFM treated group showed the reduction in histological score and endoscopic activity index. The concentrations of fecal propionate,total short-chain fatty acid, and butyrate were increased upon BFM supplementation in UC patients[60].
Mild to moderate distal UC patients were provided with a commercial probiotic blend ofBacillus mesentericusTO-A (10 mg),Clostridium butyricumTO-A (10 mg), andEnterococcus faecalisT-110 (2 mg)per tablet, called BIO-THREE (9 tablets per day) for 4 weeks. The endoscopic findings and clinical symptoms were determined as UC disease activity index and the changes in the fecal microbiota was evaluated by terminal restriction fragment length polymorphism.The results suggested that about 45% of UC patients exhibited remission because of the intervention of BIO-THREE tablets, and the bifidobacterial load in feces was increased. The BIO-THREE was considered safe and effective for the treatment of UC[61]. Another recent report by Yoshimatsuet al[62] also claimed that the BIO-THREE was effective for maintaining remission in UC patients.
About 120 UC patients were divided into three groups and supplemented with probiotic (2 × 109CFU ofB. longum), prebiotic(8.0 g of psyllium per day), and synbiotic (2 × 109CFU ofB. longumand 8.0 g of psyllium per day), for 4 weeks, respectively and the health profile of the patients was evaluated. The results showed that the probiotic intervention improved the emotional function and Inflammatory Bowel Disease Questionnaire scores, and prebiotic intervention improved bowel function and Inflammatory Bowel Disease Questionnaire scores. Whereas, the synbiotic intervention ameliorated the social and systemic functions, and Inflammatory Bowel Disease Questionnaire scores, and decreased the level of C-reactive protein.Collectively, the synbiotic intervention enhanced the life quality of UC patients, when compared to that of the probiotic and prebiotic supplements[63].
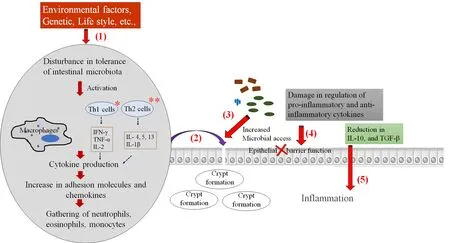
Figure 1. Schematic representation of molecular and cellular mechanism in the development of IBD.(1) Factors including environmental factors, genetic factors, lifestyle etc. affect the intestinal microbiota, which further activate the immune cells and create the inflammatory machinery. (2) The inflammatory molecules cross the epithelial barrier and form crypt abscesses, (3) which facilitate the invasion of microbes that stimulate the inflammatory cytokines. (4) The fluctuations in the expression of pro-inflammatory and anti-inflammatory cytokines that leads to failure of epithelial barrier function. (5) Reduction of anti-inflammatory cytokines, which results in loss of tolerance to antigens of na?ve microbiota, and facilitates the continuation of the inflammatory process. * In CD, T- helper 1 cells are involved and increase in IFN-γ, TNF-α, and IL-2 can be observed. Whereas, ** in UC, increased production of IL-4, IL-5, IL- 13 and IL-1β was observed with the action of T- helper 2 cells.
The supplementation of 2 400 mg per day of sulfasalazine, andLactobacillus delbruekiiandLactobacillus fermentum(10 billion CFU per day) for eight weeks diminished the inflammation by reducing the leukocyte recruitment, myeloperoxidase activity, and expression level of NF-κB p65 and TNF-α in UC patients. Moreover, the reduction in fecal calprotectin level was observed in probiotic-treated UC patients.The supplementation ofLactobacillus delbruekiiandLactobacillus fermentumalong with chemotherapy maintained the remission and prevented the relapse of UC[64]. Two capsules (thrice per day) of Probio-Tec AB-25 (a probiotic blend of equal concentration, 1.25 × 1010CFU, ofBifidobacterium animalissubsp.lactisBB-12 andL. acidophilusLA-5,respectively) has been supplemented for 52 weeks to the patients with leftsided UC. The study results showed that about 25% of patients maintained remission after one year of treatment, and also average relapse period was extended in Probio-Tec AB-25 treated group. The study revealed that the Probio-Tec AB-25 was safe, and significantly protected the patients from UC recurrence[65].
D’Incaet al[66] explained the efficiency of intervention mode of probiotic for the treatment of UC. The UC patients were grouped into three (group 1, 2, and 3) and supplemented with 5-aminosalicylic acid (2.4 g per day),5-aminosalicylic acid (2.4 per day) + oralL. caseiDG (8 × 108CFU twice a day), and 5-aminosalicylic acid (2.4 g per day) + Rectal administration ofL. caseiDG (8 × 108CFU twice a day), respectively, for 8 weeks. The colonic microflora and the level of expression of TLR were not affected in the patients of 5-aminosalicylic acid, and 5-aminosalicylic acid + oralL.caseiDG supplemented groups. Whereas, rectal administration ofL. caseiDG suppressed the IL-1β, and TLR-4 expression, and increased the IL-10 level. The reduction inEnterobacteriaceaeand increase inLactobacillusspp.have also been detected in group 3. The results proved thatL. caseiDG, as a potent probiotic, enhanced the mucosal immune system of UC patients.Another study by Olivaet al[67] proved that the rectal enema ofLactobacillus reuteriATCC 55730 (1010CFU per day) along with oral supplementation of 50 to 75 mg/kg/d of mesalazine could significantly decrease the clinical signs and histological scores. The level of IL-10 was increased, and the levels of IL-1β, IL-8, and TNF-α were found to be decreased. The results suggested that the rectal infusion ofLactobacillus reuteriwas efficient to reduce the mucosal inflammation in pediatric UC.
Synbiotic, prepared withB. breveYakult (109CFU/g; 3 g per day) and galacto-oligosaccharide (GOS) (5.5 g per day), was supplemented to UC patients for one year, and the clinical status of UC, myeloperoxidase concentration, and fecal microbiota was assessed. The results showed that the level of myeloperoxidase and the colonoscopic index were improved. The fecal pH and fecalBacteroidaceaecount were reduced.The synbiotic preparation improved the clinical condition of UC patients[68].
UC patients were supplemented with increasing dose of Profermin?, a fermented oatmeal with lecithin, barley malt,Lactobacillus plantarum299v (≥108cells/mL), and water, for 24 weeks. The results suggested that about 46% of patients exhibited the significant reduction in Simple Clinical Colitis Activity Index, and the mean time taken to reach half the reduction in repeated-measure regression analysis score was 28 days. Moreover, the authors demanded that the Profermin?was harmless and could induce the remission of UC[69]. The intervention of single probiotic strain,Bifidobacterium infantis35624, to UC patients improved the C-reactive protein level and decreased the expression of IL-6. It has been proved thatBifidobacterium infantis35624 can diminish the systemic inflammation in UC patients[70].
The UC patients with severe pouchitis have been treated with 500 mg of metronidazole (thrice per day), and 500 mg of ciprofloxacin (twice per day) for four weeks, followed by multi-strain probiotic preparation,Ecologic 825 (Bifidobacterium lactis(W51 and W52),Bifidobacterium bifidumW23,Lactococcus lactisW19,L. caseiW56,Lactobacillus plantarumW62,Lactobacillus paracaseiW20,Lactobacillus salivariusW24 andL. acidophilusW22; 2.5 × 109CFU/g; 3 g twice per day) for eight weeks. The Pouchitis Disease Activity Index was improved after treatment. The intestinal permeability level of the patients was evaluated with a representative strain ofEscherichia coli(E. coli) K12, and the results suggested thatE. coliK12 passage was reduced. The results revealed that Ecologic 825 intervention restored the mucosal barrier function in UC patients[71].
Petersenet al[72] studied the impact of the intervention ofE. coliNissle 1917 (100 mg per day for four days, and 200 mg per day until seven weeks) on the health status of UC patients along with prior standard medications (1 000 mg of ciprofloxacin per day for one week). The results suggested thatE. coliNissle was not suitable for the add-on treatment for UC along with antibiotics.
The intervention ofClostridium butyricumMIYAIRI (60 mg thrice per day) to UC patients for 24 months significantly suppressed the pouchitis development, and each group showed typical intestinal flora.The results suggested thatClostridium butyricumMIYAIRI can be a potent candidate for probiotic treatment[73]. A long-term (two years)intervention of two doses of Acronelle?(Lactobacillus salivarius,L.acidophilusandBifidobacterium bifidusstrain BGN4) along with a single dose of mesalazine (1 200 mg per day) to UC patients resulted in the reduction of clinical consequences of UC. The results also revealed that Acronelle?could be an alternative to steroid therapy for UC[74].A sum of fifty-six patients with mild to moderate UC was treated with single probiotic strain,B. longum536 (2×× 1011-3×× 1011cells per day), for eight weeks. About 63% of clinical remission was observed in the probiotic-treated group. The UC disease activity index score,endoscopic index, and Mayo sub-score were found to be reduced after the treatment. Overall,B. longum536 intervention improved the health status of UC patients[75].
About five UC and fifteen CD patients were supplemented with the yogurt containing probiotic strains,Lactobacillus reuteriRC-14 (1 ××103CFU) andLactobacillus rhamnosusGR-1 (2 × 107CFU) for 30 d. After 30 days of treatment, CD4+CD25highT cells proportion was increased, whereas the proportion of myeloid dendritic cells and TNF-α/IL-12 monocytes was reduced in both UC and DC patients. The follow-up study also suggested that the consumption of probiotic yogurt significantly suppressed the inflammation[76].
Shadnoushet al[77] also revealed the beneficial effect of probiotic yogurt on UC and CD patients. About 250 g of yogurt containing probiotic strains (106CFU/g of yogurt) namely,BifidobacteriumBB-12 andL. acidophilusLA-5 was supplemented daily to UC and CD patients for eight weeks. Stool samples were collected from the patients before and after the intervention, and the changes in microflora were analyzed by qPCR. The results showed that the mean numbers ofLactobacillus, andBifidobacteriumwere significantly increased in the treatment group compared to placebo. Significant changes were not observed in weight, and body mass index of the patients.
Patients with CD in remission were treated with lyophilized cells ofS. boulardii-17 (200 mg; 4 × 108cells), magnesium stearate (2.4 mg),and sucrose (6 mg) per day for three months. At the end of the 3rd month, the lactulose/mannitol ratio was found to be reduced in the treatment group compared to that of the placebo group. The intestinal permeability was also improved upon treatment of CD patients. The study revealed thatS. boulardiisupplementation along with baseline therapy enhanced the intestinal permeability of CD patients in remission, but complete curing was not observed[78].
Vleggaaret al[79] studied the beneficial effects of probiotic preparation,Ecologic 641 (Bifidobacterium bifidum,Lactococcus lactis,L. casei,Bifidobacterium lactis,Lactobacillus salivarius, andL. acidophilus)on patients with primary sclerosing cholangitis and concurrent IBD.Fourteen patients were treated daily with 1010cells of Ecologic 641 for three months and did the crossover after one month of the washout period. Significant changes was not observed between the treated and placebo groups in pruritus, fatigue, stool frequency, prothrombin,albumin and tested enzymes such as γ glutamyl transpeptidase, alkaline phosphatase, alanine aminotransferase and aspartate aminotransferase.The study results proved that Ecologic 641 don’t have any beneficial effect on patients with primary sclerosing cholangitis. Bourreilleet al[80]reported that the supplementation ofS. boulardii(1 g/d) for 52 weeks to CD patients does not influence the relapse and the concentration of C-reactive protein.
Clinical outcomes have been found to be improved in CD patients as a result of synbiotic (six gram of Synergy 1, and 2 × 1011CFU ofB. longum; twice a day) intervention for six months. The significant reduction in Crohns disease activity index and histological scores was observed. After synbiotic treatment, the expression of TNF-α was reduced, and the mucosal bifidobacteria level was found to be increased in CD patients[81].
Ahmedet al[82] investigated the impact of synbiotic on the colonic microflora of IBD patients. Both UC and CD patients were supplemented with three Trevis?capsules (each capsule contains 4 ×109cells ofBifidobacterium animalissubsp.lactisBB-12?,Streptococcus thermophilusSTY-31?,Lactobacillus delbrueckiisubsp.bulgaricusLBY-27, andL. acidophilusLA-5?), and oligofructose (15 g per day)for one month. After treatment, the changes in the microbiota of the patients were assessed by terminal restriction fragment length polymorphism and qPCR. The results suggested that significant changes were not observed among the treated and placebo groups of both UC and CD patients. The authors claimed that the studied synbiotic supplementation does not affect the colonic microflora of IBD patients.
5. Adverse effect of probiotics
There are no reported significant adverse effects of the probiotic intervention on IBD patients.B. longum536 supplementation caused a mild side effect (a dry cough) on one of the UC patients[75].
Though the probiotics are used for preventing, managing and treating several diseases which are common, some of the unwanted side effects like GI side effects, unwarranted immune stimulation,systemic infections, gene transfer, and lethal metabolic activities have been seen in some group of people, who have undergone the probiotic supplementation[83].
Several cases of fungemia, bacteremia, overt sepsis and endocarditis are associated with known probiotic strains namelyS. boulardii,L.casei,L. acidophilus,LactobacillusGG,Bacillus subtilis,B. breve, andLactobacillus rhamnosus[84-96]. It has been reported that the probiotic intervention prompts the inflammatory response in the small bowel region, and causes D-lactic acidosis[97,98]. The possibilities of gene transfer among the intestinal bacteria, excess stimulation of both innate and adaptive immune system, and adverse effects in GI track have also been documented[83].
Recently, Brunser[99] reviewed the safety and risk of the use of probiotics, in particular for infants, and immune-compromised patients. The reports and clinicians claimed that the use of probiotics relies on birth, age and medical conditions of an individual, and it is important to convey the unpleasant effects of probiotic to the user.
6. Conclusion
The studies suggested that the intervention of multi-strain probiotic preparation or synbiotic preparation performed better than single strain therapy. Moreover, several studies were conducted with VSL#3 and proved that the VSL#3 was safe and recommended by many clinicians to support the remission of UC, and for maintenance therapy. Most of the clinical trials, except some recent studies, were conducted with a minimum number of patients or short treatment duration or no proper follow-up. Apart from the formulation of best probiotic or synbiotic preparation, dosage, duration, mode of intervention, and form of supplementation are playing a critical role in the outcome of a clinical trial. Overall, the present review suggested that probiotics are the healthier alternatives to conventional therapy or an adjuvant for standard therapy for IBD. Further, elaborated studies are required to figure out the potent probiotic strain or combinations to treat or control the IBD, and to maintain the remission.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
The author gratefully acknowledges the Faculty of Pharmacy and Chiang Mai University, Chiang Mai, Thailand, and also wish to acknowledge the CMU Post-Doctoral Fellowship (No.6592(11)/01501, dated 24 February 2017), Chiang Mai University,Chiang Mai, Thailand.
[1] Baumgart DC, Carding SR. Inflammatory bowel disease: Cause and immunobiology.Lancet2007;369(9573): 1627-1640.
[2] Knutsson A, Boggild H. Gastrointestinal disorders among shift workers.Scand J Work Environ Health2010;36(2): 85-95.
[3] Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis.Lancet2012;380(9853): 1606-1619.
[4] Vezza T, Rodríguez-Nogales A, Algieri F, Utrilla MP, Rodriguez-Cabezas ME, Galvez J. Flavonoids in inflammatory bowel disease: A review.Nutrients2016;8(4): 211.
[5] World Cancer Research Fund and American Institute for Cancer Research.Food, nutrition, physical activity, and the prevention of cancer: A global perspective. Washington, DC: American Institute for Cancer Research; 2007.
[6] Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence,mortality, survival, and risk factors.Clin Colon Rectal Surg2009;22(4): 191-197.
[7] Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014.CA Cancer J Clin2014;64(2): 104-117.
[8] Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012.CA Cancer J Clin2015;65(2): 87-108.
[9] Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis.Nutrition2009;25(5): 520-525.
[10] Santana PS. Influence of a combination of lactobacilli and bifidobacteria upon disease activity, stool pattern and nutritional status of ulcerative colitis patients.Nutr Hosp2010;25(6): 971-983.
[11] Li K, Zhang CF, Xia YH, Li ZJ, Han Y. Efficacy of probiotics on ulcerative colitis and its mechanism.Zhonghua Wei Chang Wai Ke Za Zhi2013;16(4): 336-339.
[12] Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic.Nat Rev Gastroenterol Hepatol2014;11(8): 506-514.
[13] Sanders ME. Probiotics in 2015: Their scope and use.J Clin Gastroenterol2015;49(Suppl 1): S2-6.
[14] Ozen M, Dinleyici EC. The history of probiotics: The untold story.Benef Microbes2015;6(2): 159-165.
[15] Venugopalan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use.Emerg Infect Dis2010;16(11): 1661-1665.
[16] Food and Agriculture Organization of the United Nations, World Health Organization. Probiotics in food: Health and nutritional properties and guidelines for evaluation.FAO Food Nutr Pap2006;85: 1-50.
[17] Degnan FH. The US food and drug administration and probiotics:Regulatory categorization.Clin Infect Dis2008;46(S2): S133-136.
[18] Johnston BC, Goldenberg JZ, Vandvik PO, Sun X, Guyatt GH. Probiotics for the prevention of pediatric antibiotic-associated diarrhea.Cochrane Database Syst Rev2011;11: CD004827.
[19] Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis.JAMA2012;307(18): 1959-1969.
[20] Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibioticassociated diarrhea.J Clin Gastroenterol2008;42: S58-S63.
[21] McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea.Travel Med Infect Dis2007;5: 97-105.
[22] Chmielewska A, Szajewska H. Systematic review of randomized controlled trials: Probiotics for functional constipation.World J Gastroenterol2010;16:69-75.
[23] Grin PM, Kowalewska PM, Alhazzan W, Fox-Robichaud AE.Lactobacillusfor preventing recurrent urinary tract infections in women: Meta-analysis.Can J Urol2013;20: 6607-6614.
[24] Aloisio I, Santini C, Biavati B, Dinelli G, Cencic A, Chingwaru W, et al.Characterization ofBifidobacteriumspp. strains for the treatment of enteric disorders in newborns.Appl Microbiol Biotechnol2012;96: 561-576.
[25] Di Gioia D, Aloisio I, Mazzola G, Biavati B. Bifidobacteria: Their impact on gut microbiota composition and their applications as probiotics in infants.Appl Microbiol Biotechnol2014;98: 563-577.
[26] Dylag K, Hubalewska-Mazgaj M, Surmiak M, Szmyd J, Brzozowski T.Probiotics in the mechanism of protection against gut inflammation and therapy of gastrointestinal disorders.Curr Pharm Des2014;20: 1149-1155.
[27] Demers M, Dagnault A, Desjardins JA. Randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation.Clin Nutr2014;33(5): 761-767.
[28] Isolauri E, Rautava S, Salminen S. Probiotics in the development and treatment of allergic disease.Gastroenterol Clin North Am2012;41: 747-762.[29] Tomaro-Duchesneau C, Saha S, Malhotra M, Jones ML, Labbe A, Rodes L,et al. Effect of orally administeredL. fermentumNCIMB 5221 on markers of metabolic syndrome: Anin vivoanalysis using ZDF rats.Appl Microbiol Biotechnol2014;98: 115-126.
[30] Tung JM, Dolovich LR, Lee CH. Prevention ofClostridium difficileinfection withSaccharomyces boulardii: A systematic review.Can J Gastroenterol2009;23: 817-821.
[31] Fitzpatrick LR. Probiotics for the treatment ofClostridium difficileassociated disease.World J Gastrointest Pathophysiol2013;4: 47-52.
[32] Tompkins TA, Xu X, Ahmarani JA. Comprehensive review of post-market clinical studies performed in adults with an Asian probiotic formulation.Benef Microbes2010;1: 93-106.
[33] Richard V, Auwera P, Snoeck R, Daneau D, Meunier F. Nosocomial bacteremia caused byBacillusspecies.Eur J Clin Microbiol Infect Dis1988;7: 783-785.
[34] Buller H, Chin S, Kirschner B, Kohn J, Markowitz J, Moore D, et al.Inflammatory bowel disease in children and adolescents: Working Group Report of the First World Congress of Pediatric Gastroenterology,Hepatology, and Nutrition.J Ped Gastroenterol Nutr2002;35(2): 151-158.
[35] Podolsky DK. Inflammatory bowel disease.New Eng J Medicine2002;347:417-429.
[36] Oliveira FM, Emerick APC, Soares EG. Epidemiology aspects of inflammatory bowel disease in the east region of Minas Gerais State.Ciencia Saude Coletiva2010;15(1): 1031-1037.
[37] Shanahan F. Crohn’s disease.Lancet2002;359: 62-69.
[38] Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with disrupted interleukin-2 gene.Cell1993;75(2): 253-261.
[39] Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Büschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease.Clin Exper Immunol1995;102: 448-455.
[40] Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Büschenfelde KH. T cell specificity and cross reactivity towards enterobacteria,Bacteroides,Bifidobacterium, and antigen from resident intestinal flora in humans.Gut1999;44(6): 812-818.
[41] Matsumoto S, Hara T, Hori T, Mitsuyama K, Nagaoka M, Tomiyasu N,et al. ProbioticLactobacillusinduced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of proinflammatory cytokines in lamina propria mononuclear cells.Clin Exp Immunol2005;140(3): 417-426.
[42] Matsumoto S, Okabe Y, Setoyama H, Takayama K, Ohtsuka J, Funahashi H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain.Gut1998;43(1):71-78.
[43] Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease.Annual Rev Immunol2010;28: 573-621.
[44] Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N,et al. High prevalence of adherent-invasiveEscherichia coliassociated with ileal mucosa in Crohn’s disease.Gastroenterology2004;127(2): 412-421
[45] Sartor RB. Mechanisms of disease: Athogenesis of Crohn’s disease and ulcerative colitis.Nat Clin Pract Gastroenterol Hepatol2006;3: 390-407.
[46] Clavel T, Haller D. Molecular interactions between bacteria, the epithelium,and the mucosal immune system in the intestinal tract: Implications for chronic inflammation.Curr Issues Intestinal Microbiol2007;8: 25-43.
[47] Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I. Guidance for substantiating the evidence for beneficial effects of probiotics: Probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome.J Nutr2010;140(3):690S-697S.
[48] Champagne CP, Ross RP, Saarela M, Hansen KF, Charalampopoulos D.Recommendations for viability assessment of probiotics as concentrated cultures and in food matrices.Inter J Food Microbiol2011;149(3): 185-193.
[49] Ocon B, Anzola A, Ortega-González M, Zarzuelo A, Suarez MD,Sanchez de Medina F, et al. Active hexose-correlated compound andBifidobacterium longumBB536 exert symbiotic effects in experimental colitis.Eur J Nutr2013;52(2): 457-466.
[50] Natividad JM, Petit V, Huang X, de Palma G, Jury J, Sanz Y, et al.Commensal and probiotic bacteria influence intestinal barrier function and susceptibility to colitis in Nod1-/-; Nod2-/- mice.Inflamm Bowel Dis2012;18(8): 1434-1446.
[51] Liu YW, Su YW, Ong WK, Cheng TH, Tsai YC. Oral administration ofLactobacillus plantarumK68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities.Int Immunopharmacol2011;11(12): 2159-2166.
[52] GirishKumar B, Henry DE, Srinivasan K, Prapulla SG. Beneficial effect of a probioticLactobacillus fermentumCFR 2195 in trinitrobenzenesulfonate induced colitis in rat.Ann Food Sci Technol2012;13(2): 231-239.
[53] Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, et al. Impact on the composition of the fecal flora by a new probiotic preparation: Preliminary data on maintenance treatment of patients with ulcerative colitis.Aliment Pharmacol Ther1999;13(8): 1103-1108.
[54] Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A.Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis.Am J Gastroenterol2009;104(2): 437-443.
[55] Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-tomoderately active ulcerative colitis.Clin Gastroenterol Hepatol2009;7(11):1202-1209.
[56] Ng SC, Plamondon S, Kamm MA, Hart AL, Al-Hassi HO, Guenther T,et al. Immunosuppressive effects via human intestinal dendritic cells of probiotic bacteria and steroids in the treatment of acute ulcerative colitis.Inflamm Bowel Dis2010;16(8): 1286-1298.
[57] Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study.Am J Gastroenterol2010;105(10): 2218-2227.
[58] Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease.Clin Gastroenterol Hepatol2015;13(5): 928-935.
[59] Cui HH, Chen CL, Wang JD, Yang YJ, Cun Y, Wu JB, et al. Effects of probiotic on intestinal mucosa of patients with ulcerative colitis.World J Gastroenterol2004;10(10): 1521-1525.
[60] Kato K, Mizuno S, Umesaki Y, Ishii Y, Sugitani M, Imaoka A, et al.Randomized placebo-controlled trial assessing the effect of bifidobacteriafermented milk on active ulcerative colitis.Aliment Pharmacol Ther2004;20(10): 1133-1141.
[61] Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, et al.Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy.Scand J Gastroenterol2007;42: 1306-1311.
[62] Yoshimatsu Y, Yamada A, Furukawa R, Sono K, Osamura A, Nakamura K, et al. Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis.World J Gastroenterol2015;21(19):5985-5994.
[63] Fujimori S, Gudis K, Mitsui K, Seo T, Yonezawa M, Tanaka S, et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis.Nutrition2009;25(5): 520-525.
[64] Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis.World J Gastroenterol2010;16(33): 4145-4151.
[65] Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ. A randomized double-blind placebo-controlled trial withLactobacillus acidophilusLa-5 andBifidobacterium animalissubsp. lactis BB-12 for maintenance of remission in ulcerative colitis.J Crohns Colitis2011;5(2):115-121.
[66] D’Inca R, Barollo M, Scarpa M, Grillo AR, Brun P, Vettorato MG, et al.Rectal administration ofLactobacillus caseiDG modifies flora composition and Toll-like receptor expression in colonic mucosa of patients with mild ulcerative colitis.Dig Dis Sci2011;56(4): 1178-1187.
[67] Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, et al.Randomized clinical trial: the effectiveness ofLactobacillus reuteriATCC 55730 rectal enema in children with active distal ulcerative colitis.Aliment Pharmacol Ther2012;35(3): 327-334.
[68] Ishikawa H, Matsumoto S, Ohashi Y, Imaoka A, Setoyama H, Umesaki Y, et al. Beneficial effects of probiotic bifidobacterium and galactooligosaccharide in patients with ulcerative colitis: A randomized controlled study.Digestion2011;84(2): 128-133.
[69] Krag A, Israelsen H, von Ryberg B, Andersen KK, Bendtsen F. Safety and efficacy of Profermin?to induce remission in ulcerative colitis.World J Gastroenterol2012;18(15): 1773-1780.
[70] Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al.Bifidobacterium infantis35624 modulates host inflammatory processes beyond the gut.Gut Microbes2013;4(4): 325-339.
[71] Persborn M, Gerritsen J, Wallon C, Carlsson A, Akkermans LM,S?derholm JD. The effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis.Aliment Pharmacol Ther2013;38(7): 772-783.
[72] Petersen AM, Mirsepasi H, Halkj?r SI, Mortensen EM, Nordgaard-Lassen I, Krogfelt KA. Ciprofloxacin and probioticEscherichia coliNissle add-on treatment in active ulcerative colitis: A double-blind randomized placebo controlled clinical trial.J Crohns Colitis2014;8(11): 1498-1505.
[73] Yasueda A, Mizushima T, Nezu R, Sumi R, Tanaka M, Nishimura J, et al. The effect ofClostridium butyricumMIYAIRI on the prevention of pouchitis and alteration of the microbiota profile in patients with ulcerative colitis.Surg Today2016;46(8): 939-949.
[74] Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P,Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study.Biomed Pap Med Fac Univ PalackyOlomouc Czech Repub2016;160(3): 372-377.
[75] Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, et al.Efficacy of probiotic treatment withBifidobacterium longum536 for induction of remission in active ulcerative colitis: A randomized, doubleblinded, placebo-controlled multicenter trial.Dig Endosc2016;28(1): 67-74.
[76] Baroja LM, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients.Clin Exp Immunol2007;149(3): 470-479.
[77] Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H,Vaezjalali M. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: A double-blind, placebo-controlled clinical trial.Korean J Gastroenterol2015;65: 215-221.
[78] Garcia Vilela E, De Lourdes De Abreu Ferrari M, Oswaldo Da Gama Torres H, Guerra Pinto A, Carolina Carneiro Aguirre A, Paiva Martins F,et al. Influence ofSaccharomyces boulardiion the intestinal permeability of patients with Crohn’s disease in remission.Scand J Gastroenterol2008;43(7): 842-848.
[79] Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: A randomized placebo-controlled crossover pilot study.Eur J Gastroenterol Hepatol2008;20(7): 688-692.
[80] Bourreille A, Cadiot G, Le Dreau G, Laharie D, Beaugerie L, Dupas JL, et al.Saccharomyces boulardiidoes not prevent relapse of Crohn’s disease.Clin Gastroenterol Hepatol2013;11(8): 982-987.
[81] Steed H, Macfarlane GT, Blackett KL, Bahrami B, Reynolds N, Walsh SV, et al. Clinical trial: The microbiological and immunological effects of synbiotic consumption-a randomized double-blind placebo-controlled study in active Crohn’s disease.Aliment Pharmacol Ther2010;32(7):872-883.
[82] Ahmed J, Reddy BS, Molbak L, Leser TD, MacFie J. Impact of probiotics on colonic microflora in patients with colitis: A prospective double blind randomized crossover study.Int J Surg2013;11(10): 1131-1136.
[83] Doron S, Snydman DR. Risk and safety of probiotics.Clin Infect Dis2015;60(S2): S129-134.
[84] Munoz P, Bouza E, Cuenca-Estrella M, Eiros JM, Perez MJ, Sanchez-Somolinos M, et al.Saccharomyces cerevisiaefungemia: An emerging infectious disease.Clin Infect Dis2005;40(11): 1625-1634.
[85] Niault M, Thomas F, Prost J, Ansari FH, Kalfon P. Fungemia due toSaccharomycesspecies in a patient treated with enteralSaccharomyces boulardii.Clin Infect Dis1999;28(4): 930.
[86] Perapoch J, Planes AM, Querol A, Lopez V, Martinez-Bendayán I,Tormo R, et al. Fungemia withSaccharomyces cerevisiaein two newborns,only one of whom had been treated with ultra-levura.Eur J Clin Microbiol Infect Dis2000;19(6): 468-470.
[87] Piechno S, Seguin P, Gangneux JP.Saccharomyces boulardiifungal sepsis:Beware of the yeast.Can J Anaesth2007;54: 245-246.
[88] Trautmann M, Synowzik I, Nadji-Ohl M, Von Voigt TB, Reiter W.Fungemia due toSaccharomyces cerevisiaevar.boulardii.Chemother J2008;17: 57-61.
[89] De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR.Lactobacillus rhamnosusGG bacteremia associated with probiotic use in a child with short gut syndrome.Pediatr Infect Dis J2005;24: 278-280.
[90] Ledoux D, Labombardi VJ, Karter D.Lactobacillus acidophilusbacteraemia after use of a probiotic in a patient with AIDS and Hodgkin’s disease.Int J STD AIDS2006;17(4): 280-282.
[91] Tommasi C, Equitani F, Masala M, Ballardini M, Favaro M, Meledandri M, et al. Diagnostic difficulties ofLactobacillus caseibacteraemia in immunocompetent patients: A case report.J Med Case Reports2008;2:315.
[92] Vahabnezhad E, Mochon AB, Wozniak LJ, Ziring DA.Lactobacillusbacteremia associated with probiotic use in a pediatric patient with ulcerative colitis.J Clin Gastroenterol2013;47: 437-439.
[93] Oggioni MR, Pozzi G, Valensin PE, Galieni P, Bigazzi C. Recurrent septicemia in an immunocompromised patient due to probiotic strains ofBacillus subtilis.J Clin Microbiol1998; 36(1): 325-326.
[94] Ohishi A, Takahashi S, Ito Y, Ohishi Y, Tsukamoto K, Nanba Y, et al.Bifidobacteriumsepticemia associated with postoperative probiotic therapy in a neonate with omphalocele.J Pediatr2010;156(4): 679-681.
[95] Zein EF, Karaa S, Chemaly A, Saidi I, Daou-Chahine W, Rohban R.Lactobacillus rhamnosussepticemia in a diabetic patient associated with probiotic use: A case report.Ann Biol Clin (Paris)2008;66(2): 195-198.
[96] Mackay AD, Taylor MB, Kibbler CC, Hamilton-Miller JM.Lactobacillus endocarditiscaused by a probiotic organism.Clin Microbiol Infect1999;5:290-292.
[97] Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomized, double-blind, placebo-controlled trial.Lancet2008;371(9613): 651-659.
[98] Munakata S, Arakawa C, Kohira R, Fujita Y, Fuchigami T, Mugishima H.A case of D-lactic acid encephalopathy associated with use of probiotics.Brain Dev2010;32(8): 691-694.
[99] Brunser. Probiotics: Innocuousness, prevention and risks.Rev Chil Pediatr2017;88(4): 534-540.
 Asian Pacific Journal of Tropical Biomedicine2018年3期
Asian Pacific Journal of Tropical Biomedicine2018年3期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Synsepalum dulcificum extracts exhibit cytotoxic activity on human colorectal cancer cells and upregulate c-fos and c-jun early apoptotic gene expression
- Diet containing seeds of Buchholzia coriacea accelerates healing of acetic acid induced colitis in rats
- Antidiabetic potential of methanol extracts from leaves of Piper umbellatum L. and Persea americana Mill.
- NO-cGMP-K channel-dependent anti-nociceptive activities of methanol stem bark extract of Piptadeniastrum africanum (Mimosaceae) on rats
- Synthesis of silver and gold nanoparticles from leaf of Litchi chinensis and its biological activities
- Antioxidant and antiglycation properties of two mango (Mangifera indica L.) cultivars from Senegal
