Immunohistochemical identification of dynorphin A and Kappa opioid receptor-1 in the digestive system of scallop Chlamys farreri*
SUN Hushan (孫虎山) WANG Yiyan (王宜艷) LIU Xiaoli (劉小莉) LIU Dongwu (劉東武)
1 School of Life Science, Ludong University, Yantai 264025, China
2 Analysis and Testing Center, Shandong University of Technology, Zibo 255049, China
Abstract Little is known about the roles of dynorphin and Kappa opioid receptor (KOR) in mollusks. In this study, we aim to determine the distribution of dynorphin A and KOR-1 in the digestive system of the scallop Chlamys farreri. Using immunohistochemical staining, we confirmed the expression of dynorphin A and KOR-1 in the digestive system of C. farreri. Dynorphin A immunopositive cells were identified in intestine and hepatopancreas. In intestine, small and spherical dynorphin A immunopositive cells(4–9 μm in diameter) were scattered among the long columnar epithelial cells (ECs). In hepatopancreas,cells containing masses (5–14 μm in diameter) of dynorphin A immunopositive products were observed in epithelium of acinis. These immunopositive cells may be synthetic and/or secretory cells of dynorphin A. Dynorphin A immunoreactive products were commonly observed in epithelium and connective tissue(CT) of labial palps, mouth labia and stomach, which presented in forms of grains, fibers or flakes. KOR-1 immunoreactive material was observed in ECs and CTs of labial palps, mouth labia and stomach, intestine and hepatopancreas. The distribution of both dynorphin A and KOR-1 in the digestive organs suggests an involvement of dynorphin via KOR-1 in the functional regulation of the digestive system of C. farreri.
Keyword: Chlamys farreri; dynorphin; digestive system; kappa opioid receptors; immunohistochemistry
1 INTRODUCTION
Dynorphin, a member of endogenous opioid peptides (Ji et al., 2017), is an important mediator for behavioral and emotional responses to stress. In cases of stress, dynorphin peptides are related in the brain,which then activates the kappa opioid receptor (KOR)to produce analgesia and dysphoria (Cahill et al.,2014). Up to now, extensive studies have been focusing on activities of dynorphin and KOR in mammals (Morgan et al., 2017), and dynorphin/KOR system has been considered as a therapeutic candidate for treating pain, drug abuse (Le Merrer et al., 2009),HIV infection (Lokensgard et al., 2002) and cancer(Bohn et al., 2000).
Rare studies have been focusing on the roles of dynorphin and KOR in mollusks. Initially, a peptidase activity in extracts of the atrial gland ofAplysia Californicacleaved dynorphin A at a single arginine site (Wallace et al., 1984). Kavaliers (Kavaliers et al.,1986) reported that kappa opioid agonist (U-50,488H) induced a more potent increase in three hour food consumption by slugsLimuxmaximus.Dynorphin A, which shared some sequential similarities with nociceptin, had significant antinociceptive effects in the land snail,Cepaea nemoralis, and the effects of dynorphin were blocked by KOR antagonists (Kavaliers et al., 1986). In 1998,a prodynorphin molecule was characterized by Stefano et al. (1998) from hemocytes and hemolymph of the bivalve molluskMytilusedulis. Ten years later,Miller-Pérez et al. (2008) showed that low doses of dynorphin A produced potent antinociceptive effects in land snailHelixaspersa. Our previous study indicated that KOR, delta (DOR) and mu opioid-like receptors (MOR) were present in the gill, gonad and hemocytes of the scallopChlamysfarreri(Liu and Sun, 2010). However, our understanding on the dynorphin and KOR in mollusks is still limited.
Unlike mammals, endogenous opioids are extensively expressed in certain tissues and organs of mollusks. This highlights the importance of characterizing the expression pattern of the endogenous opioids in mollusks. Using immunohistochemical methods, we had determined the distribution of endogenous enkephalin, DOR and MOR in the digestive system of mollusks (Liu and Sun, 2008; Sha et al, 2012a, 2012b; Sha et al., 2013).Also, we confirmed the involvement of methionineenkephalin (M-ENK) in the regulation of the digestive enzymes of mollusks (Wang and Sun, 2010). To our best knowledge, there are no similar reports about the expression of dynorphin and KOR in the digestive system of mollusks. In this study, we determined the distribution of dynorphin A and KOR-1 in the digestive system of the scallopC.farreri. Our study contributes to elucidating the regulatory effects of endogenous opioid and its receptors on the digestive system of mollusks, which may provide a clue for investigating the origin of mammalian neuroendocrine.
2 MATERIAL AND METHOD
2.1 Experimental animal
Healthy scallopC.farreri(45–50 mm) were purchased from a commercial farm in Yantai, China.Then theC.farreriwere transferred to a glass aquarium tank (100 cm×30 cm×45 cm) with recirculating seawater (water temperature: 20±1°C;pH: 7.5–8.0; salinity: 31–32; and oxygen: 6–7 mg/L).Two thirds of the water was replaced by fresh seawater every morning to maintain water quality. Before experiment, scallops were subject to adaptation feeding for at least 1 week. Stocking densities were generally maintained at 12 mollusks per container and artificial feed was given twice a day. No mortality was observed during the preliminary acclimation period.
2.2 Reagent

Fig.1 Digestive system schematic graph of the scallop C. farreri
The rabbit polyclonal antiserum against dynorphin A was purchased from Abcam (Cambridge, UK).Immunogen of anti-dynorphin A antibody was a synthetic peptide (YGGFLRRIRPKLKWDNQ),which blocked with dynorphin 1–17 and 1–8. Species reactivity for the antibody included mouse, rat,human, pig and rhesus monkey. The rabbit polyclonal antiserum against KOR-1, goat anti-rabbit IgG and streptavidin biotin peroxidase complex protein (AB composite liquid) were obtained from Santa Cruz(CA, USA). KOR-1(H-70) is a rabbit polyclonal dynorphin A raised against a recombinant protein corresponding to amino acids 1–70 mapping at the amino terminus of KOR-1 of human origin. The 3,3’-diaminobenzidine (DAB) was purchased from Fluka Chemie AG (Buchs, Switzerland).
2.3 Immunohistochemistry
Tissue samples of digestive tract (i.e. labial palps,mouth labia, stomach, intestine and rectum) and digestive gland (i.e. hepatopancreas) ofC.farreri(Fig.1) were cut off from each scallop, followed by fixation for 12 h in 4% paraformaldehyde in 0.1 mol/L sodium phosphate buffer (PBS, pH 7.4, +2% sodium chlorine) at 4°C. Then the tissue samples were washed three times in PBS.
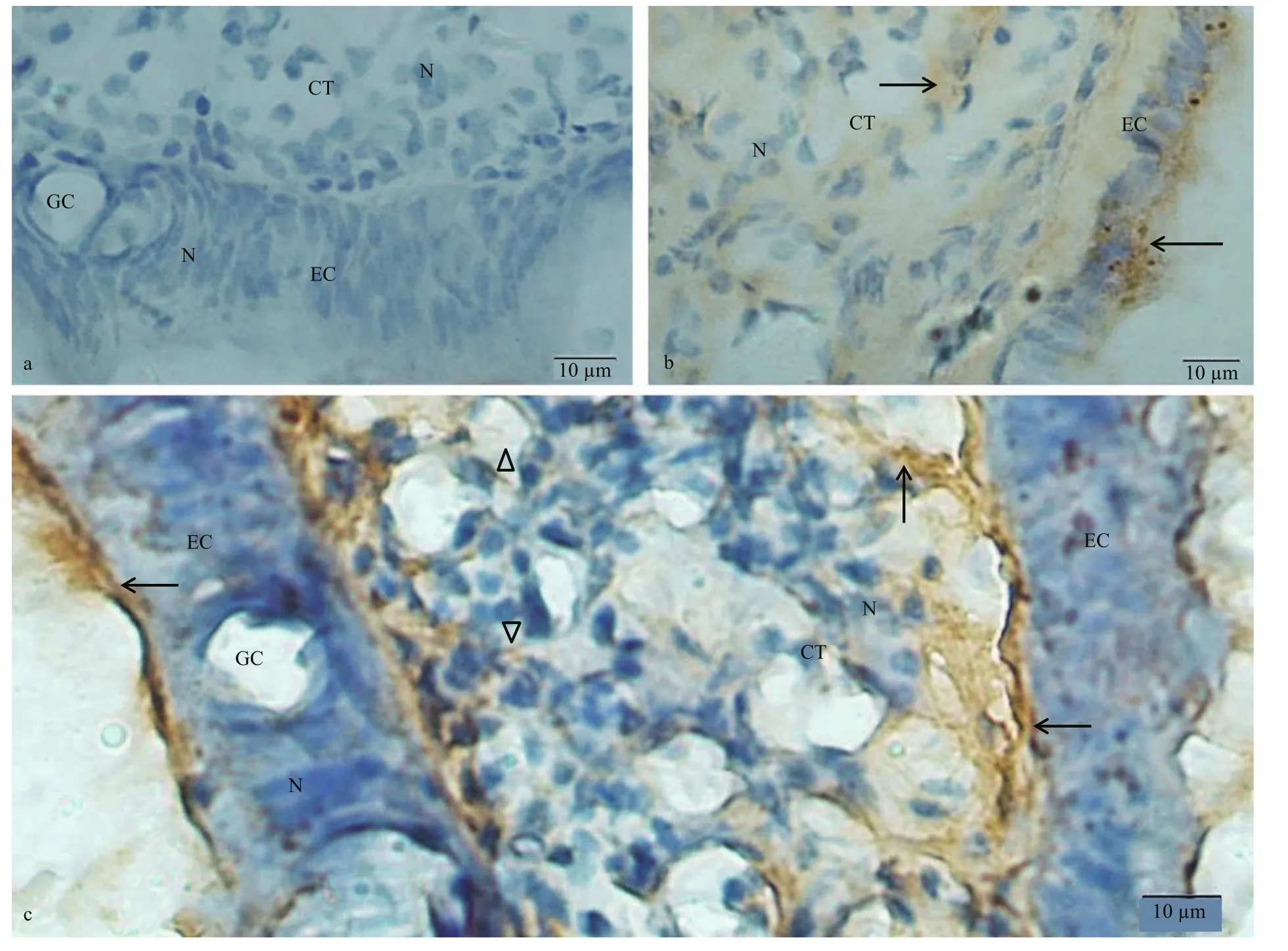
Fig.2 Immunostaining findings for the C. farreri labial palps using anti- dynorphin A or KOR-1 antibody
Immunohistochemistry was carried out according to our previous description (Liu and Sun, 2008).Cryostat sections (20 μm) were prepared based on the tissue samples of the digestive system and then mounted on chromium-gelatin-coated glass slides and rehydrated in sodium phosphate buffer (+0.3%Triton X-100, PBST). The sections were pretreated in 0.3% H2O2in methanol for 30 min, in order to eliminate the activity of endogenous peroxidases.Subsequently, the sections were incubated with rabbit anti- dynorphin A antibody (1:500, Abcam, UK) or KOR-1 antibody (1:500, Santa Cruz, US) for 4 h at room temperature. Afterwards, the tissues were washed with PBST at least in triplicate, and then incubated with goat anti-rabbit IgG (Santa Cruz, US)for 50 min at room temperature. After washing three times, the samples were incubated with AB composite liquid (Santa Cruz, US) for 30 min at room temperature. Upon rinsing with PBST, the slides were treated with 0.05% DAB (Fluka Chemie AG,Switzerland) for 10 min. Samples incubated with PBST, or with PBST instead of primary antibody, or with PBST instead of second antibody, or with PBST instead of AB composite liquid served as negative controls. Finally, the slides were observed under a light microscope (Olympus Corporation, Japan).
3 RESULT
3.1 Localization of dynorphin A and KOR-1 in the labial palps and mouth labia
The labial palp comprised an inner epithelium layer, connective tissue (CT) layer and outer epithelium layer. The epithelium layer was consisted of columnar epithelium and goblet cells (Fig.2a). In the negative control group, no immunostaining was observed in the epithelial cells (ECs) and CT of labial palps (Fig.2a). Considerable amount of KOR-1 immunoreactive material was observed in the EC and CT of the labial palps (Fig.2b). The dynorphin A immunoreaction product was detected in the outer and inner EC and the CT of labial palps (Fig.2c).Besides, immunohistochemical staining of dynorphin A in CT was more intense in the areas close to the EC than that close to the center of CT layer. The dynorphin A immunoreaction product observed in the CT presented in the form of fibers or flakes.
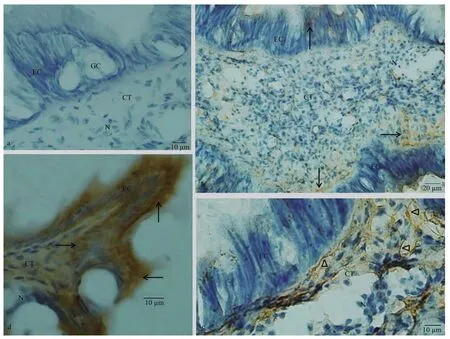
Fig.3 Immunostaining findings for mouth labia
Similar with the labial palp, mouth labia was consisted of an inner epithelium layer, CT layer and outer epithelium layer (Fig.3a). No immunoreaction was noticed in the control sections from the mouth labia incubated without a primary antibody, second antibody or ABC staining system (Fig.3a). Few dynorphin A immunoreactive products were observed in the EC, and more dynorphin A immunoreactive materials were located in the CT. The dynorphin A immunoreactive product stained in brown was in a form of fiber-like extensions in the CT (Fig.3b, c). The KOR-1 immunoreactive material was observed both in EC and CT of the mouth labia. Strong immunostaining was noted in the single layer of ECs (Fig.3d).
3.2 Localization of dynorphin A and KOR-1 in the stomach and intestine
In stomach ofC.farreri, control sections incubated with no primary antibody, second antibody or ABC staining system exhibited no immunoreaction(Fig.4a). KOR-1-like immunoreactive materials were observed both in the epithelium and the CT, and more positive signals were observed at the free end of the epithelium (Fig.4b). The dynorphin A immunoreactive product was observed in the stomach epithelium in a grainy or fibrous form (Fig.4c).
In intestine, no immunoreaction was noticed in control (Fig.5a). Most of the columnar epithelial cells of the intestine presented KOR-1 immunoreactivity.More KOR-1 immunoreactive materials were detected in epithelium of the longitudinal folds. Few KOR-1 immunoreactive materials or fibers were observed in the CT (Fig.5b). The immunoreactivity of dynorphin A in the long columnar epithelial cells and CT was weak, and the dynorphin A immunoreaction products were usually observed in the form of fibers.Large amounts of small spherical dynorphin A immunoreactive cells (4–9 μm in diameter) were scattered in the epithelial layer of the intestine, which presented as either individual cells or small cell clusters with small spherical, or irregular shaped nuclei. The number of these dynorphin A immunoreactive cells in areas of the epithelial tissue layer close to the CT was larger than that in areas close to the lining lumen of the intestine (Fig.5c, d).
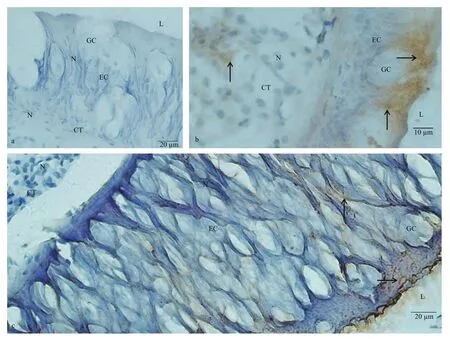
Fig.4 Immunostaining findings for stomach
3.3 Localization of dynorphin A and KOR-1 in the hepatopancreas
The hepatopancreas ofC.farreriis a type of compound tubuloacinar gland. Adjacent acinus were separated from each other by connective tissue.Acinus was comprised of hepatocytes surrounding a lumen. Some hepatic tubules were seen in the sections of hepatopancreas (Fig.6a).
KOR-1 immunoreactive material was observed in nearly all of the hepatocytes. Most of the hepatocytes showed weak positive reaction, and some of hepatocytes showed very strong KOR-1 immunoreactivity. Most of the CT between the acinus showed strong KOR-1-like immunoreactivity. KOR-like immunoreactivity was also observed in the ECs and CT of some hepatic tubules (Fig.6b).
The immunoreactivity of dynorphin A was negative in most of the hepatocytes in the acinus. A few cells in the acinus were strongly immunostained for the dynorphin A. Masses of dynorphin A immunoreactive product were observed in these immunoreactive cells.The shape of the masses was irregular, triangular or polygonal. The maximal diameters of these dynorphin A immunoreactive masses were 5.56–14.44 μm(9.65±2.51 in mean maximum diameter). The dynorphin A immunoreactive product was occasionally observed in the connective tissue, which was in the walls of hepatic tubules and between the acinus. These immunoreactive products were in grainy or fibrous forms (Fig.6c, d).
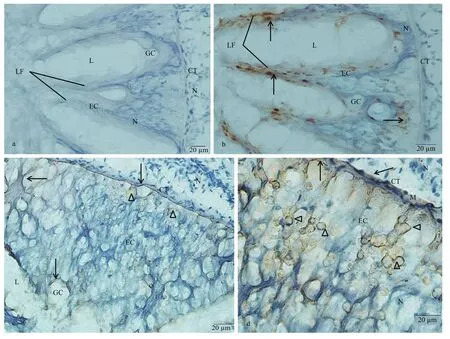
Fig.5 Immunostaining findings for C. farreri intestine
4 DISCUSSION
Increasing evidence shows that dynorphin A can stimulate feeding in vertebrates, which appears to be primarily depending on KOR, as feeding is reduced after pretreating with kappa antagonist,binaltorphamine, or antisense oligodeoxynucleotides probes directly against exons 1 or 2, other than exon 3 of theKOR-1gene (Le Merrer et al., 2009). In rats,KOR-1 agonist U50, 488H and the KOR-3 agonist naloxone benzoylhydrazone were reported to enhance the intake of food by animals (Ruegg et al., 1997). In mice, KOR knockout was associated with reduced body weight and decrease of fat mass in response to a high-energy diet (Czyzyk et al., 2010). In scallops,the feeding is called filter feeding through which the gills utilize their ciliary tracts to remove suspended particles from water by pumping via mantle cavity. In this process, labial palps is mainly involved in sorting and rejection of the particles from the gills (Espinosa et al., 2009). In this study, immunoreactivity of dynorphin A and KOR-1 was observed in epithelium and CT in the labial palps and mouth labia ofC.farreri, which indicated an involvement of dynorphin A via KOR in the functional regulation of the food intake. The dynorphin A immunoreactive product,observed in the epithelium and CT of the labial palps and mouth labia, was usually in the form of fibers or flakes. As the immunoreactive fibers in the CT of the digestive tract may be nerve fibers (Sun et al., 2014),we speculate that the immunoreactive product in labial palps and mouth labia ofC.farrerimay be produced in other parts of the body and then is transported by nerve fibers or hemolymph.
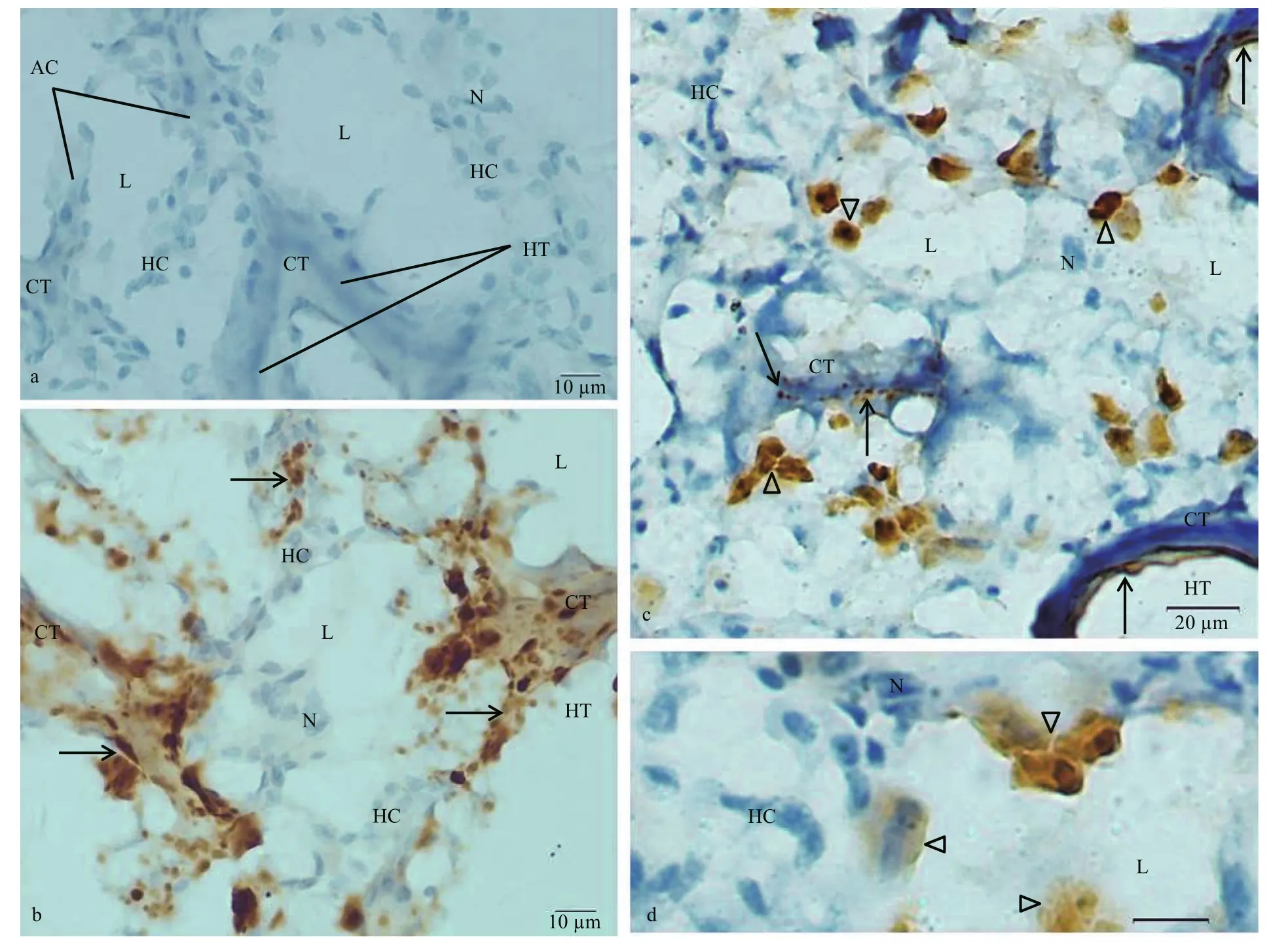
Fig.6 Sections of C. farreri hepatopancreas immunostained with anti-dynorphin A or anti-KOR-1 antibody and counterstained with hematoxylin
Opioid receptors in the gastrointestinal tract contributed to mediating the effects of endogenous opioid peptides and exogenously administered opioid analgesics on several physiological functions associated with motility, secretion and visceral pain(Sobczak et al., 2014). The effects of opioid peptides on these organ-level gastrointestinal functions reflect actions on electrical and synaptic behavior of neurons in the enteric nervous system (Wood and Galligan,2004). Dynorphin A and a synthetic KOR-3 agonist stimulated gastric acid secretion via KOR in the central nervous system of rats in vivo (Capasso et al.,2008). KOR and MOR but not DOR agonists reduced the tension of ferret esophageal vagal afferent mechanosensitivity (Sengupta, 2009). The neural circuits controlling peristalsis in the guinea-pig small intestine were inhibited by endogenous and exogenous opioids acting via KOR and MOR other than DOR(Winters et al., 2017). KOR agonist inhibiting plasma extravasations showed a 26.3-fold increase during chronic intestinal inflammation compared with the acute counterpart (Rubin et al., 2012). KOR agonists showed high potential for clinical use in gastrointestinal disorders as they were located on visceral primary afferents and were able to mediate the antinociceptive behavior (Xiao et al., 2016).Meanwhile, kappa receptor inhibited the cholinergic transmission by opiates in ileal myenteric plexus(Sobczak et al., 2014). Our data showed the immunoreactivity of dynorphin A and KOR-1 in the epithelium layer of the stomach and intestine ofC.farreri. Therefore, we speculate an involvement of dynorphin via KOR in functional regulation of the stomach and intestine ofC.farreri. Small and spherical cells with positive dynorphin A staining in the epithelial layer of intestine might be the major source for secretion of dynorphin A.
Rare studies have been carried out to investigate the interaction between the dynorphin and hepatic or pancreatic systems. In rats with thioacetamideinduced fulminant hepatic failure, pituitary and brainstem dynorphin A levels increased, whereas,plasma dynorphin A levels decreased (Yurdaydin et al., 1995). In rat pancreas, all glucagon cells exhibited immunoreactivities for dynorphin A, and the pancreas might be another source for dynorphin with a biosynthetic pathway different from that in the pituitary or in other locations (Cetin, 1985). Besides,as a very potent stimulus for insulin secretion,dynorphin may contribute to the calcium ion movement and elevation of islet c-AMP (Green et al.,1983). Additionally, the sensitivity to KOR agonists was elevated in the insulin-resistant glucoseinsensitive mouse model of obesity and non-insulindependent diabetes, which probably mediated hyperglycemic and hyperinsulinemic responses in a peripheral and independent manner (Khawaja et al.,1990). In this study, we found that isolated cells with dynorphin A immunoreactivity were scattered in the acinis of hepatopancreas of the scallop, which was consistent with the previous study (Varaksin et al.,1990). On this basis, we speculate that these dynorphin A positive cells may be a source for the secretion of dynorphin and other hormones.
5 CONCLUSION
Dynorphin A and KOR-1 were present in the digestive system ofC.farreri. This suggests an involvement of dynorphin via KOR in the functional regulation of the digestive system ofC.farreri. Many dynorphin A immunopositive cells were observed in the intestine and hepatopancreas. These cells may be a source for the generation of dynorphin A inC.farreri. The dynorphin A immunoreactive product observed in the labial palps, mouth labia and stomach was usually in the form of grains, fibers or flakes.These product in these areas may be produced in intestine, hepatopancreas or other parts of the body and then were transported by hemolymph or nerve fibers.
6 DATA AVAILABILITY STATEMENT
Authors can confirm that all relevant data are included in the article. The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
 Journal of Oceanology and Limnology2018年6期
Journal of Oceanology and Limnology2018年6期
- Journal of Oceanology and Limnology的其它文章
- Neuroanatomy and morphological diversity of brain cells from adult crayfish Cherax quadricarinatus*
- On the influence of season and salinity on the phenology of invertebrates in Australian saline lakes, with special reference to those of the Paroo in the semiarid inland
- Man-made plutonium radioisotopes in the salt lakes of the Crimean peninsula
- Cladophora mats in a Crimean hypersaline lake: structure,dynamics, and inhabiting animals
- Antimony speciation at the sediment-water interface of the Poyang Lake: response to seasonal variation*
- Seasonal variations of phosphorus species in the Tuohe River,China. Part I. Sediments*
