The correlation and overlaps between PD-L1 expression and classical genomic aberrations in Chinese lung adenocarcinoma patients: a single center case series
Jianghua Wu, Wei Sun, Haiyue Wang, Xiaozheng Huang, Xinyu Wang, Weiyang Jiang, Ling Jia, Ping Wang, Qin Feng, Dongmei Lin
1Department of Pathology, Peking University Cancer Hospital & Institute, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Beijing 100142, China; 2Department of Pathology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin 300060, China.
ABSTRACT Objective:To investigate the correlation and overlaps between PD-L1 expression and classical genomic aberrations in Chinese lung adenocarcinoma (LADC) patients.Methods:We reviewed 428 consecutive, surgically resected cases of LADC from October 2015 to December 2016 from our center.PD-L1 expression was evaluated based on tumor proportion score (TPS). Correlation and co-occurrence of PD-L1 expression level with those of classical driver genes, such as EGFR, ALK, ROS-1, and KRAS and with clinical variables and disease-free survival(DFS) were analyzed.Results:Seventy of the 428 cases (16.4%) showed TPS ≥ 1%, and 21 cases (4.9%) showed TPS ≥ 50%. PD-L1 positive expression was significantly associated with male gender, smoking, advanced TNM stage, and solid histologic subtype. Both TPS ≥ 1% and ≥50% were correlated with the absence of an EGFR mutation (P < 0.001) and the presence of ALK rearrangement (P = 0.024).KRAS mutation was associated with TPS ≥ 50% (P = 0.035). PD-L1 positivity commonly overlapped with the alterations of classical driver oncogenes (58.5% with TPS ≥ 1% and 42.9% with TPS ≥ 50%). Approximately three-quarters of PD-L1 positive cases co-occurred with classical therapeutic-gene aberrations in cases with stage III/IV cancer or cancer progression. LADC could be divided into four subgroups based on the expression profile of current routine biomarkers for potential therapeutic strategies.Conclusions:PD-L1 expression is not only closely correlated with classic gene alterations but also commonly overlaps with the aberrations of classical driver oncogenes in Chinese LADC patients. These findings provide a useful overview of clinical strategies that rely on the profile of routinely used molecular biomarkers.
KEYWORDS Lung adenocarcinoma; PD-L1 expression; classical genomic aberrations; molecular biomarkers
Introduction
Lung adenocarcinoma (LADC) is the most frequent histologic type of lung cancer and one of the most common causes of cancer death in China1,2. The development of molecular-targeted therapy has greatly increased the survival of LADC patients over the past few years. Despite significant improvements in therapeutic strategies, the overall prognosis of LADC patients remains poor, especially in patients who do not benefit from targeted therapy. Programmed death 1 (PD-1) /programmed death-ligand 1 (PD-L1) targeted immunotherapy has been proven to be efficacious in the treatment of nonsmall cell lung cancer (NSCLC)3-5. Immunohistochemical(IHC) for PD-L1 was recommended to identify patients most likely to respond to immunotherapy. The FDA has approved IHC assay for PD-L1 utilizes a cut-off of 50% tumor proportion score (TPS) for first-line and 1% TPS for secondline therapy with pembrolizumab6. This breakthrough exerts a profound influence on the routine investigation of therapeutic biomarkers.
Testing for alterations in classic driver genes, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1),and kirsten rat sarcoma viral oncogene homolog (KRAS) has been in practice as routine pre-treatment molecular evaluations in NSCLC patients. Expression of PD-L1, a wellknown biomarker for PD-1/PD-L1 targeted immunotherapy,has also been recommended in the recent guidelines of nonsmall cell lung cancer (NSCLC)6. This update of the routine biomarker profile of NSCLC impacts the tissue molecular detection approach currently used in clinics to identify patients who might benefit from tyrosine kinase inhibitors(TKIs) or PD-1/PD-L1 targeted immunotherapy.
Many studies on NSCLC have been conducted regarding the correlation between major driver gene alterations and PD-L1 expression. However, these studies occasionally showed conflicting results, and a consensus has not been reached7,8. It is known that the occurrence of EGFR and KRAS mutations significantly differs among diverse ethnic populations. EGFR mutation is found in 10% of Caucasian and up to 60% of East Asian lung adenocarcinoma patients9,10. In contrast, KRAS mutation is found in 20% to 30% of Caucasian lung adenocarcinoma patients but in much fewer East Asian patients11. More importantly, IHC staining for PD-L1 and investigation of driver genes are vital for triaging treatments for NSCLC patients in clinical practice. However, to date, these molecular alterations, as a biomarker profile in routine clinical care, have not been well elucidated concerning their overlaps in Chinese patients that might significantly affect first-line therapy choices8,12,13.Therefore, the correlation and overlaps between PD-L1 expression and classical genomic aberrations need to be evaluated in a cohort of Chinese LADC patients. In this study, we focused on studying the impact of PD-L1 expression as an additional biomarker for the routine clinicopathological investigation of LADC, including its relation with driver genes and clinicopathologic features.This study will explore whether IHC expression of PD-L1 and classic driver oncogene aberrations frequently cooccurred in Chinese patients. It is crucial for identifying subsets of LADC patients who might benefit from individual therapies.
Materials and methods
Patients
A total of 451 consecutive medical records and tissue samples of surgically resected LADC cases were collected from October 2015 to December 2016 at Peking University Cancer Hospital & Institute. Twenty-three patients were excluded since they were receiving neoadjuvant therapy, which might affect PD-L1 IHC staining, and the rest of 428 patients were reviewed in this study. The clinical data for this study included age, gender, disease-free survival (DFS) and the status of EGFR, ALK, ROS1, and KRAS. TNM stage was determined according to the guidelines mentioned in the 8th edition of TNM classification14. Informed consent of patients and ethical approval was obtained from the Ethics Committee Board of the Peking University Cancer Hospital and Institute, Beijing, China (Approval No. 2018KT88).
Histologic evaluation
Morphological evaluation was performed according to the criteria specified by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS).The histologic pattern was defined by the pattern of the largest percentage15.
Immunohistochemistry and interpretation of PD-L1 expression
Tissue sections of 4 μm thickness were used for the analysis of PD-L1 staining. The sections were stained with antihuman PD-L1 monoclonal antibody (clone SP142; Spring Bioscience, Ventana, Tucson, AZ, USA), and a matched immunoglobulin G-negative control. The assay was performed using an OptiView DAB IHC Detection Kit along with an OptiView Amplification Kit on the BenchMark ultra automated staining platform (Ventana Medical Systems Inc.,Tucson, AZ, USA). Two pathologists (W.S., D.L.) interpreted the staining pattern of PD-L1. Protein expression of PD-L1 was evaluated based on the tumor proportion score (TPS),which is defined as the percentage of tumor cells showing partial or complete membranous staining at any intensity.PD-L1 expression was considered to be positive when TPS ≥1% and a TPS ≥ 50% indicated high expression.
Investigation of classical genomic aberrations in routine clinical data
Genomic DNA of EGFR and KRAS was isolated from paraffin-embedded tissues using the QIAamp DNA mini-Kit(Qiagen, Valencia, CA) according to the manufacturer’s instructions. EGFR tyrosine kinase exons 18, 19, 20, 21, and KRAS exon 2 were detected by amplification refractory mutation system (ARMS) based polymerase chain reaction(PCR), using the ACCB Gene mutation Detection Kit (ACCB Biotech Ltd, Beijing, China).
ROS1 was detected by IHC staining (clone D4D6, Cell Signaling Technology Inc, Shanghai, China) and ROS1 gene rearrangement was verified by PCR using ROS1 fusion gene detection kit (Ed biopharmaceutical company, China). ALK was detected only by IHC with intense cytoplasmic granular staining, for a high consistency between IHC positive expression and gene rearrangement of ALK. Automated IHC for ALK rearrangement was performed using an anti-ALK monoclonal antibody (D5F3, Roche) using the OptiView DAB IHC Detection kit and OptiView Amplification kit(Spring Bioscience, Ventana, Tucson, AZ, USA). IHC staining of ALK and ROS1 was performed automatically using Ventana BenchMark ultra automated staining platform(Ventana Medical Systems Inc., Tucson, AZ, USA).
Statistical analysis
Correlations between PD-L1 expression and gene alterations and clinicopathologic factors were examined by the Chisquare test and Fisher’s exact test. DFS was evaluated by the Kaplan-Meier method. The log-rank test was applied to compare cumulative survival time. A P-value less than 0.05 was considered as significant. Statistical analyses were performed using the SPSS software version 22.0 (Chicago, IL,USA).
Results
Correlation between PD-L1 expression and clinicopathologic features
Among 428 patients, 173 were male (40.4%) and 255 were female (59.6%); the median age of patients was 60.5 years(range, 32.0-83.0 years). Disease-free survival (DFS) was measured from the date of surgery until relapse or metastasis.Three hundred and twenty-four cases were followed-up with 59 cases of relapse or metastasis. The follow-up time ranged from 12 to 40 months, with a median time of 28 months.Overall survival was not measured since the follow-up time was inadequate. The clinical profile of patients is presented in Table 1, and representative PD-L1 IHC staining is shown in Figure 1.
Seventy of 428 cases (16.4%) showed PD-L1 positive staining with TPS ≥ 1%. Forty-nine cases showed low PD-L1 expression with 1% ≤ TPS < 50% and 21 cases (4.9%)showed high PD-L1 expression with TPS ≥ 50%. The positive expression of PD-L1 (TPS ≥ 1%) was significantly associated with male gender (P = 0.040), smoking (P = 0.005), and advanced TNM stage (stage I-II vs stage III-IV, P = 0.020).The correlation between PD-L1 expression and age was not significant (P = 0.553). High expression of PD-L1 (TPS ≥50%) was significantly associated with male gender (P =0.040) and smoking (P = 0.003). Histologically, LADC with positive PD-L1 expression was less likely of the minimally invasive adenocarcinoma (MIA), lepidic predominant (LPA),and invasive mucinous adenocarcinoma (IMA) subtypes and more likely of the solid predominant (SPA) subtype (P<0.001).
Correlation and overlaps between PD-L1 expression and classical genomic aberrations in Chinese LADC patients
Among the 428 LADC cases assessed, 287 cases had (67.1%)EGFR mutation, 11 had (2.6%) ALK rearrangement, 3 had(0.7%) ROS1 rearrangement, and 34 had (7.9%) KRAS mutation. PD-L1 expression was significantly associated with the absence of EGFR mutation (P< 0.001) and the presence of ALK rearrangement (P = 0.008 with TPS ≥ 1%, P = 0.039 with TPS ≥ 50%). KRAS mutation was significantly associated with high expression of PD-L1 (P = 0.006). ROS1 rearrangement was not correlated with PD-L1 expression(Table 2).
There were rare co-occurrences among classical driver gene alterations. However, the co-occurrence of PD-L1 expression and classical gene alterations was common. These overlaps are shown in Figure 2 and 3. Of the 70 cases with TPS ≥ 1%, 35 cases (50%) had EGFR mutation, 5 cases(7.1%) had ALK rearrangement, 1 case (1.4%) had ROS1 rearrangement and 9 cases (12.9%) had KRAS mutation. Of the 21 cases with TPS ≥ 50%, 6 cases (28.6%) had EGFR mutation, 2 cases (9.5%) had ALK rearrangement, 1 case(4.8%) had ROS1 rearrangement and 5 cases (23.8%) had KRAS mutation. Only 20 cases (28.6%) with TPS ≥ 1% and 7 cases (33.3%) with TPS ≥ 50% did not carry these gene alterations in Chinese LADC patients. There were 41 cases out of 70 (58.5%) with TPS ≥ 1% and 9 cases out of 21(42.9%) with TPS ≥ 50%, which showed the presence of driver oncogenes, targeted therapies against which have been approved (EGFR, ALK, and ROS-1).
Overlaps between PD-L1 expression and classical genomic aberrations in advanced stage LADC cases or with disease progression
We further analyzed the overlaps between PD-L1 expression and classical genomic aberrations in advanced stage cases or with disease progression (Figure 4). There were 80 cases outof 428 with TNM stage III or IV. Among these, 20 cases(25.0%) showed PD-L1 positivity with TPS ≥ 1%, and 7 cases(14.0%) had a TPS ≥ 50%. Among the 20 cases with TPS ≥1%, 12 cases (60%) had EGFR mutation, 2 cases (10%) had ALK rearrangement, 1 case (5%) had ROS1 rearrangement,and 2 cases (10%) had KRAS mutation. Only 3 LADC cases(15%) did not harbor major oncogenes.
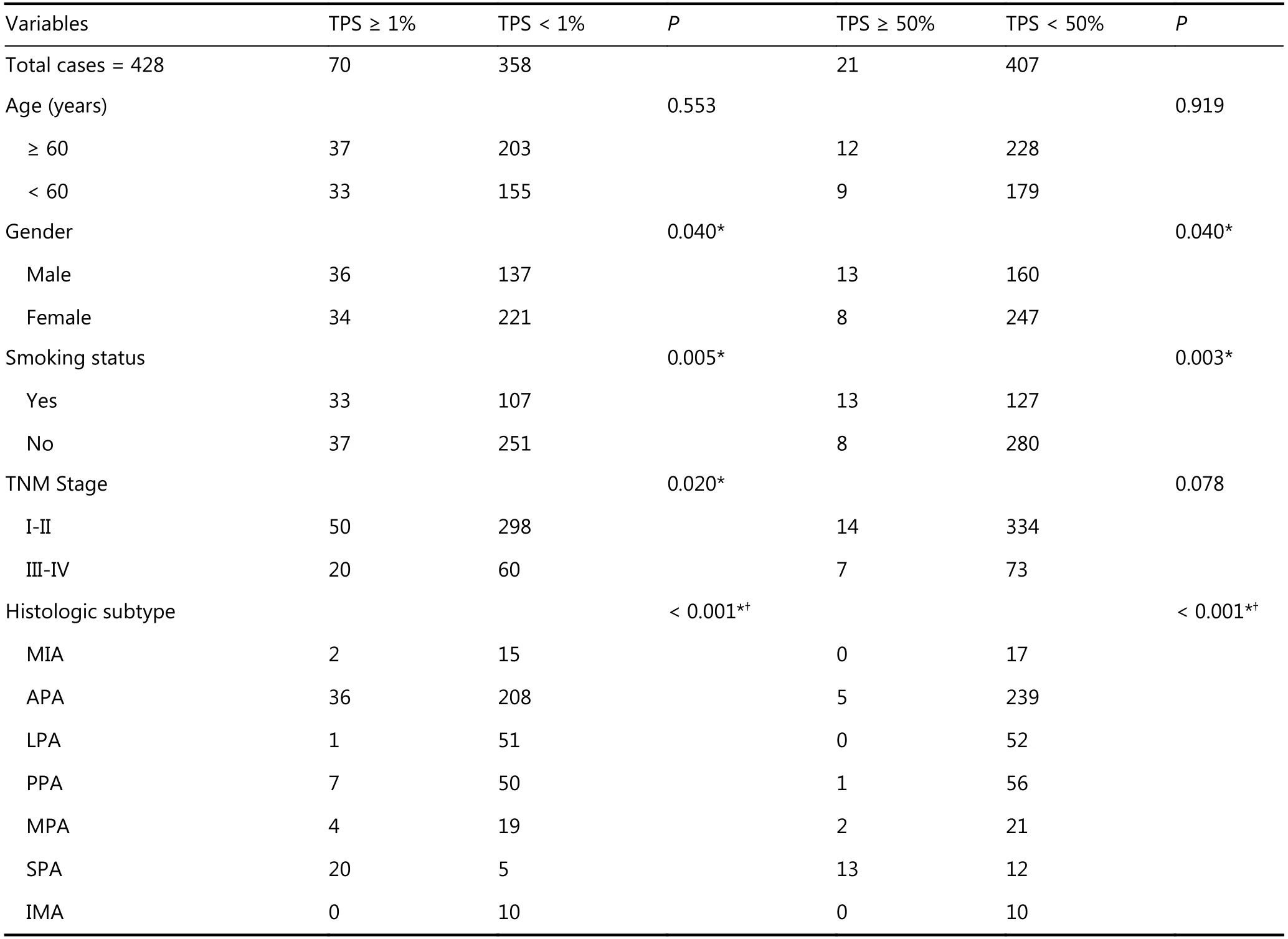
Table 1 Correlations of PD-L1 expression and clinicopathological parameters in Chinese LADC patients
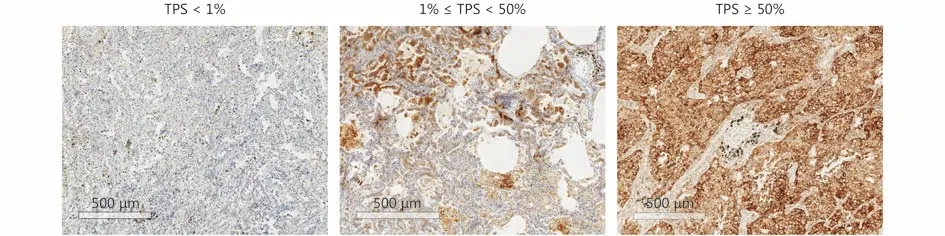
Figure 1 Representative images of IHC staining for PD-L1 expression in LADC with different levels of TPS.
Among 59 of the 324 cases with relapse or metastasis, 42were (71.2%) EGFR mutated, 4 (6.8%) had ALK rearrangement, 2 (3.4%) had ROS1 rearrangement, and 5 cases had (8.5%) KRAS mutation. Eleven cases (18.6%)showed PD-L1 positive expression with TPS ≥ 1% and 3 cases(5.1%) had a TPS ≥ 50%. Among 11 cases with TPS ≥ 1%, 5 cases (45.5%) had EGFR mutation, 2 cases (18.2%) had ALK rearrangement, 1 case (9.1%) had ROS1 rearrangement and 3 cases (27.3%) had KRAS mutation. All PD-L1 positive cases(100%) had at least one classical genomic aberration.

Table 2 Correlation between PD-L1 expression and classical genomic aberrations in Chinese LADC patients
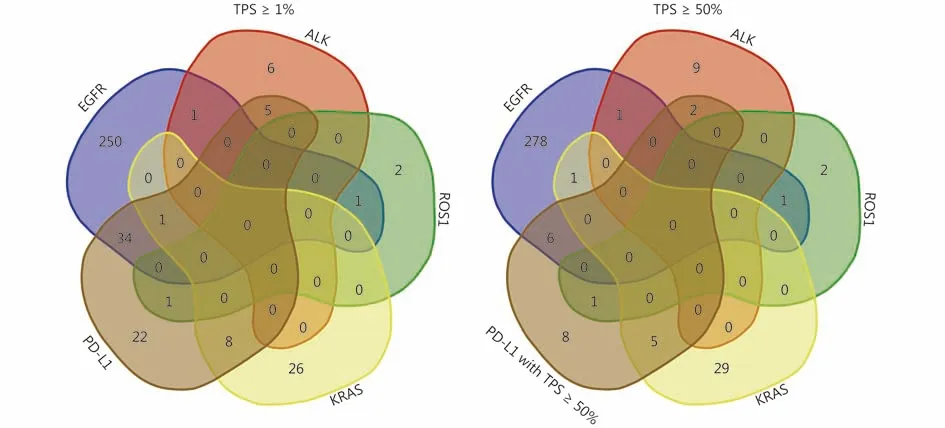
Figure 2 Venn diagrams showing the details of co-occurrence of classical genomic aberrations and PD-L1 expression with different levels of TPS in the routinely used biomarker profile.
Classification of LADC into four subgroups based on the routinely used molecular biomarker profile as a strategy for clinical therapy
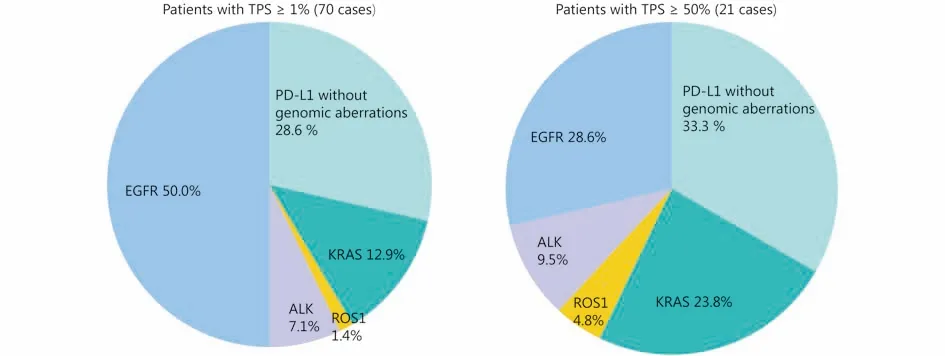
Figure 3 Graphic pie charts illustrating the frequency of classical driver oncogenes aberrations with PD-L1 positive expression with different levels of TPS.
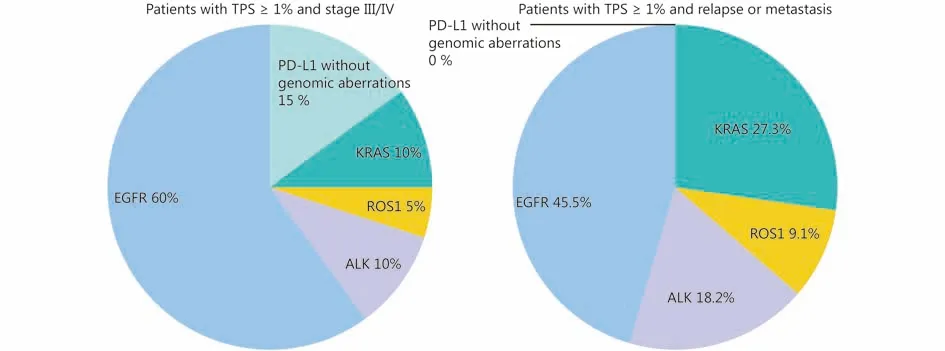
Figure 4 Graphic pie charts showing the frequent overlaps of classical genomic aberrations and PD-L1 positive expression (TPS ≥ 1%) in LADC cases with a TNM stage of III or IV and disease progression.
To provide an overview of the impact of the routinely used molecular biomarker profile in clinical strategies, we classified LADC cases into four subgroups based on the results above.Each group was defined based on its optimal pairing with an appropriate treatment strategy, as follows: Group 1 was defined as LADC cases harboring genotypes for which an inhibitor (EGFR, ALK, ROS-1) has been approved and PDL1 expression is negative. Group 2 was defined as LADC cases with PD-L1 positive expression but no aberrations in classical therapeutic genes. Group 3 was defined as LADC cases with co-occurrence of aberrations in classical therapeutic genes and PD-L1 positive expression. Group 4 was defined as LADC cases with negative expression of current therapeutic biomarkers or the presence of KRAS mutation. There were 258 cases with TPS ≥ 1%, (60.3%) and these were categorized in group 1, 29 cases (6.8%) were categorized in group 2, 41 cases (9.6%) were categorized in group 3, 100 cases (23.4%)were categorized in group 4. Figure 5 illustrates this overview of the four subgroups of LADC cases.
The molecular changes and clinical features of each group are summarized in Table 3. Analysis of these subgroups showed that apparent clinical discrepancies existing in group 3 for age (P < 0.001), gender (P < 0.001), smoking, (P <0.001) and TNM stage (P = 0.005). Comparison of the histopathologic types among groups showed significant differences such as, groups 3 and 4 included cases of the acinar predominant adenocarcinoma (APA) subtype, group 2 included cases of the micropapillary predominant adenocarcinoma (MPP) subtype, groups 2 and 3 included cases of the SPA subtype and group 4 included cases of the invasive mucinous adenocarcinoma (IMA) subtype (P < 0.05).
Correlation between PD-L1 expression and disease-free survival in Chinese LADC patients

Figure 5 Four subgroups of LADC cases based on routinely used molecular biomarker investigations. The graphic pie chart shows the frequency of LADC patients for the potential pairing of systemic therapies.
Analysis of DFS showed that there was no significant correlation between PD-L1 expression and DFS in LADC patients with stage I-II disease (P = 0.273, TPS ≥ 1%; P =0.261, TPS ≥ 50%). Investigation of DFS in the four subgroups defined above showed that there were no significant differences among these subsets (Figure 6).
Discussion
In the era of targeted therapy and immunotherapy,management of LADC has improved due to the discovery of molecular biomarkers that form the basis for the development of precise treatments. This advance requires an examination of the correlation between the molecular profiles of biomarkers. In this study, we analyzed the correlation and overlaps between the expression of the immunotherapeutic biomarker PD-L1, as detected by IHC,and classical genomic aberrations in Chinese LADC patients from our left. Our results indicated that PD-L1 expression is closely correlated with classic gene alterations and more than half of the PD-L1 positive Chinese cases, as well as the cases with advanced stage cancer or disease progression,synchronously harbor driver gene alterations. The overlaps among these biomarkers might significantly affect personalized therapeutic choices for appropriate tyrosine kinase inhibitors (TKIs) or PD-1/PD-L1 targeted immunotherapy in Chinese LADC patients.
We initially analyzed the correlation between PD-L1 expression and clinicopathologic features. PD-L1 expression detected by SP142 assay was positive in 70 of 428 cases(16.4%) assessed, which was consistent with the results of previous reports using the same antibody clone16,17. PD-L1 expression was significantly associated with male gender,smoking, advanced clinical stage, and solid predominant subtype. These results were similar to those of previous studies conducted in Asian populations8,18-21. However,analysis of DFS did not show a significant correlation with PD-L1 expression in patients with stage I-II disease, which might be due to the relatively short follow-up time. Many previous studies have reported the association between PDL1 expression and driver gene aberrations in LADC with conflicting results. Our findings showed that PD-L1 positive expression was associated with wild-type EGFR and ALK rearrangement in Chinese patients. These results might show consistency or discrepancy with some studies13,20,22-24.However, it is difficult to draw a definite conclusion due to reasons including variations in sample selection, different occurrences of driver gene alterations among ethnic populations, inconsistencies in PD-L1 antibody assay results,and the usage of multiple cut-off values.
It is noteworthy that the status of biomarkers, such as PDL1, and molecular target genes, such as EGFR, ALK, and ROS1, was more crucial for classifying patients with therapeutic benefits. However, the overlaps among these routine biomarkers received less attention in Asian patients25.An analysis of the Caucasian population from real-world data showed that high expression of PD-L1 and alterations in EGFR, ALK, and ROS1 seldom overlapped26. In contrast, in our cohort of Chinese LADC patients, PD-L1 positivity overlapped with the presence of classical driver oncogenes in more than half of the cases. The overlaps increased in patients with a higher TNM stage or disease progression who required adjuvant therapy. Since LADC patients who should preferentially receive approved first-line EGFR- or ALKdirected TKIs has been proposed27, subsets based on current routine biomarkers indicated that there might only be 5% of Chinese LADC patients who can be categorized based onIHC expression of PD-L1 for immunotherapy. There are nearly 20% of patients with negative expression of current routine supportive therapy-oriented biomarkers. The overlaps between current biomarkers might exert a significant impact on screening the population for PD-1/PD-L1 targeted immunotherapy as a first-line treatment in Chinese patients.As IHC expression of PD-L1 screened only a small number of LADC cases for immunotherapy, other biomarkers, such as tumor mutation burden (TMB), the number of neo-antigens,and diversity of T cell repertoire, need to be evaluated in order to overcome the limitations of PD-L1 as a single biomarker to realize the benefits of immunotherapy28.
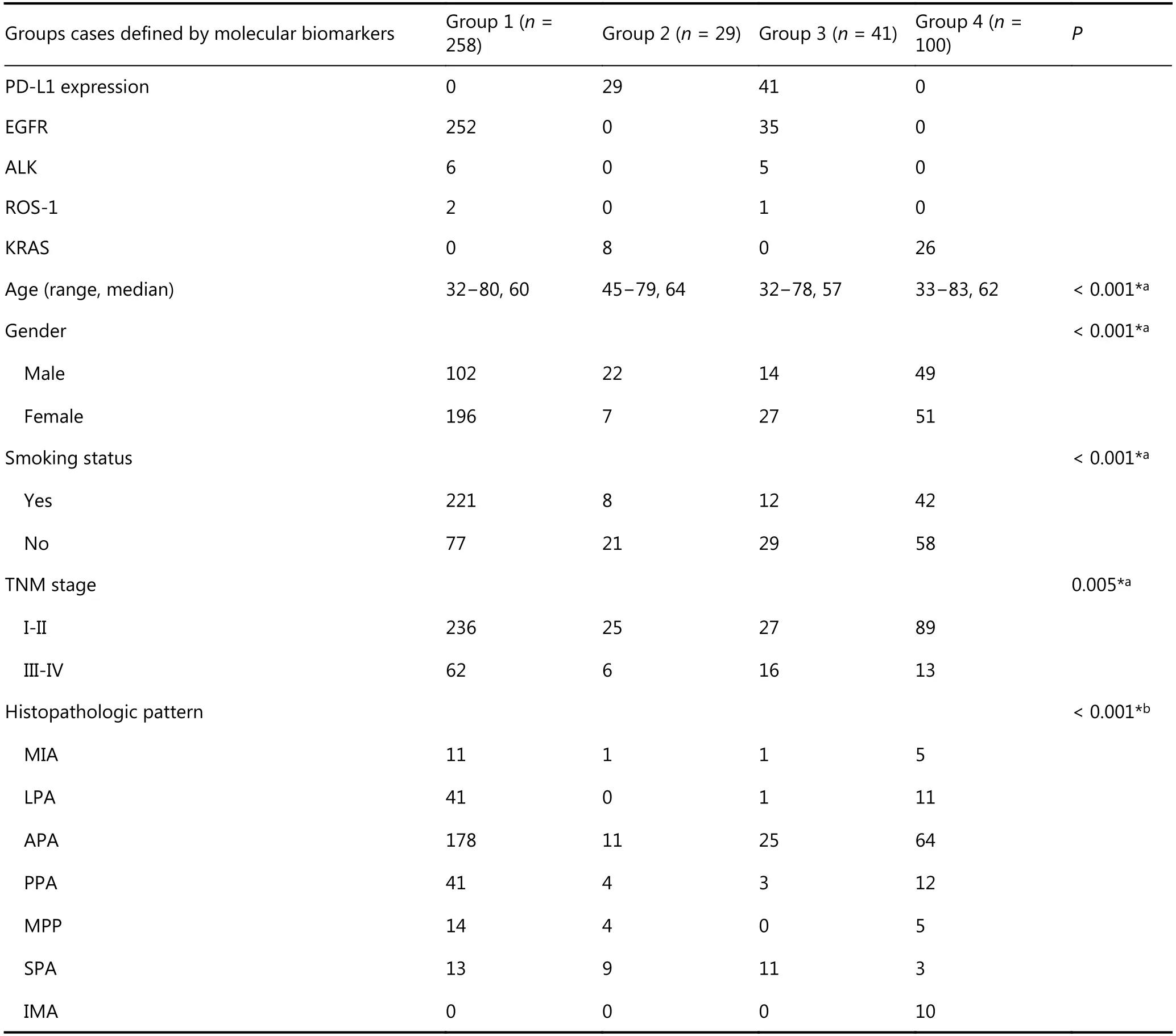
Table 3 Characteristics of four subgroups defined by current molecular biomarker profile in Chinese LADC patients
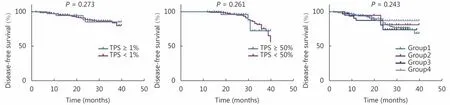
Figure 6 Disease-free survival according to the different cutoff values for PD-L1 in stage I-II cases, and four subgroups defined based on the current molecular biomarker.
Based on the evolving paradigm of molecular detection, we can preliminarily divide patients into four subgroups; this categorization has highly relevant therapeutic implications.Interestingly, these subgroups had specific clinical characteristics that may contribute to identifying effective therapeutic strategies for Chinese LADC patients. Especially,group 3, cases included in which harbored both PD-L1 expression and classical genomic aberrations had unique clinical features, such as a younger population, a majority of females and non-smokers, patients of a relatively higher TNM stage, and presentation of more solid subtypes compared to those in other groups. In this study, we classified the cases with co-occurrence of PD-L1 expression and genotype with TKIs as a separate group accounting for about 10% of LADC patients. Although previous clinical trials have suggested that EGFR-mutated NSCLC patients might exhibit a low response to PD-1/PD-L1 checkpoint blockade29, other studies have demonstrated the efficacy of nivolumab treatment after disease progression with T790M mutation during EGFR-TKI treatment30. Considerable evidence is required to investigate the efficacy of immune checkpoint inhibitors in EGFR-mutated lung cancers in Chinese patients. To improve the treatment strategies for oncogene-driven NSCLC, it is necessary to design clinical trials comparing TKIs to immunotherapy, evaluating different treatment combinations or sequences, including immunotherapy. Besides, our results showed that KRAS mutation was significantly associated with high expression of PD-L1, consistent with the findings of another study demonstrating the co-occurrence of PD-L1 upregulation and KRAS mutations31. Some patients with KRAS-mutations might potentially benefit from anti-PD-1/PD-L1 immunotherapy.
There are some limitations to our study. First, our data were retrospectively analyzed with the data available from clinical practice in a single left. Thus, the possibility of bias may exist because of the limited cases, especially only a small number of patients with TPS ≥ 50%. Second, the study was limited to a single PD-L1 antibody clone, SP142, which is currently commonly used for the detection of PD-L1 expression in Chinese patients. There are several IHC assays for detecting PD-L1. Each IHC assay was developed using a unique primary antibody against PD-1/PD-L1. According to the report by the Blueprint Working Group, the PD-L1-positivity rate using the SP142 clone might be lower than that observed using other antibodies32,33. Additional prospective studies in collaboration with multiple institutions and obtaining data using different assays are warranted.
Conclusions
Our study demonstrated the correlation and overlaps between PD-L1 expression and classical genomic aberrations in surgically resected samples from Chinese LADC patients.Our data revealed that only a small number of these patients could be classified based on the expression profile of the single biomarker PD-L1 as potential first-line immunotherapy, and approximately 20% of patients showed a negative expression for current routine supportive therapeutic biomarkers. We also classified Chinese LADC patient cases into four subgroups to provide a useful overview of this disease in the Chinese population. This categorization could help in developing clinical strategies for adjuvant therapy for LADC.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81871860).
Conflict of interest statement
No potential conflicts of interest are disclosed.
 Cancer Biology & Medicine2019年4期
Cancer Biology & Medicine2019年4期
- Cancer Biology & Medicine的其它文章
- Interpretation of breast cancer screening guideline for Chinese women
- Breast cancer screening guideline for Chinese women
- Erratum to Simultaneous inhibition of PI3Kα and CDK4/6 synergistically suppresses KRAS-mutated non-small cell lung cancer
- Nomogram based on albumin-bilirubin grade to predict outcome of the patients with hepatitis C virus-related hepatocellular carcinoma after microwave ablation
- Omics-based integrated analysis identified ATRX as a biomarker associated with glioma diagnosis and prognosis
- ILF2 cooperates with E2F1 to maintain mitochondrial homeostasis and promote small cell lung cancer progression
