Decomposition behaviors of methane hydrate in porous media below the ice melting point by depressurization☆
Yu Zhang ,Tian Wang,4 ,Xiaosen Li, *,Kefeng Yan ,Yi Wang ,Zhaoyang Chen
1 Guangzhou Institute of Energy Conversion,Key Laboratory of Gas Hydrate,Chinese Academy of Sciences,Guangzhou 510640,China
2 Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development,Guangzhou 510640,China
3 Guangzhou Center for Gas Hydrate Research,Chinese Academy of Sciences,Guangzhou 510640,China
4 University Science&Technology of China,Nano Science&Technology Institute,Suzhou 215123,China
Keywords:Methane hydrate Depressurization Porous media Decomposition Ice-melting point
ABSTRACT The decomposition behaviors of methane hydrate below the ice melting point in porous media with different particle size and different pore size were studied.The silica gels with the particle size of 105-150 μm,150-200 μm and 300-450 μm,and the mean pore diameters of 12.95 nm,17.96 nm and 33.20 nm were used in the experiments.Methane recovery and temperature change curves were determined for each experiment.The hydrate decomposition process in the experiments can be divided into the depressurization period and the isobaric period.The temperature in the system decreases quickly in the depressurization process with the hydrate decomposition and reaches the lowest point in the isobaric period.The hydrate decomposition in porous media below ice-melting point is very fast and no self-perseveration effect is observed.The hydrate decomposition is influenced both by the driving force and the initial hydrate saturation.In the experiments with the high hydrate saturation,the hydrate decomposition will stop when the pressure reaches the equilibrium dissociation pressure.The stable pressure in the experiment with high hydrate saturation exceeds the equilibrium dissociation pressure of bulk hydrate and increases with the decrease of the pore size.
1.Introduction
Natural gas hydrate has an energy density equivalent to the highly compressed gas and has been found all over the world in sediments in deep sea and permafrost regions[1,2].It is considered as an ideal alternative energy source with the advantages of high energy density and large reservation[3-5]and attracted considerable attention due to its potential hazards in the oil and gas industry[6].With approximately 164 volume of gas contained in per volume of gas hydrate,the hydrate method is also considered as a promising commercial application and has attracted great attentions[7,8].
Many researchers have investigated hydrate decomposition characteristics below the ice melting point in laboratory systems in an effort to understand decomposition morphologies and mechanism of the gas hydrate[9,10].Water produced after the decomposition of hydrate will transform into ice at the surface of hydrate crystal when the temperature is lower than the ice melting point.The released gas should spread from the hydrate and ice blend to the volume of gas phase.An anomalously low rate of gas hydrates dissociated into gas and ice was found preciously when the temperature was lower than the ice melting point[11,12].This phenomenon is called the self-preservation effect.Many researchers paid considerable attention in self-preservation because of its characteristics,moreover,the gas hydrate self-preservation has been used to develop the commercial technology to store and transport the natural gas[9].In addition,the investigations into the hydrate decomposition in porous media below the ice melting point are also helpful for the development of the exploitation technology of the natural gas hydrate.Therefore,the hydrate decomposition rate has been studied widely in order to have a deeper understanding of hydrate decomposition behaviors below the ice melting point.Stern et al.[10]found hydrate decomposition is more difficult in the temperature ranges of 255-264 K than that in the temperature ranges of 248-254 K and 265-271 K through the experiments.Takaya et al.[11]verified that an ice layer will appear surrounding the hydrate particles,retarding the hydrate to dissociate.Zhan et al.[12]demonstrated experimentally that the size of ice particles has significant influence on the hydrate decomposition.Wu et al.[13]also found that methane hydrate dissociates rapidly at the beginning and the decomposition rate is gradually decreased with time.The decomposition of methane hydrate at a temperature of 269 to 272 K was much slower than that when the temperature was between 261 and 266 K.Methane hydrate formed from ice particles of larger than 250 μm may have obviously unusual preservation.The hydrates could stably exist at the pressure just below the equilibrium dissociation pressure if the residual water is supercooled.
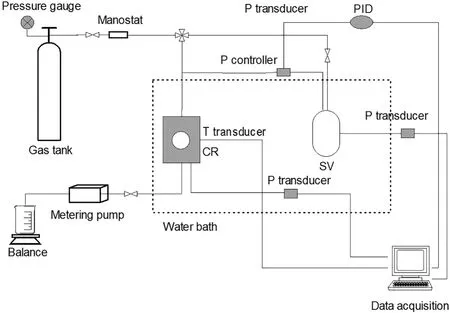
Fig.1.Schematic of the experimental apparatus.
Till now,most of the studies focused on the bulk hydrate decomposition below the ice melting point,such as the decomposition rate and anomalous preservation[11-13].There is still a lack of understanding of the methane hydrate decomposition in porous media below the ice melting point.It has been revealed that the porous media has significant influences on the hydrate equilibrium and kinetic behaviors[14].By the effect of capillary pressure,the equilibrium dissociation pressure of hydrate in porous media is enhanced compared to that in bulk.The gas diffusion and reaction surface in porous media will be notably influenced by the complex states and the phase transition of water[15].Many elements on the hydrate decomposition,such as the decomposition temperature,the hydrate saturation and the properties of the porous media,should need further investigations.
In this article,we experimentally investigated the methane hydrate decomposition in porous media with the various particle and pore sizes below the ice melting point.The effects of the initial hydrate saturation,decomposition temperature,particle size and pore size on the decomposition behaviors of methane hydrate are discussed and analyzed.
2.Experimental
2.1.Equipment and material
The schematic of the experimental apparatus is shown in Fig.1[16,17].The high-pressure hydrate crystallizer(CR)with an effective maximum volume of 419 ml is used as the reactor.The supply vessel(SV)with a volume of 1091 ml is used to supply the gas to the crystallizer.A Pt1000 thermoprobe(JM6081)is placed in the crystallizer with the estimated error of±0.05 K to measure the temperature.The pressure is measured using an MBS3000 absolute pressure transducer (0-25 MPa)with the uncertainty of ±0.02 MPa.The crystallizer is immersed in a water bath with the uncertainty of±0.1 K.Silica gels(produced by Qingdao Makall Group Inc.)were used in the experiments.Table 1 gives the properties of the silica gels used in the experiments.The pore diameter of the silica gels was measured using a Micromeritics ASAP 2010 pore-size analyzer.The pore volume and surface area were determined based on BET analysis.CH4(mass fraction 0.999)was provided by Foshan HaoWen Gas Co.
2.2.Experimental procedure
The experimental procedures have been partly described in detail in the previous paper[16].Briefly,the known amount of pore saturated silica gel was packed into the crystallizer.In different experiments,the water contained in the porous media is 148 g and the gas volume is 208.4 ml.The hydrate was formed at the constant bath temperature and reaction volume.The hydrate formation was ended when the pressure in the crystallizer kept constant for 3 h.The gas consumption was calculated by the pressure change in the crystallizer[16].
After the hydrate formation,the decomposition experiment of the methane hydrate by depressurization was performed.The outlet valve was opened and the gas in the crystallizer was released rapidly until the pressure reached the atmospheric pressure.The outlet valve was then closed and the hydrate in the crystallizer dissociated gradually.When the pressure in the crystallizer maintained constant,the decomposition experiment was finished and the crystallizer was depressurized to the atmospheric pressure again.In the experiments,the temperature and pressure changes were recorded and the moles of the gas dissociated from the hydrate were calculated.
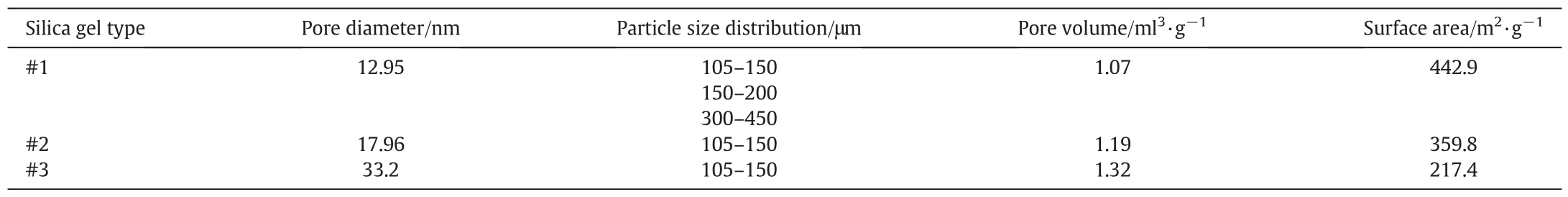
Table 1 Silica gel properties
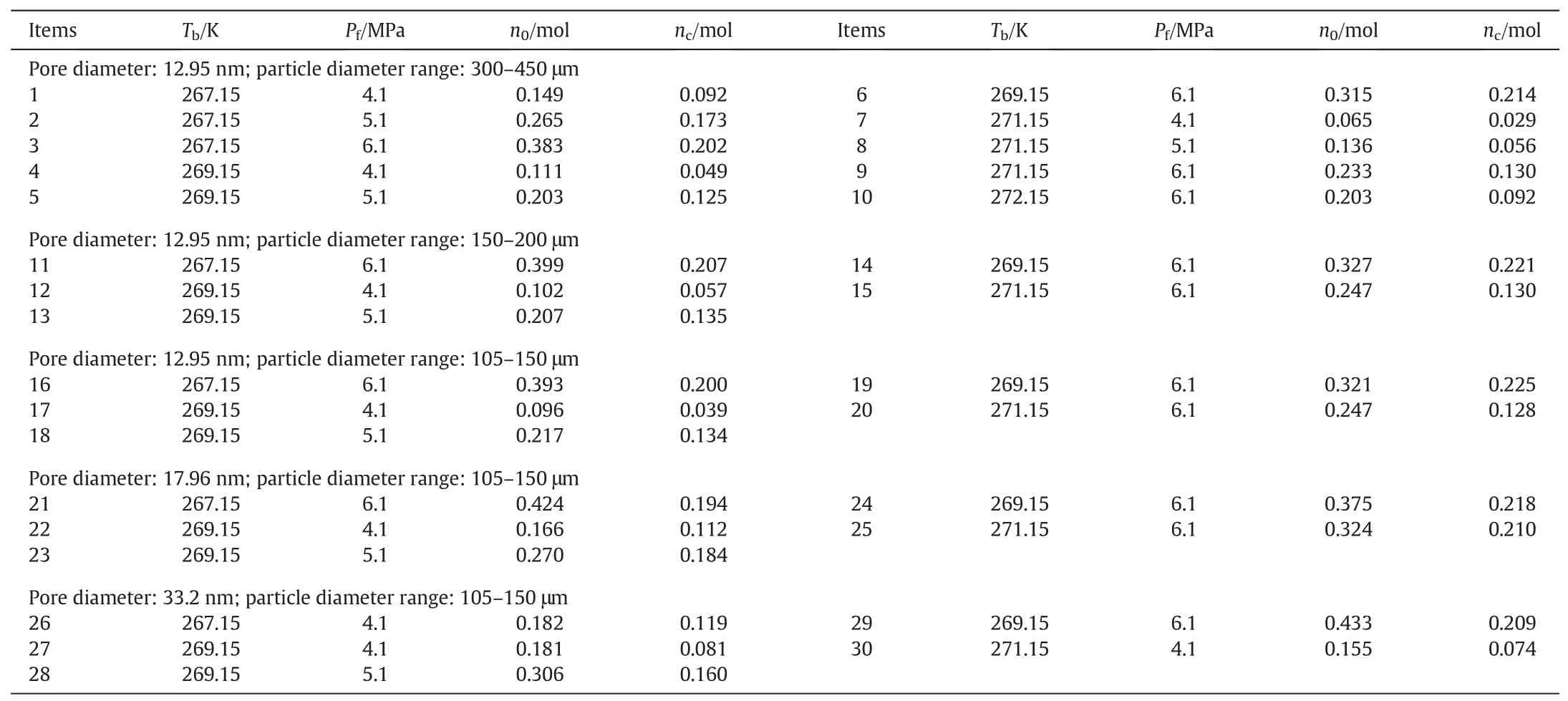
Table 2 The experimental conditions and results
3.Results and Discussions
The experimental conditions and results are given in Table 2.In Table 2,P0is the initial formation pressure of the experiment,n0is the total moles of the gas in hydrate before the hydrate decomposition,Tbis the bath temperature for the hydrate decomposition,and ncis the total moles of the gas collected in the crystallizer at the end of the decomposition experiment.
3.1.Influence of the hydrate saturation
Fig.2a gives the profiles of the pressure and temperature in the crystallizer for the experiments at 269.15 K and the different initial formation pressure of 4.1 MPa(experiment 17),5.1 MPa (experiment 18)and 6.1 MPa (experiment 19).The particle diameter range and the mean pore diameter of the porous media used in the experiments are 105-150 μm and 12.95 nm,respectively.Fig.2b shows the details of the first 10 min of the experiments given in Fig.2a.As shown in Table 2,the total moles of methane(n0)in hydrate before the decomposition increase with the increase of the initial formation pressure.The higher n0means the higher hydrate quantity in the crystallizer before the decomposition experiment.From the typical experiment conducted at 6.1 MPa(experiment 19),it can be noted that there are two periods during the hydrate decomposition.The first period is the depressurizing process,which is from the beginning of the experiment to Point A.In this period,the pressure decreases quickly to the atmospheric pressure with the gas release.The temperature inside the crystallizer also drops dramatically in the first period.The heat of hydrate phase transformation is more than the heat transmitted from the circumstance and the sensible heat in the system is consumed rapidly due to the hydrate decomposition,which causes the temperature to decrease.The second period is an isobaric process,which starts from Point A,as shown in Fig.2b.In this period,the crystallizer is kept in an isobaric condition and the pressure inside the crystallizer increases gradually due to the gas release from the dissociated hydrate.The temperature inside the crystallizer still decreases in the second period before it reaches the lowest value at Point B,indicating that the heat transmitted from the circumstance is still lower than that required for the hydrate decomposition due to the high decomposition rate.After Point B,the temperature inside the crystallizer increases gradually to the setting bath temperature.As the experiment processes,the hydrate decomposition rate and the required heat decrease gradually.Therefore,the temperature inside the crystallizer to the bath temperature increases gradually with the heat transmission from the circumstance.
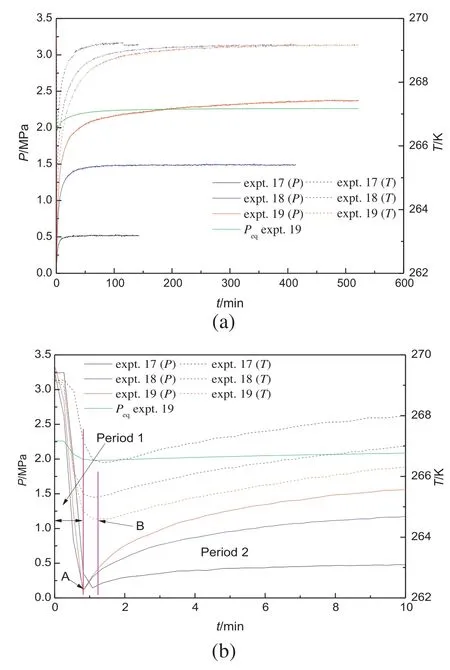
Fig.2.Pressure and temperature changes during hydrate decomposition for experiments with different hydrate saturation.
As shown in Fig.2,the highest temperature drop compared with initial temperature for experiments 17,18 and 19 is 2.69 K,3.84 K and 4.55 K,respectively,which decreases as the initial hydrate quantity increases.Because the duration of the depressurization period is similar and the temperature drop mostly occurs in the depressurization period,a higher temperature drop should mean a higher hydrate decomposition rate,indicating that the rate of the gas released from the dissociated hydrate is higher for the experiment with a higher hydrate quantity in the first period.In the second period,it also can be noted that the hydrate decomposition in the experiment with the higher initial hydrate saturation is faster.The final pressure inside the crystallizer and the decomposition duration both increase with the increase of the initial hydrate saturation.For experiments 17 and 18,the pressure inside the crystallizer reaches stability quickly.For experiment 19,this has the highest initial hydrate saturation,but the pressure increases slowly in the later stage.It may be because that with a large amount of hydrate dissociated,the pressure is close to the equilibrium dissociation pressure of the methane hydrate,resulting in the lower driving force for the hydrate decomposition in the later stage.In Fig.2,the equilibrium dissociation pressure of the bulk methane hydrate corresponding to the temperature in the crystallizer for experiment 19 is given[14].As shown,the equilibrium dissociation pressure of the methane hydrate increases when the temperature inside the crystallizer increases.The pressure inside the crystallizer gradually increases to the equilibrium dissociation pressure of the methane hydrate in bulk as the hydrate decomposition processes.It indicates that the driving force of the hydrate decomposition drops quickly,which will become the controlling factor of the hydrate decomposition.Due to the low stable pressure,the hydrate decomposition will be easier to be stopped and less gas could be recovered below the ice melting point.In addition,the pressure in the later stage is higher than the equilibrium dissociation pressure of the methane hydrate in bulk.The system pressure in porous media below the ice-melting point can reach a higher value than that for bulk hydrate decomposition.It may be caused by the higher equilibrium dissociation pressure of the methane hydrate in porous media than bulk hydrate for a given temperature.It illustrates that the decomposition conditions of the hydrate in porous media are milder than bulk hydrate.For the experiments 17 and 18,the pressure is very low,and the pressure increase rate drops quickly as the decomposition finishes quickly.
It should be pointed out that no self-preservation was observed in the experiments,indicating that the hydrate decomposition in porous media is easier than bulk hydrate.Due to the capillary pressure of the porous media,the temperature of the ice-melting point is lower than 0°C and is lower in the smaller pores[11].Therefore,there is a part of water that is in liquid state in porous media below 0°C.In these experiments,the silica gel particles packed in the crystallizer were incompact.Meanwhile,silica gels were water saturated and no water exists among the silica gel particles.The gas dissociated from the hydrate can diffuse easily to the gas phase.Therefore,the hydrate dissociates more quickly than that in bulk.
3.2.Influence of the bath temperature
Fig.3 gives the pressure changes in the crystallizer versus time in the experiment with different bath temperature.The hydrate was formed at the same initial pressure of 6.1 MPa.The particle diameter range and the mean pore diameter of the porous media used in the experiments are 105-150 μm and 12.95 nm,respectively.The experiments were conducted out at 267.15 K (experiment 3),269.15 K (experiment 6),271.15 K(experiment 9)and 272.15 K(experiment 10),respectively.Fig.4 shows the temperature profiles of experiments 3,6,9 and 10.As shown,the biggest drop of the temperature during the hydrate decomposition is approximately 1.69 K,2.40 K,2.93 K and 3.44 K for the experiments 3,6,9 and 10,respectively.The highest drop of temperature increases as the bath temperature increases.

Fig.3.Pressure changes during hydrate decomposition for experiments at different bath temperature.
In the second period,the pressure increases more quickly at the higher experimental temperature.Meanwhile,in the experiments with lower bath temperature,the final pressure is higher and the decomposition duration is longer.As shown in Table 2,more hydrate can form in the formation process at a lower temperature,resulting in higher hydrate saturation before the decomposition experiments.Therefore,the decomposition rate of the methane hydrate is higher even though the decomposition driving force is lower at a lower experimental temperature.However,for the experiment at 267.15 K,in the early stage,the hydrate dissociates faster than that at 269.15 K due to the higher hydrate saturation.It has been found that the lowest temperature terminus in the hydrate decomposition is lower in the experiment with lower experimental temperature.The higher temperature terminus means the higher hydrate decomposition rate.It is different to that observed in the second period.As the temperature decreases,the equilibrium dissociation pressure of the methane hydrate also decreases.Thus,the hydrate decomposition rate may be lower at a lower temperature even though the hydrate saturation is higher in the porous media.It indicates that the hydrate decomposition rate will be affected both by the driving force and the initial hydrate saturation,and presents different in the depressurization period and the isobaric period.

Fig.4.Temperature changes during hydrate decomposition for experiments at different bath temperature.
In the later stage of the second period,the hydrate decomposition at 267.15 K slows down gradually to be lower than that at the temperature of 269.15 K.Meanwhile,the final pressure in the experiment at 267.15 K is lower than that at 269.15 K.Because the initial hydrate saturation of the experiments at the temperature of 267.15 K and 269.15 K is enough high,the final pressure inside the crystallizer can reach the equilibrium dissociation pressure of the methane hydrate in porous media under the experimental conditions.Meanwhile,the equilibrium hydrate decomposition pressure at 269.15 K is higher than that at the temperature of 267.15 K.Therefore,the pressure inside the crystallizer at 269.15 K can reach a higher pressure than that at 267.15 K although the initial hydrate saturation at 269.15 K is lower.
3.3.Influence of the particle diameter
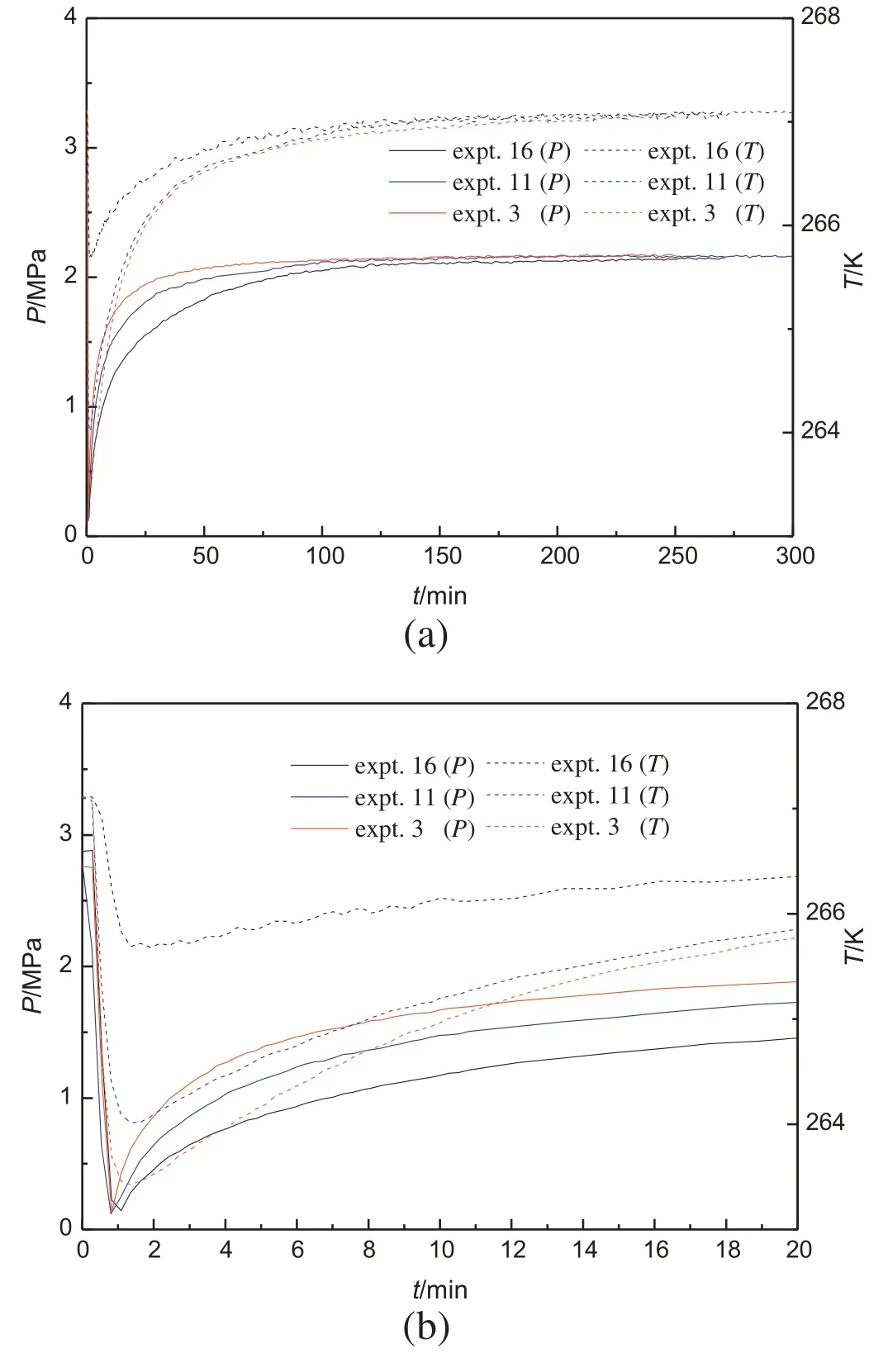
Fig.5.Pressure and temperature changes during hydrate decomposition for experiments with different particle size of porous media.
To investigate the influence of the particle size of the porous media on the hydrate decomposition,the hydrate was formed and dissociated at the same temperature and pressure conditions in the 12.95 nm porous media with the particle diameters of 105-150 μm (experiment 16),150-200 μm(experiment 11)and 300-450 μm(experiment 3),respectively.Fig.5a gives the temperature and pressure profiles for the experiments 3,11 and 16.Fig.5b shows the details of the first 20 min of the experiments given in Fig.5a.As shown in Table 2,the total gas consumed in the hydrate formation is similar for experiments 3,11 and 16,indicating a similar initial hydrate saturation before the decomposition experiments.It can be found that the temperature decreases to the lowest temperature of 267.00 K,265.70 K and 265.31 K during the hydrate decomposition for the experiments 3,11 and 16,respectively.The temperature terminus shows that the hydrate decomposition rate in the depressurization period increases with the decrease of the particle diameter.
In the second period,it can be observed from the pressure change that the hydrate decomposition rate is lower in porous media with the larger particle size and it is obviously low when the particle diameter ranges from 300 to 450 μm,same to that in the first period.Hence,it is concluded that the particle size has a significant influence on the decomposition process.It may be mainly attributed to that for the porous media particle with large size,the gas dissociated from hydrate is difficult to diffuse from the inside to the outside of the particle.The temperature in the crystallizer increases more quickly for the larger particle diameter.It also can be seen that the final pressure for experiments 3,11 and 16 has little difference,indicating that the stable pressure in the hydrate decomposition is not affected by the particle size.
3.4.Influence of the pore diameter
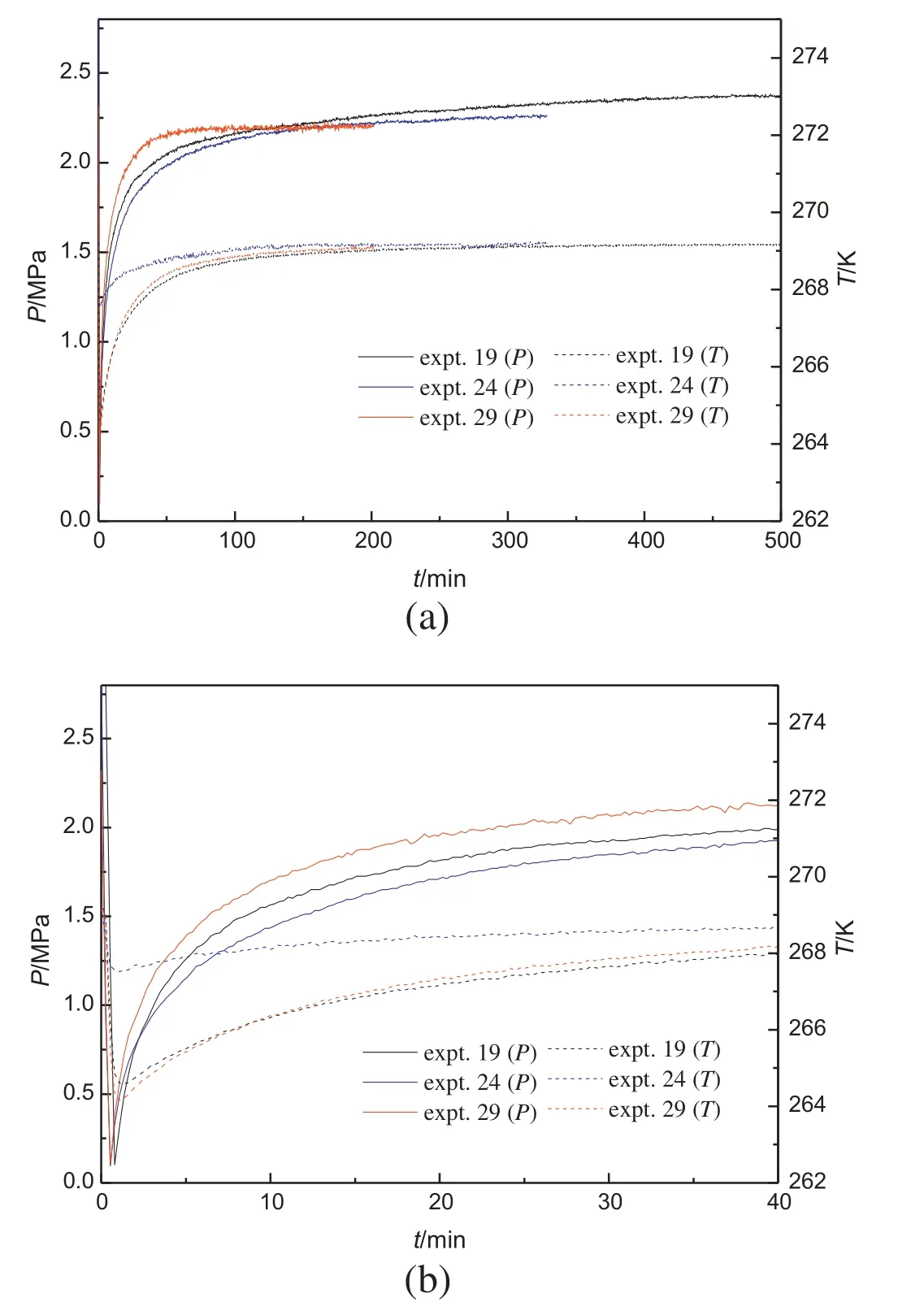
Fig.6.Pressure and temperature changes during hydrate decomposition for experiments in different pore size of porous media.
Fig.6a gives the temperature and pressure profiles for the experiments in porous media with different pore size.Fig.6b shows the details of the early stage of the experiments given in Fig.6a.The methane hydrate was formed at 269.15 K with the initial formation pressure of 6.1 MPa,and dissociated by depressurization.The mean pore diameters are 12.95 nm(experiment 19),17.96 nm(experiment 24)and 33.2 nm(experiment 29),with the same particle size of 105-150 μm.As shown in Table 2,the final hydrate quantity formed in porous media with larger pore size is higher at the same formation conditions because of the lower equilibrium pressure in the larger pores.However,the order of the lowest temperature in the depressurization process is experiment 29 <experiment 19 <experiment 24,same to the pressure increase rate in the second period,in which the hydrate decomposition rate in 33.20 nm porous media is the highest.It may be due to that the hydrate quantity in experiment 29 before the hydrate decomposition is the highest.In smaller pores,the hydrate quantity before the decomposition experiment decreases,but the driving force for hydrate decomposition increases.Therefore,the hydrate decomposition rate for the experiments in 12.95 nm and 17.96 nm porous media is similar.The decomposition rate in 17.96 nm porous media even is a little lower than that in 12.95 nm porous media.It may be due to that the driving force of hydrate decomposition at 17.96 nm is lower than that at 12.95 nm.The final pressure increases as the pore size increases.As calculation,the equilibrium dissociation pressure of bulk methane hydrate at 269.15 K is 2.26 MPa,the final pressure for experiments 19,24 and 28 is 2.37,2.26 and 2.21 MPa,respectively.It can be found that the final stable pressure is close to or a little higher than the equilibrium dissociation pressure of the bulk methane hydrate and increases with the decrease of the pore size.It should be due to that the equilibrium dissociation pressure of the methane hydrate is higher in smaller pores[14].
4.Conclusions
To investigate the decomposition behaviors of the methane hydrate in porous media below the ice melting point,the methane hydrate in silica gels was dissociated by depressurization.The following conclusions were obtained:
1)The hydrate decomposition in these experiments has two periods,which are the depressurization period and the isobaric period.The temperature drops rapidly during the depressurization with the hydrate decomposition and reaches the lowest point in the isobaric period and then increases gradually.
2)The hydrate decomposition in porous media below the ice melting point is very fast and no self-perseveration was observed.The hydrate decomposition in silica gels with different initial hydrate saturation is influenced both by the driving force and the initial hydrate saturation and presents different in the depressurization period and the isobaric period.The dissociation rate increases with the initial hydrate saturation in the depressurization period,and decreases with the initial hydrate saturation in the isobaric period due to the lower driving force.
3)The hydrate decomposition rate increases with the increase of the initial hydrate saturation,and decreases with the increase of the particle size.For the different experimental temperature,the hydrate decomposition rate increases as the bath temperature increases in the depressurization period,and increases as the bath temperature decreases in the isobaric period.For the experiments with different pore size,the hydrate decomposition rate increases with the increase of the pore size in the depressurization period.In the isobaric period,the decomposition rate at 33.2 nm is the highest,but the decomposition rate at 17.96 nm is a little slower than that at 12.95 nm due to the lower driving force.
4)The hydrate decomposition will stop when the pressure reach the equilibrium dissociation pressure of the methane hydrate when the initial quantity of the hydrate is high enough.The stable pressure in high hydrate saturation experiments exceeds the equilibrium dissociation pressure of bulk hydrate and increases with the decrease of the pore size.
 Chinese Journal of Chemical Engineering2019年9期
Chinese Journal of Chemical Engineering2019年9期
- Chinese Journal of Chemical Engineering的其它文章
- Advances of experimental study on gas production from synthetic hydrate reservoir in China☆
- Experimental characterization of guest molecular occupancy in clathrate hydrate cages:A review☆
- Hybrid versus global thermostatting in molecular-dynamics simulation of methane-hydrate crystallisation
- Growth kinetics of hydrate formation from water-hydrocarbon system☆
- Investigation of the hydrate formation process in fine sediments by a binary CO2/N2gas mixture☆
- Investigation on the effect of oxalic acid,succinic acid and aspartic acid on the gas hydrate formation kinetics
