Recyclable, non-leaching antimicrobial magnetic nanoparticles
Wei Ye,Lingren Wang,Jie Zhao,Quentin Q.Fang,Weihua Ming
a Department of Chemistry and Biochemistry, Georgia Southern University, Statesboro, GA 30460, United States
b Department of Mechanical and Materials Engineering, Huaiyin Institute of Technology, Huaian 223001, China
c Key Laboratory of Bionic Engineering, Ministry of Education, Jilin University, Changchun 130022, China
d Department of Biology, Georgia Southern University, Statesboro, GA 30460, United States
Keywords:
Antimicrobial
Quaternary ammonium compound (QAC)
Contact killing
Magnetic particles
Recyclability
ABSTRACT
Pathogenic bacterial contaminations in water cause serious or even lethal threats.Strategies that effectively kill bacteria without causing environmental contamination are urgently needed in a wide range of applications.We prepared recyclable antimicrobial magnetic nanoparticles, Fe3O4@P(St-co-AcQAC),through surfactant-free seeded emulsion polymerization involving a polymerizable,hydrophobic quaternary ammonium compound (QAC).Fe3O4 particles were first synthesized by a solvothermal reaction,followed by functionalization with a methacrylic silane(MPS),and then copolymerized with a QAC-containing acrylic monomer (AcQAC), leading to Fe3O4 @P(St-co-AcQAC) nanoparticles.As confirmed by antibacterial assays, these Fe3O4@P(St-co-AcQAC) nanoparticles exhibited strong antimicrobial action against both Gram-positive Staphylococcus epidermidis and Gram-negative Escherichia coli, without leaching out any bactericidal agent.An additional benefit of antimicrobial magnetic particles is that they can be easily recycled while maintaining excellent antimicrobial efficacy.
Pathogenic bacterial contaminations involved in water purification and disinfection can cause serious or even lethal threats in the related areas of safe drinking water,livestock,food production and processing [1-3].Therefore, developing a strategy that can effectively kill bacteria and guarantee human and livestock health and safety is highly desired.One commonly adopted approach is to use antimicrobial agents such as silver [4,5], halogens [6,7],quaternary ammonium compounds(QACs)[8-11],and antibiotics[12,13].Although effective, most of the traditional antimicrobial strategies involve the use of free biocides, and function via the release of biocides into the surroundings, so the antimicrobial property will diminish in time.Even worse, leaching of biocides may cause environmental concern and trigger antibiotic resistance[14,15].In addition,these antimicrobial materials are not suitable for reuse.Therefore, recyclable antibacterial materials without leaching out biocides have attracted great interests.
Magnetic nanomaterials are a new type of adsorbent materials with high adsorption capability and super magnetic separation performance [16].They are easy to be separated from liquid medium under external magnetic field.Compared with traditional adsorbents,magnetic nanomaterials possess superior merits,such as fast adsorption,high adsorption efficiency,low preparation cost,simple desorption process, and recyclability [17,18].In particular,magnetic nanoparticles with functional groups on the surface have unique advantages in the process of water purification and disinfection, so their applications in the field of wastewater treatment have attracted recent attentions [19,20].There have been numerous reports on the preparation of magnetic antimicrobial particles[21-24], which however mostly employed Ag for antimicrobial action and would lead to the concerns of heavy metal contamination and triggering of antibiotic resistance.
In this work,we first synthesized Fe3O4magnetic nanoparticles by using a modified solvothermal reaction as reported [25], and then modified the particle surface with a silane coupling agent 3-(methacryloyloxy) propyltrimethoxysilane (MPS) to introduce polymerizable acrylic double bonds (designated as Fe3O4@MPS,Scheme 1).The surface acrylic moiety was further copolymerized with styrene,a QAC-containing monomer (AcQAC[9],Scheme 1),and a cross-linker, divinyl benzene (DVB) via seeded surfactantfree emulsion polymerization to obtain antimicrobial magnetic particles.Gram-positive Staphylococcus epidermidis(S.epidermidis)and Gram-negative Escherichia coli (E.coli) were used as representative microorganisms to examine antibacterial properties of these nanoparticles.
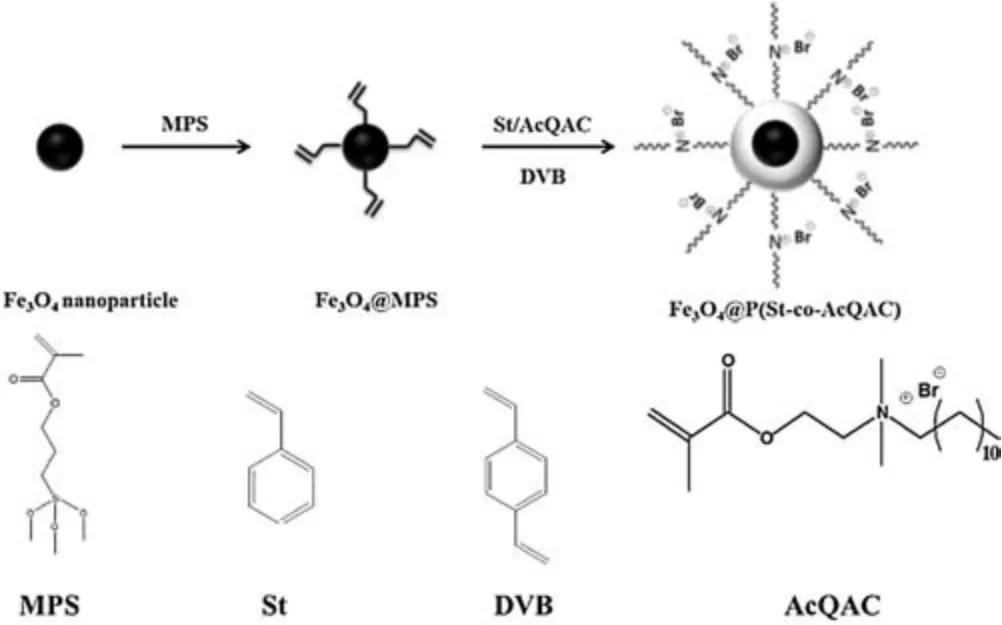
Scheme 1.Synthesis of Fe3O4@P(St-co-AcQAC) nanoparticles.
The QAC-containing monomer(AcQAC,Scheme 1) was synthesized by a one-step quaternization by reacting DMAEMA with 1-bromodecane [9].We used seeded emulsion polymerization to obtain antimicrobial magnetic particles.A typical procedure is as follows.Fe3O4@MPS nanoparticles(40 mg)were dispersed in 50-mL deionized water under mechanical stirring in a three-neck flask equipped with a condenser, a pressure-equalizing addition funnel and N2inlet and outlet, followed by gentle bubbling with N2.The dispersion was then heated to 75°C, followed by the addition of 0.6 mL of potassium persulfate(KPS)aqueous solution(10 mg/mL).Next,amonomermixturecomprising0.2 gofAcQAC,0.2 gofstyrene,and0.05 g of DVB was addeddropsizeto the flask.The reactionlasted for 10 h to ensure complete monomer conversion.
The as-synthesized Fe3O4nanoparticles were uniform in size(~260 nm in diameter) with negatively charged surface (Table 1),as determined by dynamic light scattering on a Malvern Zetasizer Nano.There was no significant difference in the particle size for the Fe3O4nanoparticles before and after MPS modification(Fig.1 and Table 1).The zeta potential (the absolute value), however,decreased slightly for the Fe3O4@MPS particle but remained negative (Table 1), obviously due to the fact that, during MPS modification, some metal hydroxyl groups at the Fe3O4surface were consumed.As shown in Fig.1a, most of the Fe3O4particles were of 200 nm to 250 nm in diameter,consistent with the results obtained from dynamic light scattering.The subsequent introduction of MPS did not lead to obvious difference on the morphology of Fe3O4@MPS nanoparticles(Fig.1b).Following the seeded emulsion polymerization,there was a substantial increase of the diameter to about 360 nm for the Fe3O4@P(St-co-AcQAC) nanoparticles(Fig.1c), evidently due to the formation of the QAC-containing copolymer layer on the particle surface.All magnetic nanoparticles showed good dispersibility in water ( Figs.2a-c).When placed above a magnet, all three nanoparticles exhibited excellent magnetic separation performance ( Figs.2d-f), with all the nanoparticles being completely attracted by the magnet thus settling at the bottom of the vial.Furthermore, once the magnet was removed,all magnetic nanoparticles became well dispersed in water again by gentle shaking.This magnetically induced separation would allow the antimicrobial nanoparticles to be recycled and reused after sterilization.
To evaluate antibacterial properties of Fe3O4@P(St-co-AcQAC)nanoparticles, antimicrobial tests were performed according to a standard antimicrobial susceptibility test protocol such as ISO22196 and JIS Z 2801.Briefly,cultures of E.coli(Carolina#155065A)and S.epidermidis (Carolina #155556) were grown aerobically at 37°C for 24 h in sterile Luria-Bertani (LB) broth.These active growing cultures were diluted in sterile distilled water to obtain a concentration of 105bacteria/mL(determined by the cell counter),then Fe3O4@P(St-co-AcQAC) nanoparticle dispersion (overall concentration: 2 mg/mL) was added to the bacterial suspension.Fe3O4and Fe3O4@MPS nanoparticles were also tested as control.After 2, 4, and 6-h incubation at 37°C, the suspensions were appropriately diluted,and each dilution(500 μL)was plated onto a sterile LB agar culture plate and incubated at 37°C overnight to give visible colonies.

Table 1 Particle size and zeta potential of different nanoparticles.
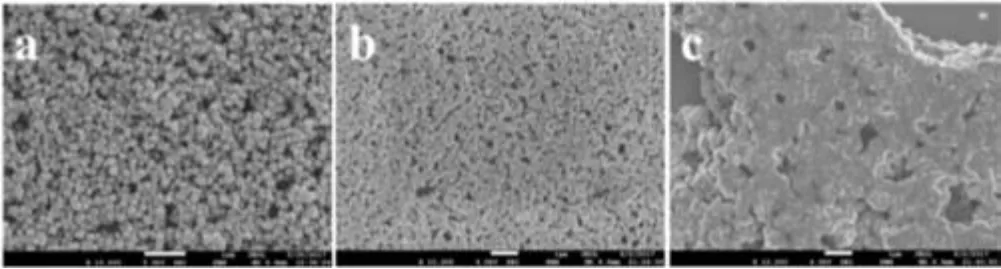
Fig.1.SEM images of different magnetic particles:(a)Fe3O4,(b)Fe3O4@MPS and(c)Fe3O4@P(St-co-AcQAC).

Fig.2.Separation of magnetic particles in aqueous dispersions with a magnet:(a,d)Fe3O4, (b, e) Fe3O4@MPS, and (c, f) Fe3O4@P(St- co-AcQAC) nanoparticles.
As shown in Figs.3a, 3b, 4a, 4b, a large number of colonies were found on the culture plates treated by both Fe3O4and Fe3O4@MPS nanoparticles,respectively,demonstrating that these two nanoparticles were not bactericidal after 6-h incubation.In contrast, the Fe3O4@P(St-co-AcQAC) nanoparticles demonstrated remarkable antimicrobial activity against both Gram-negative E.coli (Fig.3c) and Gram-positive S.epidermidis (Fig.4c), and the bacteria-killing capability for the antimicrobial magnetic nanoparticles was more pronounced against S.epidermidis.It also became evident from Figs.3c and 4c that bacteria-killing of these magnetic particles was time dependent; this is not surprising considering that the antimicrobial mode of action is contact based.After 2-h incubation with the antimicrobial magnetic particles,the number of bacteria(both E.coli and S.epidermidis)only decreased slightly (Figs.3-c1and 4-c1).Notably, the 4-h incubation led to more pronounced bacteria-killing, especially for S.epidermidis(Fig.4-c2); after 6-h incubation, only a small number of E.coli colonies were observed (Fig.3-c3) and S.epidermidis was completely killed (Fig.4-c3).
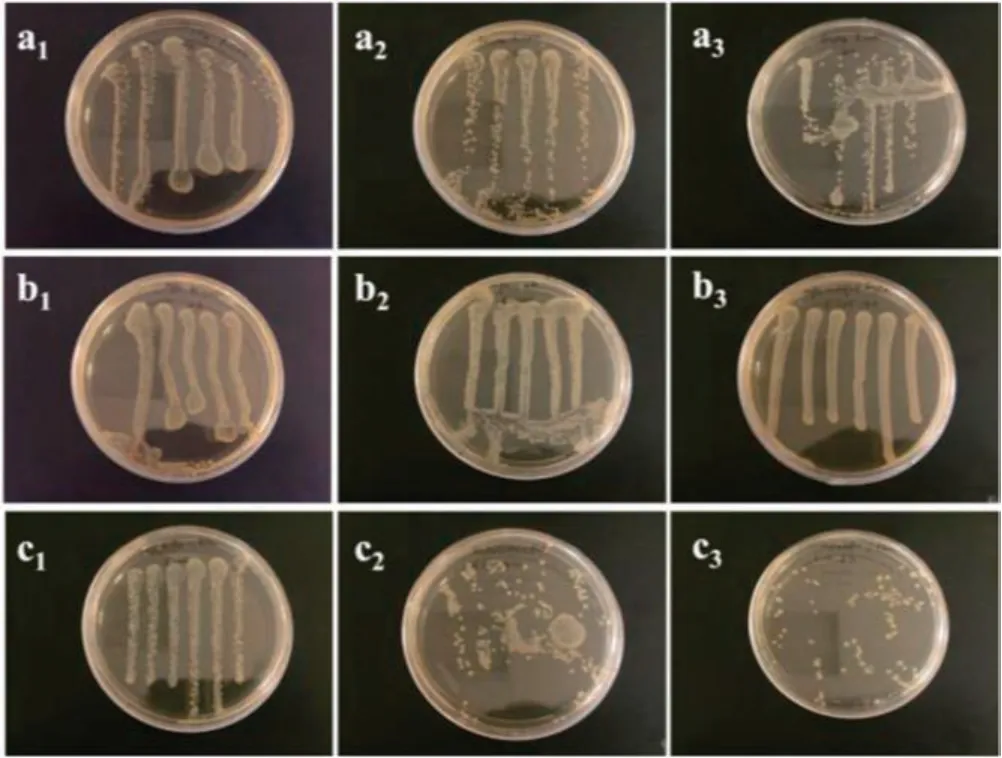
Fig.3.Antimicrobial test results for three magnetic nanoparticles: (a) Fe3O4, (b)Fe3O4@MPS,and(c)Fe3O4@P(St-co-AcQAC)against E.coli after different incubation time: (1) 2 h, (2) 4 h and (3) 6 h.

Fig.4.Antimicrobial test results for three magnetic nanoparticles, (a) Fe3O4, (b)Fe3O4@MPS and (c) Fe3O4 @P(St-co-AcQAC) against S.epidermidis after different incubation time: (1) 2 h, (2) 4 h and (3) 6 h.
Free QACs pose a serious hazard to the aquatic environment,mainly due to bacterial resistance that may be triggered by the release of free QACs [26,27].To investigate the possible effects of antimicrobial nanoparticles on the environment,we separated the supernatant of the aqueous nanoparticle dispersion and added it to the bacterial suspension for co-cultivation.500 μL of the supernatant was added into the bacterial solution, followed by 6-h incubation.As shown in Fig.5, the supernatant of the antimicrobial nanoparticle dispersion had no obvious antimicrobial efficacy,and the number of colonies was comparable to that of the control (sterile deionized water).The results clearly demonstrated that the QAC immobilized on the nanoparticle surface by polymerization did not leach out into the environment and, thus,would not cause environmental concern.
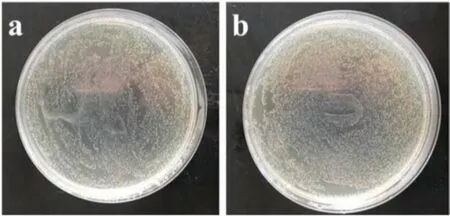
Fig.5.Antimicrobial test of (a) the supernatant (after removal of antimicrobial magnetic Fe3O4@P(St-co-AcQAC nanoparticles)and(b)sterile deionized water(the control) against E.coli.
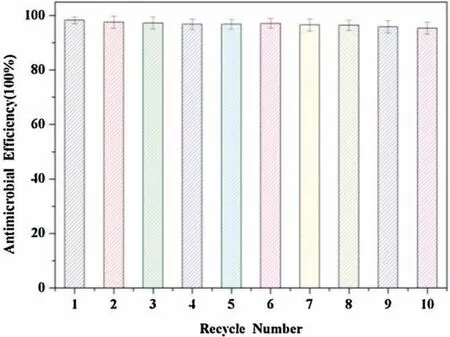
Fig.6.Antimicrobial efficacy against E.coli of Fe3O4@P(St-co-AcQAC)nanoparticles after 10 cycles of use.
We further examined the antimicrobial performance of recycled Fe3O4@P(St-co-AcQAC) nanoparticles.The recycled antimicrobial magnetic particles were incubated with a fresh bacterial sample (1 mL suspension;magnetic particle concentration:2 mg/mL) at 37°C for 6 h under mild stirring.This procedure was repeated for up to 10 times.At the end of each cycle,the Fe3O4@P(St-co-AcQAC) nanoparticles were collected by using a magnet,washed with deionized water,and sterilized for next use.As shown in Fig.6, the Fe3O4@P(St-co-AcQAC) nanoparticles exhibited high antimicrobial efficiency(≥95%)against E.coli even after 10 cycles.The test results have clearly demonstrated that the bactericidal activity of the Fe3O4@P(St-co-AcQAC) nanoparticles can be effectively maintained after multiple cycles.These antimicrobial magnetic particles appear to be promising,water-insoluble,highefficacy antimicrobial agents for water purification and disinfection.
In summary, we have designed and prepared recyclable, nonleaching antimicrobial magnetic nanoparticles through surfactant-free seeded emulsion polymerization.The QAC-containing magnetic nanoparticles have demonstrated excellent antimicrobial activity against both Gram-positive and Gram-negative bacteria, without leaching out biocidal species into the environment.The antimicrobial magnetic nanoparticles can be easily separated from aqueous dispersions and reused to kill bacteria.These recyclable antimicrobial magnetic nanoparticles may find promising applications in safe water, livestock, food production and processing.
Acknowledgment
The authors thank the Distinguished Chair in Materials Science Endowment Fund at Georgia Southern University for the partial financial support of this research.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
