TsCl-promoted sulfonylation of quinoline N-oxides with sodium sulfinates in water
Sh Peng,Yn-Xi Song,Jun-Yi He,Shn-Shn Tng,Ji-Xi Tn,Zhong Co,Ying-Wu Lin,Wei-Min He,*
a Department of Chemistry, Hunan University of Science and Engineering, Yongzhou 425100, China
b Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China
d School of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
Keywords:
Quinoline N-oxides
2-Sulfonylquinolines
Sodium sulfinates
Sulfonylation
In water
ABSTRACT
An eco-friendly protocol for the synthesis of various 2-sulfonyl quinolines/pyridines through sulfonylation of heteroaromatic N-oxides with sodium sulfinates in water at ambient temperature under metal- and oxidant-free conditions has been developed.The mild reaction conditions, high reaction efficiency, operational simplicity, short reaction time and remarkable functional-group compatibility make the developed protocol very attractive for the preparation of 2-sulfonyl N-heteroaromatic compounds.
Green and sustainable chemistry has become an all-important subject in modern chemistry,which emphasizes the development of sustainable chemical processes.With this important goal,numerous traditional synthetic reactions need to be re-designed to make them more efficient and sustainable.However, the majority of value-added chemicals were synthesized in volatile organic solvents(often highly harmful),which usually account for 90% of the mass of the reaction system.In this regard, aqueous organic reactions represent the ideal approach since they are in conformity with at least two requirements of green chemistry: “first principle:prevent waste”and“fifth principle:safer solvents and auxiliaries”.During the past years, remarkable efforts and much progress has been made in the research area of aqueous synthetic organic [1].
N-Containing compounds have continued to capture the interest of chemists worldwide due to their attractive applications[2].Among them,the quinoline frameworks are widely present in natural products, synthetic drugs, agricultural chemicals and functional organic materials[3].Therefore,the efficient construction of diverse functionalized quinoline molecules has received significant attention.Due to the easy availability of quinoline N-oxides and excellent site selectivity, it is not surprising that various C2-functionalizations of quinoline N-oxides[4]have been developed since Cui-Wu coupling reaction reported in 2009 [5].Given the central role of 2-sulfonylquinolines in organic synthesis and pharmaceutical chemistry, the sulfonylation [6]of quinoline N-oxides was one of the most important quinoline functionalization reactions [[7]].Sodium sulfinates are the most widely employed sulfonating reagents,because they are stable to ambient air and moisture as well as are safe to humans.In 2016, Han and Pan pioneered the copper-catalyzed sulfonylation of quinoline N-oxides with sodium sulfinates by using K2S2O8as the oxidant under argon atmosphere in the mixed solvent of nitromethane and dichloroethane (Scheme 1a) [7c].In 2017, Yotphan's group reported molecular iodine promoted sulfonylation of quinoline N-oxides by employing 3 equiv.of TBHP as the oxidant in N,N-dimethylformamide (Scheme 1b) [7g].Recently, our group developed the catalyst-free sulfonylation of quinoline N-oxides with 2 equiv.of K2S2O8as the oxidant in dichloroethane(Scheme 1c) [7f].Although these protocols described above have expanded the scope of sulfonylation reactions of quinoline N-oxides, from an eco-friendly and sustainable point of view, all methodologies run counter to the first(Prevention),second(Atom economy),fifth(Safer solvents and auxiliaries:explosive and toxic nitromethane, harmful N,N-dimethylformamide and dichloroethane) and sixth (design for energy efficient: high temperature and long reaction time) principles of green chemistry.Therefore,despite the remarkable advances,the establishment of a mild and eco-friendly route to construction of 2-sulfonylquinolines is still necessary.
Following our consistent program in the development of green chemistry [8], herein, we wish to report a mild, efficient and sustainable route for the synthesis of various 2-sulfonylquinolines through TsCl-promoted sulfonylation of quinoline N-oxides with sodium sulfinates at ambient temperature in water (Scheme 1d).The present method is metal-, oxidant-, and organic solvent-free,featuring mild reaction conditions, simple operation and energyefficient.
The sulfonylation reaction of quinolineN-oxide(1a)with sodium p-toluenesulfinate(2a)was initially chosen as a template reaction to optimization of the reaction conditions.Treatment of 1a and 1 equiv.of 2a in the presence of 1.5 equiv.of p-tosyl chloride(TsCl)at ambient temperature in water for 50 min gave the desired 2-sulfonylquinoline(3aa)in 90%NMR yield(Table 1,entry 1).Only a trace yield of the expected product was observed when MsCl,benzoyl chloride and oxalyl chloride were used as the activating agent (entries 2-4 vs.entry 1).Increasing the amount of TsCl to 2 equiv.did not improve the yield of 3aa(entry 5).Reduction of the amount of TsCl to 1.3 equiv.was feasible; however, further decreasing to 1 equiv.led to a lower yield (entry 7).It should be noted that no reactionwas detected in the absence of TsCl(entry 8).The investigation of the concentration of 1a (entries 9 and 10)revealed that performing the sulfonylation reaction in aconcentration of 0.4 mol/L not only led to minimum amount of water but also resulted in shortest reaction time.Performing the reaction under ultrasound conditions led to lower reaction efficiency(entry 11).
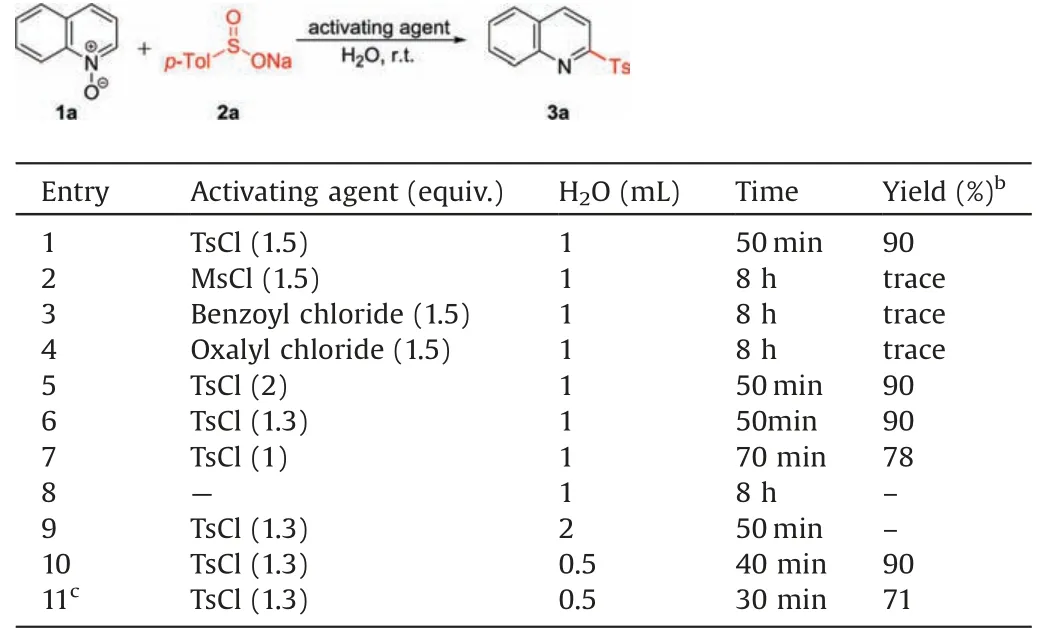
Table 1 Optimization of reaction conditions.a
After deciding the optimized reaction conditions(Table 1,entry 10),the substrate scope of the present sulfonylation reaction with respect to sodium sulfinates was explored (Scheme 2).Sodium benzenesulfinates with either electron-donating or electronwithdrawing substituents such as alkyl (3aa and 3ac), alkoxy(3ad and 3ae), halide (3af-3ah), trifluoromethyl (3ai) and ester group (3aj) underwent sulfonylation reaction efficiently to produce the expected products in good yields.Different positions of substituted groups on the phenyl ring of sodium benzenesulfinates hardly exhibited significant influence in the reaction outcome.For example, in comparison to the reaction substrates with meta- and para-substituents (3ak and 3aa), the ortho-one(3 al) led to a similar isolated yield.Sodium disubstituted benzenesulfinate, naphthalene-2-sulfinate and sodium thiophene-2-sulfinate were all compatible in this protocol, delivering the desired products in good yields (3am-3ao).Moreover, the sulfonylation reaction of a sodium aliphatic sulfinates such as sodium benzenemethane sulfinate (1p) and sodium propane sulfonate (1q) generated the desired products in good yields.Subsequently, a series of quinoline N-oxides were involved to investigate the generality of the developed approach.Quinoline N-oxides bearing diverse functional-groups were well tolerated under the optimized conditions (3ba-3oa) regardless of their steric and electronic properties.To our delight, the reaction substrates with functional-groups such as methoxyl,fluoro,chloro,and bromo all delivered the desired products in good yields(3 da and 3ea, 3ha-3ma), which extended the substrate scope of further transformation on the quinoline ring.Remarkably, this protocol was also applied for other heteroaromatic N-oxides,such as pyridine N-oxide and isoquinoline N-oxide, yielding the corresponding products (3pa and 3qa) in 67% and 71% yields,respectively.No reaction occurred when quinoxaline N-oxide was used as the substrate.
To demonstrate the practicality of the developed method, a large-scale sulfonylation reaction (5 mmol) was performed under the standard reaction conditions.Pleasingly,this reaction provided 2-sulfonylquinoline (3aa) in 86% yield (1.22 g) (Scheme 3a),showing good potential in industrial scale-up application.Finally,a one-pot sequential oxidation of quinoline (4a) followed by sulfonylation reaction to afford product 3aa was achieved(Scheme 3b).The one-pot sequential transformation is expected to be of high synthetic utility.
A plausible reaction mechanism for the formation of 2-sulfonylquinoline based on the related literature[7g]is shown in Scheme 4.At first,tautomerization of sulfinate anion A exists in equilibrium S-centered anionic intermediate B in water.Simultaneously,p-tosyl chloride acting as an activate reagent,attacks the oxygen atom of quinoline N-oxide 1 to form a reaction intermediate C.The C2 of intermediate C was regio-selectively nucleophilically attacked by sulfonyl anion B and delivered an intermediate D, which subsequently re-aromatized to form 2-sulfonylquinoline 3 along with the release of p-toluenesulfonic acid (detected by MS).
In conclusion,we have developed a general protocol to sulfonylation of quinoline N-oxides with sodium aryl, heteroaryl and alkyl sulfinates in the absence of any metal or oxidant at ambient temperature in water.Importantly, the mild reaction conditions were compatible with some pharmaceutical derivatives.The high reaction efficiency,operational simplicity,short reaction time,airand moisture-insensitive conditions and remarkable functional group compatibility make the developed protocol very attractive for the preparation of 2-sulfonyl N-heteroaromatic compounds.
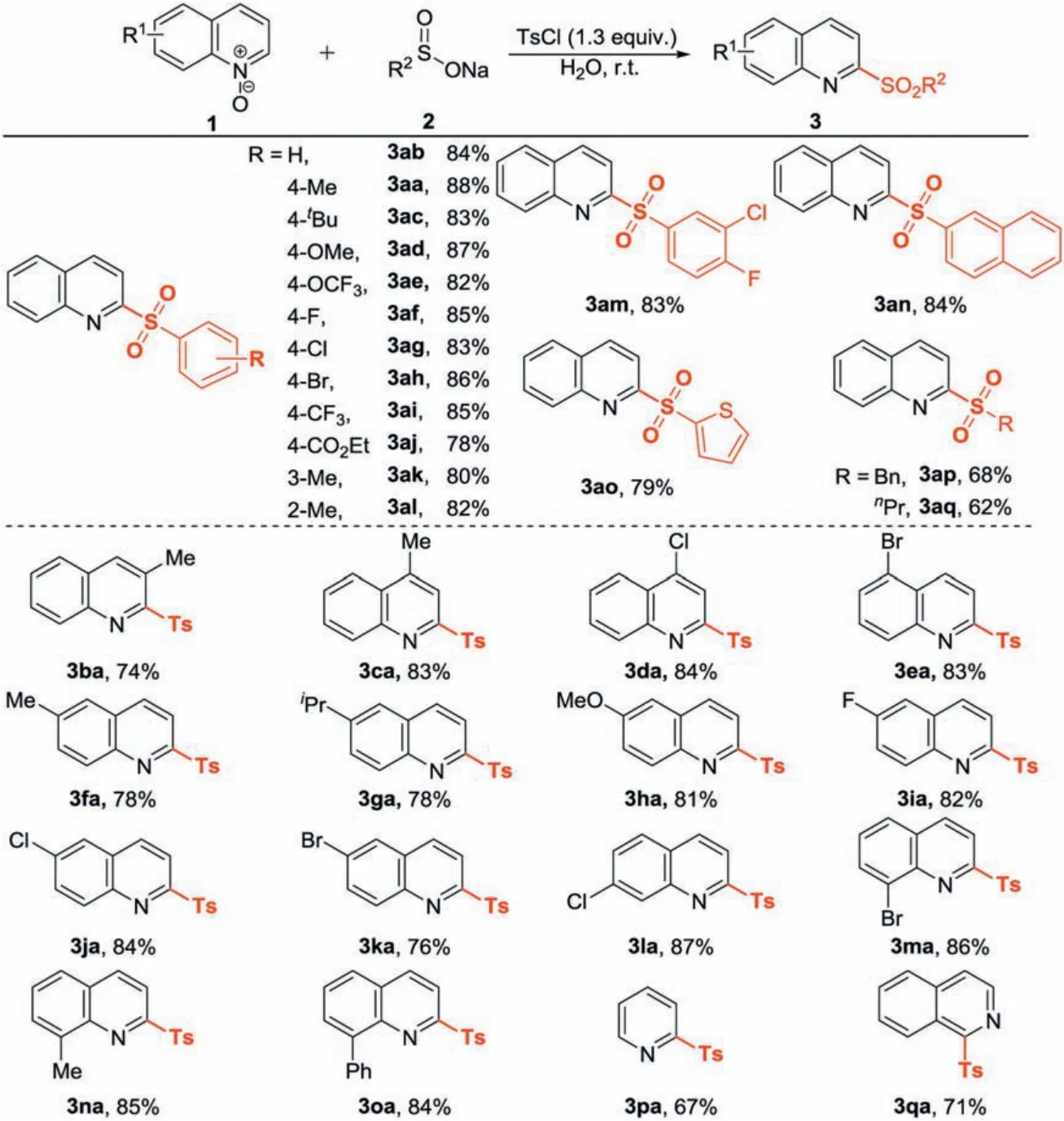
Scheme 2.Substrate scope.All reactions were carried out in a seal tube in the presence of 1(0.2 mmol),2(0.2 mmol),TsCl(0.26 mmol),H2O(0.5 mL),r.t.Isolated yields are reported here.

Scheme 3.Large-scale synthesis of 3aa.
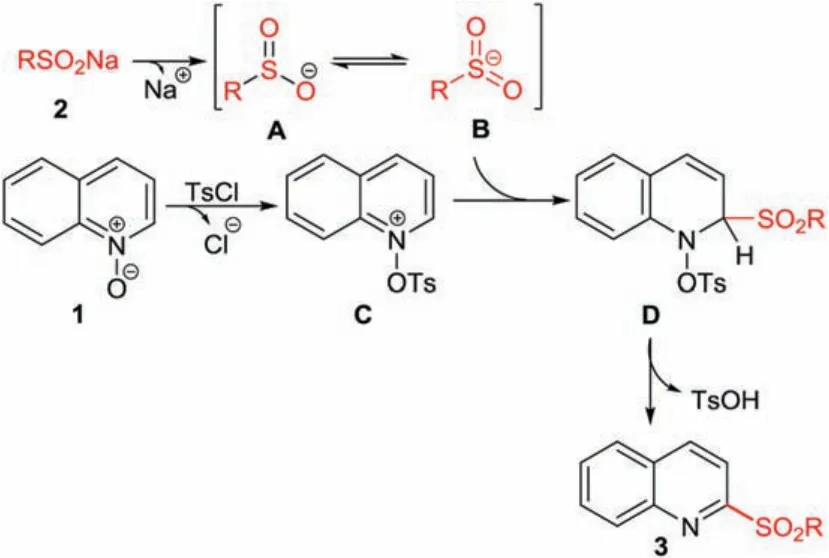
Scheme 4.Plausible reaction mechanism.
Acknowledgement
We are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (No.2019JJ20008).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.08.002.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- Post-self-repair process of neuron cells under the influence of neutral and cationic nanoparticles
- CdS nanocrystallites sensitized ZnO nanorods with plasmon enhanced photoelectrochemical performance
- A simple visual method for DNA detection based on the formation of gold nanoparticles
- Self-assembly of L-tryptophan on Cu(111)studied by low-temperature scanning tunneling microscopy
- Functional delivery vehicle of organic nanoparticles in inorganic crystals
- Facile assembly of mesoporous silica nanoparticles with hierarchical pore structure for CO2 capture
