GC-NICI-MS analysis of acetazolamide and other sulfonamide(R-SO2-NH2)drugs as pentafluorobenzyl derivatives[R-SO2-N(PFB)2]and quantification of pharmacological acetazolamide in human urine
Olga Begou ,Kathrin Drabert ,Georgios Theodoridis ,Dimitrios Tsikas
a Institute of Toxicology,Core Unit Proteomics,Hannover Medical School,Carl-Neuberg-Strasse 1,D-30625,Hannover,Germany
b Department of Chemistry,Aristotle University of Thessaloniki,54124,Thessaloniki,Greece
c BIOMIC_AUTh,Center for Interdisciplinary Research and Innovation(CIRI-AUTH),Balkan Center,10th Km Thessaloniki-Thermi Rd,P.O.Box 8318,GR 57001,Thessaloniki,Greece
Keywords:
ABSTRACT
1.Introduction
Acetazolamide (N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)acetamide,MM 222.25;CAS number 216665-38-2;see Fig.S1)is one of the oldest therapeutically used diuretic drugs.Acetazolamide is a strong inhibitor of carbonic anhydrase(CA)which catalyzes the reversible reaction between CO2and H2O to form bicarbonate and protons(CO2+H2O?HCO3-+H+)[1].Acetazolamide and other sulfonamide(R-SO2-NH2)drugs(see Fig.S1)are widely used for the treatment of glaucoma,mountain sickness,sleep apnea,epilepsy and hypertension[1].The pharmacological action of acetazolamide and relatives in several organs such as eyes,brain and kidney is based on the inhibition of CA activity.In glaucoma and epilepsy,inhibition of CA activity by acetazolamide results in reduction of intraocular and cerebral pressure.Inhibition of CA activity in the kidney results in enhanced excretion ofand inorganic nitrite[2,3].Acetazolamide-induced loss of nitrite may be of particular importance becauseis considered an abundant reservoir of the endogenous potent vasodilator nitric oxide(NO).The urinary nitrate-to-nitrite molar ratio,i.e.,UNOxR,may be a useful measure of renal CA-dependent reabsorption of urinary nitrite in humans[4].Besides its therapeutic use,acetazolamide gained importance as a potential performance-enhancing drug in sport doping[5].
Several analytical methods based on liquid chromatography(LC)[6-12]and gas chromatography(GC)[13-21]have been reported for the determination of acetazolamide and related sulfonamides in various biological samples including plasma and urine.For GC analysis,sulfonamides such as acetazolamide require conversion of their sulfonamide group to thermally stable and volatile derivatives.The amine group of the sulfonamide group of acetazolamide and many other sulfonamides is the only functional group that is accessible for chemical derivatization.Methylation of the amine group of sulfonamides has been often performed using methyl iodide(iodomethane,CH3I)[13-19].Silylation of the amine group of sulfonamides for GC has also been reported[20,21].
Pentafluorobenzyl(PFB)bromide(PFB-Br)is a versatile derivatization reagent in chromatography(GC,LC)and mass spectrometry(MS)[22].It can be used for the derivatization of nucleophilic substances and inorganic and organic anions both in aqueous phase such as plasma and urine and in anhydrous organic solvents such as acetonitrile[2,23-25].Analogous to the derivatization of amine groups such as those in creatinine with PFB-Br[24],we assumed that PFB-Br may be useful as a derivatization reagent for the amine group of acetazolamide and other sulfonamides in GC-based analytical methods.The reaction of R-SO2-NH2with PFB-Br may lead to the formation of mono-PFB derivatives(R-SO2-NHPFB)and di-PFB derivatives(R-SO2-N(PFB)2).To the best of our knowledge,the derivatization of acetazolamide or other sulfonamides with PFB-Br has not been reported thus far.
In the present work,we demonstrate that PFB-Br is useful for the N-alkylarylation of the sulfonamide group of acetazolamide representing the group of sulfonamides.We found that the derivatization of acetazolamide is best performed in anhydrous acetonitrile using N,N-diisopropylethylamine(i.e.,Hünig base)as the catalyst under very mild conditions(30 ℃)(Fig.S1).As acetazolamide is excreted in the urine without metabolization,we developed and validated a GC-MS method for the quantitative determination of acetazolamide in human urine using commercially available[acetylo-2H3]acetazolamide as the internal standard(IS).Negative-ion chemical ionization(NICI)of the PFB derivative of acetazolamide provided the lowest limit of detection(LOD)thus far reported for acetazolamide.We also tested the utility of PFB-Br for the derivatization of other sulfonamide drugs.The applicability of the method in clinical-pharmacologic settings was demonstrated upon ingestion of a 250 mg acetazolamide tablet by a healthy volunteer.
2.Experimental
2.1.Materials and chemicals
[acetylo-2H3]Acetazolamide(d3-AZM;declared amount,1 mg)was obtained from Hycultec(Beutelsbach,Germany)and was diluted in dimethyl sulfoxide(DMSO)in the original flask.The chemical and isotopic purity of d3-AZM had not been declared by the supplier.2,3,4,5,6-Pentafluorobenzyl bromide,N,N-diisopropylethylamine,pentafluoropropionic anhydride(PFPA,>99%),2,3,4,5,6-pentafluorobenzoyl chloride(>99%),unlabeled acetazolamide(d0-AZM;>99%)and all the other sulfonamides(>98%)used in the present study were obtained from Sigma-Aldrich(Darmstadt,Germany).DMSO and acetonitrile(GC grade;dried over molsieve)were purchased from Merck(Darmstadt,Germany).Toluene(p.a.)was purchased from Baker(Deventer,The Netherlands).The silylation reagent N,O-bis(trimethylsilyl)trifluoroacetamide(BSTFA,>99%)was obtained from Macherey-Nagel(Düren,Germany).
2.2.Safety considerations
PFB-Br and PFPA are corrosive.PFB-Br is an eye irritant.Inhalation and contact with skin and eyes should be avoided.All work should be performed in a well-ventilated fume hood.
2.3.Preparation of calibration standards
Acetazolamide is freely soluble in DMSO.Solutions and dilutions of acetazolamide and all other sulfonamides were prepared in DMSO.Stock solutions of d0-AZM(100 mM)and d3-AZM(50 mM)and their working dilutions(each 10 mM)were stored at 8 ℃ and-18 ℃,respectively.
2.4.Biological samples and preparation of urine samples
Acetazolamide-free urine samples used in method development and validation were obtained from healthy volunteers after conformed consent.These volunteers did not ingest acetazolamide at least two weeks before.Samples(1 mL aliquots)were kept frozen at-18 ℃ until analysis.Prior to sample derivatization,urine samples were thawed and centrifuged(3350×g,5 min).
In quantitative analyses,50μL aliquots of the clear urine supernatants were placed in 1.8 mL glass vials and 5μL aliquots of a 10 mM solution of d3-AZM in DMSO were added.Urine and DMSO were evaporated to dryness under a stream of nitrogen gas.To remove effectively remaining water,ethanol(100μL)was added,the vials were vortex-mixed for about 10 s and then ethanol was evaporated to dryness under a stream of nitrogen gas.
2.5.Derivatization procedure with PFB-Br in water-free phase
Solid residues were dissolved in anhydrous acetonitrile(100μL).Then,10μL of N,N-diisopropylethylamine and 10μL of 30 vol%PFBBr in anhydrous acetonitrile were added,the glass vials were tightly sealed and vortex-mixed for about 10 s.Subsequently,the samples were heated for 60 min at 30 ℃ on a thermostatic block model MBT250-4 from Kleinfeld Labortechnik(Gehrden,Germany).After cooling to room temperature the samples were evaporated again to dryness under a stream of nitrogen gas and the residues were reconstituted with 200μL aliquots of toluene and vortexed for 2 min.Clear and particle-free aliquots(about 180μL)of the toluene phases were transferred into 1.8 mL autosampler glass vials equipped with 200μL microinserts for GC-MS analysis as described below.
2.6.Validation of the method
The GC-MS method was validated for acetazolamide in urine samples donated by three healthy humans.Pharmacologically relevant concentrations ranges(0-1000μM acetazolamide)were used.Validation experiments included linearity,accuracy(recovery,%)and precision(RSD,%).For the sake of clarity,the validation experiments are described in detail in the sections Results and Discussion.
2.7.GC-MS conditions
GC-MS analyses were performed on a single-quadrupole mass spectrometer model ISQ directly interfaced with a Trace 1310 series gas chromatograph equipped with an autosampler AS 1310 from ThermoFisher(Dreieich,Germany).The gas chromatograph was equipped with a 15 m long fused-silica capillary column Optima 17(0.25 mm I.D.,0.25μm film thickness)from Macherey-Nagel(Düren,Germany).In quantitative analyses,the following oven temperature program was used with helium(at a constant flow rate of 1 mL/min)as the carrier gas:1.0 min at 90 ℃,then increased to 250 ℃ at a rate of 35 ℃/min and to 320 ℃ at a rate of 35 ℃/min,respectively.The column was held at 320 ℃ for 6 min.Interface,injector and ion-source were kept constant at 260 ℃,200 ℃ and 250 ℃,respectively.Electron energy was set to 70 eV and electron current to 50μA.Methane(2.4 mL/min)was used as the reagent gas for NICI.Aliquots(1μL from the toluene phase)were injected in the splitless mode by means of the autosampler.Quantification of acetazolamide was performed in the NICI mode by selected-ion monitoring(SIM)the ions with m/z 581 and m/z 83 for d0-AZM and m/z 584 and m/z 86 for d3-AZM(IS)with a dwell-time of 50 ms for each ion.The electron multiplier voltage was set to 2025 V.Deviations from the conditions described above are mentioned in the sections Results and Discussion.
2.8.Data analysis and presentation
If not otherwise specified,quantitative analyses were performed in triplicate.Unpaired t-test was used to check statistical significance.Values are presented as mean±SD.
3.Results
3.1.Testing derivatization methods for acetazolamide
Acetazolamide(Fig.S1)and methazolamide have a single functional group that is accessible to chemical derivatizaton.This is the amine group of the sulfonamide functionality;other sulfonamides such as dorzolamide possess additional derivatizable functional groups.We tested the utility of several derivatization agents for acetazolamide representing the group of sulfonamide drugs for GC-NICI-MS analysis.These derivatization reagents included pentafluorobenzyl bromide(PFB-Br),pentafluorobenzoyl chloride(PFBzoyl-Cl)and pentafluoropropionic anhydride(PFPA).Previously,we found these reagents to be useful for amine groupscontaining substances such as creatinine[24],dimethylamine(DMA)[26]and amino acids[27],respectively.
We also tested BSTFA,which is a versatile reagent for different functionalities including hydroxyl and amine groups.In our tests,BSTFA was found to be not suitable,because derivatization(60 min,60 ℃)resulted in loss of the acetyl group in d0-AZM and d3-AZM,thus not allowing quantitative analysis of acetazolamide(data not shown).Procedures previously reported for the derivatization of methyl esters of amino acids[27]revealed that PFPA is not useful for the derivatization of acetazolamide(data not shown).By using a previously reported procedure for the extractive derivatization of urinary DMA with PFBzoyl-Cl[26],acetazolamide could also not be derivatized(data not shown).
PFB-Br is a versatile derivatization reagent in nucleophilic substitution reactions[22].Its utility in GC-MS has been demonstrated for different classes of substances in aqueous and anhydrous systems[22].We found that acetazolamide cannot be derivatized in aqueous buffered solutions including phosphate and Tris buffer of neutral pH value and in human urine(pH range,5.5-7.8)(data not shown).In contrast,acetazolamide was found to be readily derivatized with PFB-Br in anhydrous acetonitrile using N,N-diisopropylethylamine as the base catalyst under very mild conditions and short derivatization times as has been reported for carboxylic acids including prostaglandins[28],i.e.,warming the derivatization mixture for 60 min at 30 ℃.Higher derivatization temperatures tested(i.e.,40 ℃,50 ℃,60 ℃)were found to provide yellow-tobrownish colored derivatization solutions,most likely due to decomposition reactions(data not shown).For these reasons we selected PFB-Br derivatization of acetazolamide in anhydrous acetonitrile and tested the utility of this derivatization for methazolamide and other sulfonamide drugs.
3.2.GC-NICI-MS characterization of the PFB derivatives of sulfonamides
Each 50 nmol of d0-AZM and d3-AZM were mixed,derivatized with PFB-Br(60 min,30 ℃),the sample was evaporated to dryness and the residue was reconstituted with toluene as described in Experimental section.The appearance of the originally clear and colorless solution did not change upon derivatization.GC-NICI-MS mass spectra were obtained by injecting 1μL aliquots of the toluene phases(180μL)each corresponding to 250 pmol of d0-AZM and d3-AZM(assuming quantitative derivatization).GC-NICI-MS analysis of the sample in scan mode resulted in few GC peaks.The mass spectrum obtained from the GC peak of the PFB derivatives of the mixture of d0-AZM and d3-AZM eluting at about 8.35 min is shown in Fig.1.It contains five pairs of ions differing by 3 Da each,most likely due to the presence of the intact acetyl groups in the PFB derivatives of d0-AZM and d3-AZM.
The largest anions at m/z 581 and m/z 584 in the mass spectrum can be assigned to the N,N-dipentafluorobenzyl derivatives of d0-AZM(R-SO2-N(PFB)2;molecular mass,582)and d3-AZM(R(d3)-SO2-N(PFB)2;molecular mass,585),respectively.The ions m/z 375 and m/z 357 are common to the d0-AZM and d3-AZM derivatives and are likely produced from breaking of the derivatized sulfonamide groups.Presumably,m/z 357 derives from m/z 375 by loss of a water molecule(H2O,18 Da).The ion m/z 167 is also common to d0-AZM and d3-AZM and is due to[C6F5]-.The ions m/z 83 and m/z 86 carry each one acetyl group and their structures are likely to be[CH3CO-N-CN]-and[CD3CO-N-CN]-,respectively.The most intense and common ion in the mass spectra of d0-AZM-(PFB)2and d3-AZM-(PFB)2is m/z 58 and is likely to be the negatively charged thioethene epoxide[(CH=CH)S]-.
It is worth mentioning that the retention time of the PFB estermethoxime(MO)-trimethylsilyl(TMS)ether derivative of prostaglandin E2(i.e.,PGE2-PFB-MO-(TMS)2;molecular mass,705)under the same GC-MS conditions was 8.32 min.This observation indicates that the C34-species PGE2-PFB-MO-(TMS)2is as volatile as the C18-species d0-AZM-(PFB)2and d3-AZM-(PFB)2,presumably because of its two TMS ether functionalities.
The mass spectrum obtained from the GC peak of the PFB derivative of methazolamide(retention time,7.8 min)is shown in Fig.2.The ions at m/z 357 and m/z 375 in this mass spectrum indicate that dorzolamide reacts with PFB-Br to form the N,Ndipentafluorobenzyl derivative R-SO2-N(PFB)2(molecular mass,596)analogous to acetazolamide.However,the R-SO2-N(PFB)2derivative of methazolamide ionizes quite differently than the RSO2-N(PFB)2derivative of acetazolamide.The intense ion at m/z 415 is likely to be due to[M-PFB]-.The most intense ion at m/z 220 in the mass spectrum of the N,N-dipentafluorobenzyl derivative of methazolamide is likely to be due to[M-NH(PFB)2]-which is absent in the mass spectrum of N,N-dipentafluorobenzyl derivative of acetazolamide.The reason for this disparity could be the formation of the electrically neutral[M-NH(PFB)2]or radical species[MNH(PFB)2]·.A likely explanation of the remarkable differences in the NICI mass spectra of the N,N-dipentafluorobenzyl derivatives of the structurally closely acetazolamide and methazolamide and the shorter retention time of the methazolamide derivative could be the methyl group on the amidic N atom of methazolamide.This methyl group makes the derivative more lipophilic,volatile,presumably more stable,and influences the NICI as well(see Fig.S2).
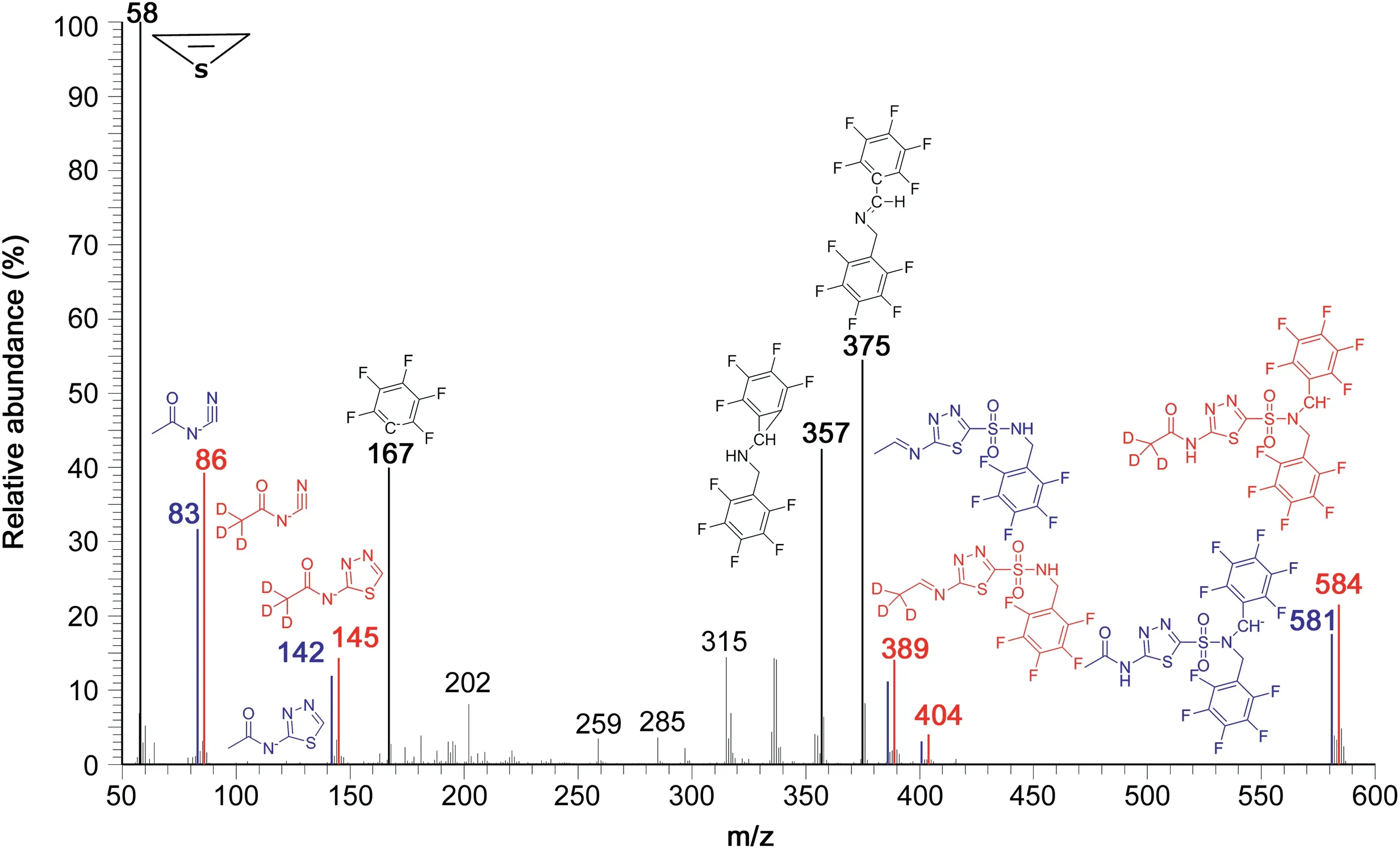
Fig.1.GC-NICI-MS spectrum obtained from a mixture containing each about 250 pmol of unlabeled acetazolamide(d0-AZM)and[acetylo-2H3]-acetazolamide(d3-AZM)as pentafluorobenzyl(PFB)derivatives eluting at about 8.35 min.Inserts indicate the proposed structures for the anions of the PFB derivatives of d0-AZM and d3-AZM.Mass fragments differing by 3 Da are shown in blue and red.For simplicity the charge of the anions is not indicated.
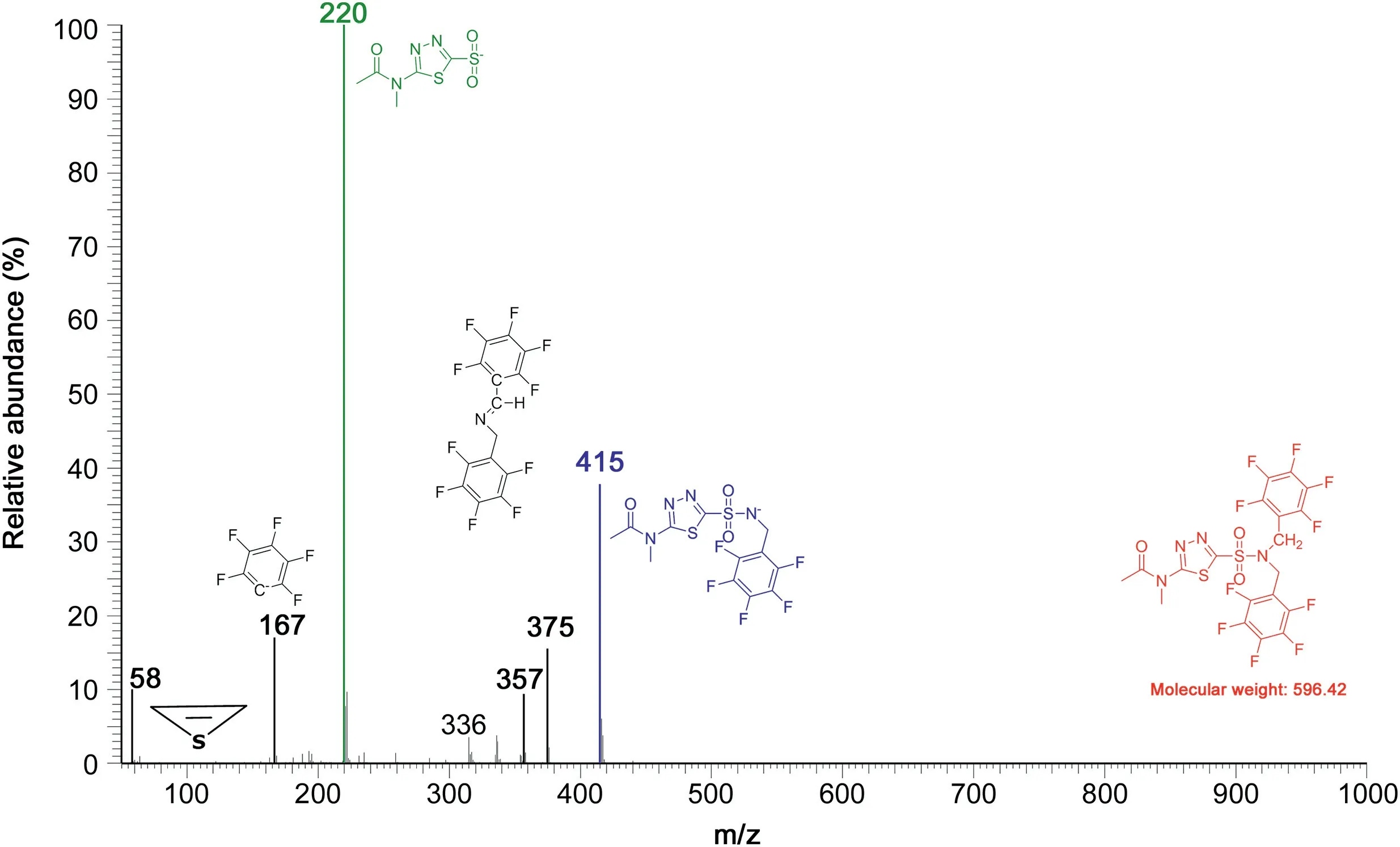
Fig.2.GC-NICI-MS spectrum obtained from unlabeled methazolamide(about 250 pmol)as pentafluorobenzyl derivative eluting at about 7.8 min.Insets indicate the proposed structures for the PFB derivative of methazolamide and of mass fragments.
The mass spectrum obtained from the GC peak of the PFB derivative of dorzolamide(retention time,10.2 min)is shown in Fig.S3.The ions at m/z 357 and m/z 375 in this mass spectrum indicate that dorzolamide reacts with PFB-Br to form the N,Ndipentafluorobenzyl derivative R-SO2-N(PFB)2(molecular mass,684)analogous to acetazolamide and methazolamide.Yet,in contrast to the mass spectra of the PFB derivatives of acetazolamide and methazolamide,the NICI mass spectrum of the dorzolamide derivative does not contain the anion at m/z 58([(CH=CH)S]-).This could be due to the lack of the thiadiazo structure in dorzolamide.
3.3.GC-NICI-MS characterization and standardization of[acetylo-2H3]acetazolamide(d3-AZM)
Declared amounts of commercially available stable-isotope labelled analogs,especially of those delivered in very small,not(accurately)weighable amounts,may differ from those obtained experimentally.The chemical and isotopic purity of the d3-AZM used in our study had not been declared by the supplier and was,therefore,determined experimentally as follows.
Aliquots(5μL)of separate 10 mM solutions of d3-AZM and d0-AZM in DMSO were derivatized and analyzed separately.Aliquots(1μL)of the toluene phases assuming to contain 250 pmol of d3-AZM or d0-AZM were injected on three different days each in triplicate per day and SIM of m/z 581,m/z 584,m/z 83 and m/z 86 was performed.The peak area ratio(PAR)values for d3-AZM in the absence of synthetic d0-AZM were determined to be(mean±SD,n=9)0.00257±0.001 for the PAR m/z 581 to m/z 584 and 0.00477±0.002 for the PAR m/z 83 to m/z 86.These data indicate an isotopic purity of the commercially available d3-AZM preparation of about 99.7%at2H and no appreciable contribution of d0-AZM to d3-AZM in the commercially available d3-AZM.The PAR values for d0-AZM in the absence of d3-AZM were determined to be(mean±SD,n=9)0.0233±0.001 for the PAR m/z 584 to m/z 581 and 0.00122±0.0007 for the PAR m/z 86 to m/z 83,indicating no appreciable contribution of d3-AZM to d0-AZM.
The standardization procedure of d3-AZM and the linearity of the method were performed in matrix-free samples,i.e.,by using solutions of d3-AZM(10 mM)and d0-AZM(10 mM)in DMSO.Standard curves were prepared on three different days by derivatizing mixtures containing a fixed amount of d3-AZM(50 nmol;5μL)and different amounts of d0-AZM(0 nmol,0μL;40 nmol,4μL;80 nmol,8μL;120 nmol,12μL;160 nmol,16μL;200 nmol,20μL)and by analyzing the samples by GC-MS in the SIM mode:m/z 83,m/z 86,m/z 581 and m/z 584 each a dwell-time of 50 ms.Triplicate injections were performed for each concentration point.The concentration of d3-AZM in the toluene phases(each 200μL)was considered(assuming quantitative derivatization and extraction)to be 250μM.The concentrations of do-AZM in the toluene phases(each 200μL)were considered(assuming quantitative derivatization and extraction)to be 0,200,400,600,800 and 1000μM.These concentrations were chosen in order to simulate pharmacologically relevant concentrations for acetazolamide in human urine.
Linear regression analysis between the PAR of m/z 581 to m/z 584(y1)measured on three days and the nominal acetazolamide concentration (x1) yielded the regression equation y1=0.0581(±0.016)+0.0031(±0.00003)x1(r=0.997).Linear regression analysis between the PAR of m/z 83 to m/z 86(y2)measured and the nominal acetazolamide concentration derivatized (x2) yielded the regression equation y2=0.1024(±0.0190)+0.0028(±0.00003)x2(r=0.994).The reciprocal slope values of the regression equations provide the concentration of the internal standard d3-AZM.In this experiment,the d3-AZM concentration was calculated to be 323μM using the PAR of m/z 581 to m/z 584,and 357μM using the PAR of m/z 83 to m/z 86.Both values differ from the nominal d3-AZM concentration of 250μM considering the delivered amount of d3-AZM.The concentration of the internal standard was corrected using the experimentally observed data.Accuracy and precision data obtained using the PAR of m/z 581 to m/z 584(corrected d3-AZM concentration,323μM)and the PAR of m/z 83 to m/z 86(corrected d3-AZM concentration,357μM)are summarized in Table S1.GC-MS chromatograms from the analysis of acetazolamide(at 600μM)in DMSO and urine are shown in Fig.S4.
Linear regression analysis between the corresponding PAR values of m/z 83 to m/z 86(y)and the PAR values of m/z 581 to m/z 584(x)resulted in the regression equation y=0.05(±0.02)+0.913(±0.009)x(r=0.995).The slope value of this regression equation is very close to the ratio of the corrected concentrations of d3-AZM,i.e.,323/357=0.905.In method validation experiments and in quantitative analyses of acetazolamide in urine samples,the above mentioned corrected d3-AZM concentrations were used.
The retention time of the R-SO2-N(PFB)2derivatives in the standardization experiment described above was determined to be(mean±SD,n=124)8.33±0.008 min(RSD,0.09%)for d3-AZM and 8.35±0.007 min(RSD,0.09%)for d0-AZM(paired t-test,P<0.0001).The ratio of the retention times of d0-AZM and d3-AZM was calculated to be(mean±SD)1.002±0.001(RSD,0.1%),indicating a highly reproducible GC behaviour of the R-SO2-N(PFB)2derivatives of AZM.The PFB derivative of d3-AZM eluted constantly in front of the PFB derivative of d0-AZM,indicating an interaction of the acetyl groups of the PFB derivatives of d3-AZM and d0-AZM with the stationary phase of the GC column.
3.4.Accuracy and precision of the method for urinary acetazolamide
Method validation was performed using urine samples from three healthy volunteers.Native urine samples were used without dilution,yet centrifugation was performed to remove solid material.Aliquots(50μL)of clear supernatants were used throughout.The internal standard d3-AZM was added to urine samples at a final added concentration to cover a pharmacologically relevant concentration range(0-1000μM).The method was validated on three days in triplicate for each concentration using the SIM of m/z 83,m/z 86,m/z 581 and m/z 584.d0-AZM was calculated by multiplying the PAR of m/z 581 to m/z 584 with the d3-AZM concentration of 323μM and the PAR of m/z 83 to m/z 86 with the d3-AZM concentration of 357μM(see above).The results from the validation of the GC-MS method for acetazolamide in the human urine samples from three healthy subjects are summarized collectively in Table 1.In the urine samples spiked with d3-AZM only,the concentration of d0-AZM was determined to be around 1μM,likely originating from unlabeled acetazolamide(d0-AZM)present in the commercially available d3-AZM preparation(see above).The PAR values(y)were linear in the entire concentration range of added AZM(x).Precision(RSD)ranged between 5.3%and 12.9%for the spiked urine samples.Accuracy(recovery)ranged between 97.8% and 109.1% for AZM added to the urine samples.These data indicate that the GC-MS method is precise and accurate for the measurement of AZM in human urine.
3.5.Limit of detection(LOD)of the method for acetazolamide
For the determination of the LOD value of the GC-MS method,50 nmol of d0-AZM and 50 nmol of d3-АΖМ(each 5μL of two separate 10 mM solutions in DMSO)were combined and derivatized as described above.The residue was reconstituted in 200μL toluene aliquots corresponding to an amount of 250 pmol per 1μL.Subsequently,serial 1:1-dilutions(by volume)with toluene were performed to construct samples containing 125,62.5,31.3,15.6,7.8,3.9 pmol per 1μL toluene.Each 1μL of the samples was injected in triplicate and analyzed by SIM of m/z 83,m/z 86,m/z 581 and m/z 584.
The PAR values of m/z 581 to m/z 584 and of m/z 83 to m/z 86 were determined to be(mean±SD,n=21)0.745±0.011 and 0.797±0.035(P<0.0001,Wilcoxon test),respectively.The ratio of the signal-to-noise(S/N)value of the peak areas of m/z 581 and m/z 584 was determined to be(mean±SD,n=21)1.33±1.43.The S/N values of the peak areas of m/z 83 and m/z 86 was calculated to be(mean±SD,n=21)1.16±0.57.The S/N values of m/z 581,m/z 584,m/z 83 and m/z 86 were correlated with the injected amounts of d0-AZM and d3-AZM derivatives(P<0.0001 for all).The corresponding Spearman correlation coefficients were 0.948,0.916,0.940 and 0.918.Linear regression analysis between the S/N values of m/z 581,m/z 584,m/z 83 and m/z 86(y)and the injected amounts of d0-AZM and d3-AZM(x,pmol)resulted in the regression equations y=-395+53x(r=0.944),y=601+30x(r=0.633),y=68+2.1x(r=0.876),and y=83+1.98x(r=0.813),respectively.
The S/N values of the lowest injected amount(each 3.9 pmol of d0-AZM and d3-AZM)were determined to be 38.7±3.2(RSD,8.3%)for m/z 581,41.0±8.0(RSD,19.5%)for m/z 584,18.3±4.55(RSD,24.6%)for m/z 83,and 14.7±6.7(RSD,45%)for m/z 86.These data indicate that SIM of m/z 581 and m/z 584 is associated with a higher precision compared to SIM of m/z 83 and m/z 86 for very low AZM concentrations.Extrapolation of the mean S/N value of 39 to an S/N value of 3(for m/z 581)yields an LOD value of about 0.3 pmol(300 fmol,67 pg)for the d0-AZM derivative(SIM of m/z 581,m/z 584,m/z 83 and m/z 86;50 ms dwell-time for each ion).

Table 1Inter-and intra-day GC-MS validation data for acetazolamide in urine samples of three healthy volunteers(1,2,3)using SIM of m/z 581,584,83 and 86.
3.6.Limit of quantitation(LOQ)for acetazolamide in human urine
The LOQ of the method for urinary acetazolamide was determined in the same urine samples(each three 50μL aliquots)used in the method validation.In this experiment,urine samples were spiked with d0-AZM to reach final added concentrations of 0,25,50,100,150 and 200μM.The concentration of d3-AZM added to the urine samples was 250μM.Each toluene extract was injected three times and SIM of m/z 581,m/z 584,m/z 83 and m/z 86 with 50 ms dwell-time for each ion was performed.
Plotting the PAR of m/z 581 to m/z 584(y1)versus the concentration of d0-AZM added to urine(x1,μM)yielded the regression equation y1=0.018+0.00381x1(r=0.9983).Plotting the PAR of m/z 83 to m/z 86(y2)versus the concentration of d0-AZM added to urine (x2, μM) yielded the regression equation y2=0.018+0.0035x1(r=0.9943).The reciprocal slope values correspond to d3-AZM concentrations of 262μM and 283μM,respectively,which are close to the nominal concentration of 250μM.Acetazolamide was determined in the urine samples of this experiment with a precision ranging between 1.9% and 7%,and with a recovery ranging between 101%and 120%.The lowest added d0-AZM concentration of 25μM was determined with a precision of 5%(using m/z 581 and m/z 584)and 7%(using m/z 83 and m/z 86).The corresponding recovery values were 120.5% and 120%.These data suggest that the LOQ value for acetazolamide in 50μL aliquots human urine samples is 25μM or lower using SIM of m/z 581,m/z 584,m/z 83 and m/z 86.The results of this experiment are summarized in Table 2.
3.7.Stability of PFB derivatives of acetazolamide in toluene
The stability of the PFB derivatives of d0-АΖМa(chǎn)nd d3-AZM in toluene extracts from 50μL aliquots of a human urine sample spiked with 0,250,500,750 and 1000μM of d0-AZM and with the fixed concentration of 250μM of d3-АΖМ(see above)were analyzed immediately after sample work up and after storage at room temperature(about 18 ℃)for 21 days.
Linear regression analysis between the PAR values of m/z 581 to m/z 584(y1)and d0-АΖМconcentration in urine(x1)resulted in the regression equations y1=0.074+0.0036x1(r=0.9950)for day 1 and y1=-0.006+0.0039x1(r=0.9969)for day 21.Linear regression analysis between the PAR values of m/z 83 to m/z 86(y2)and the d0-АΖМconcentration in urine(x2)resulted in the regression equations y2=0.135+0.0034x2(r=0.9877)for day 1 and y2=0.011+0.0037x2(r=0.9965)for day 21.Considering the data of all samples of this experiment,the peak area(arbitrary unit,a.u.)of the d3-АΖМderivative(m/z 584)decreased by a mean factor of 2.6(mean±SEM;from 7,186,105±71,508 a.u.on day 1 to 2,807,011±98,903 a.u.on day 21).The corresponding values for the peak area of m/z 86 decreased by a mean factor of 1.5(mean±SEM;from 4,379,094±86,864 a.u.to 3,001,832±183,046 a.u.).The PAR of m/z 86 to m/z 584 was(mean±SEM)0.586±0.022 on day 1(PAR1d)and 1.096±0.039 on day 21(PAR21d),resulting in a PAR21d/PAR1dratio of 1.94±0.16.These data indicate that the PFB derivatives of d0-АΖМ(from urine samples spiked with 0-1000μM d0-АΖМ)and d3-АΖМ(from the same urine samples spiked at 250μM)in toluene are relatively stable when stored for 21 days at 18 ℃.
3.8.Biomedical application
We tested the usefulness of our GC-MS method in the setting of a pilot study.One healthy volunteer(female,29 years of age,70 kg),being an author of this article,ingested 250 mg acetazolamide(one tablet Acemit?from medphano,Germany).Before(time zero)and after(up to 5 h)drug administration urine samples were collected in polypropylene bottles pre-cooled in an ice bath.The pH of the urine samples was measured,the urine specimens were aliquoted,and the samples were analyzed for nitrate,nitrite and creatinine with previously reported GC-MS methods after acidification by using a 20 vol%acetic acid solution to remove CO2/NaHCO3/Na2CO3[2,3].The urinary concentration of acetazolamide was measured in 50μL aliquots as described in this article by SIM of m/z 581,m/z 584,m/z 83 and m/z 86.The concentration of the internal standard was250μM in all urine samples.Urinary excretion of nitrate,nitrite,creatinine and acetazolamide was corrected for urinary creatinine excretion and the creatinine-corrected excretion rates are expressed asμmol analyte per mmol creatine(μmol/mmol).
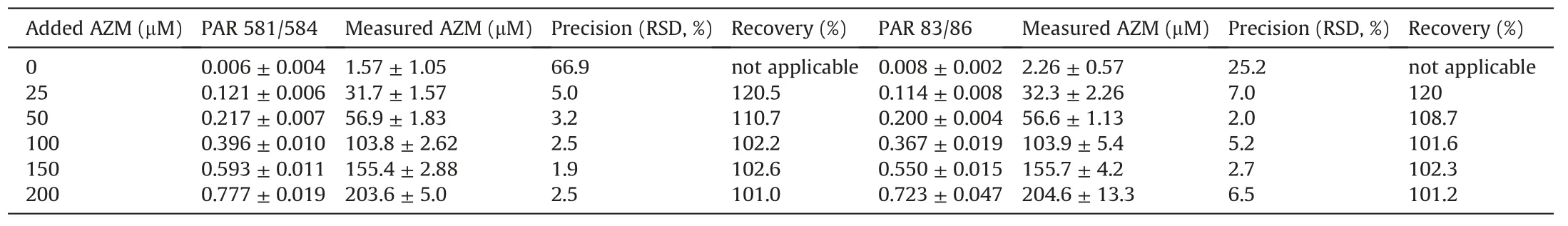
Table 2Results of the experiment performed to determine the LOQ of the GC-MS method for acetazolamide(AZM)in human urine.
After a lag-time of about 0.5 h,the creatinine-corrected excretion of acetazolamide increased almost linearly within the subsequent 1.5 h to reach the value 96μmol/mmol,which remained almost unchanged for the next 3 h(Fig.3A).SIM of m/z 581 and m/z 584,and SIM of m/z 83 and m/z 86 resulted in closely correlating acetazolamide concentrations using urine volumes of 50μL(r=0.929).Similar results were also obtained by using urine volumes of 40,30,20,10 and 5μL from this study(Fig.S5).The creatinine-corrected excretion rates of nitrite and nitrate increased in parallel to reach their maximum values about 1 h after acetazolamide ingestion(Fig.3B).The pH value of the urine samples collected in this experiment increased from 7.10 before acetazolamide ingestion to 7.65 after 1.5 h(Fig.3C).The highest concentration of acetazolamide measured in the urine samples of this experiment was 278μM.GC-MS chromatograms from analyses of urine samples from this experiment are shown in Fig.4.The acetazolamide concentrations in the urine samples measured in the present study are in agreement with previously reported data using other analytical methods such as HPLC[7,8,29,30],indicating a relatively constant and long-lasting excretion of acetazolamide.The acetazolamide concentrations in blood of humans ingested acetazolamide have also been reported to be relatively constant for many hours upon acetazolamide ingestion at therapeutical doses[31,32].
The results with respect to urinary excretion of nitrite and nitrate collaborate with our previous observations,indicating reversible acetazolamide-dependent inhibition of nitrite and nitrate reabsorption in the kidney[2,3].
4.Discussion
The sulfonamide group(R-SO2-NH2)is common to many classes of drugs.Acetazolamide is one of the oldest and structurally simplest and still therapeutically used sulfonamide drugs,administered for the treatment of many diseases[1],but also used as a potential performance-enhancing drug in sport doping[5].We have recently shown that acetazolamide ingested at therapeutic doses by healthy subjects enhanced excretion of HCO3-and inorganic nitrite(NO2-)in urine[2-4],most likely by inhibiting nitritedependent renal CA activity and by additional not yet well understood mechanisms.This newly recognized pharmacological action of acetazolamide may be of particular importance,since NO2-is considered an abundant reservoir of NO,one of the strongest endogenous vasodilators.In order to better understand the effects of acetazolamide on nitrite excretion in vivo in humans,reliable analytical methods for the quantitative measurement of acetazolamide in urine are required.
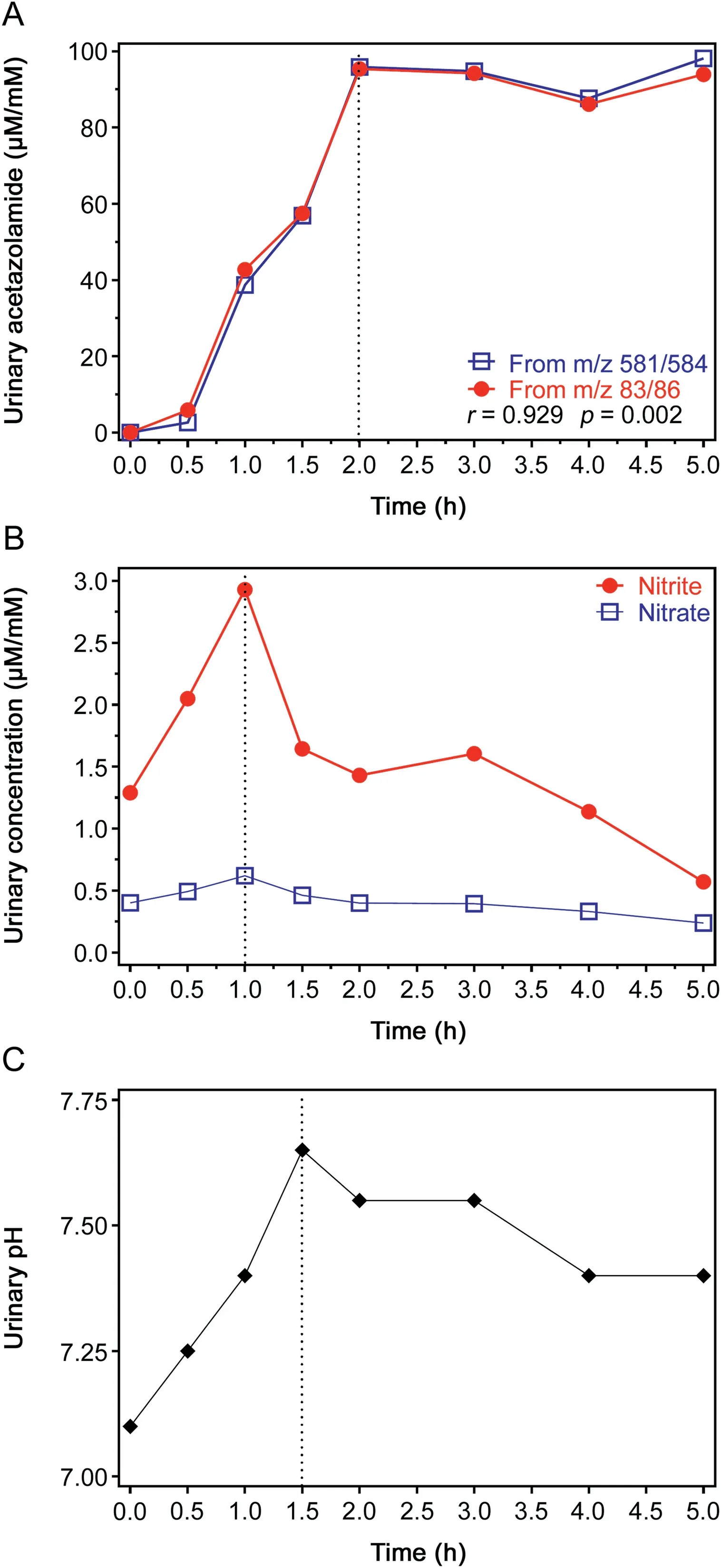
Fig.3.Creatinine-corrected urinary excretion of(A)acetazolamide and(B)nitrate and nitrite,and(C)urinary pH before(0 h)and after ingestion of a 250 mg tablet acetazolamide.The arrows indicate the time point of ingestion.
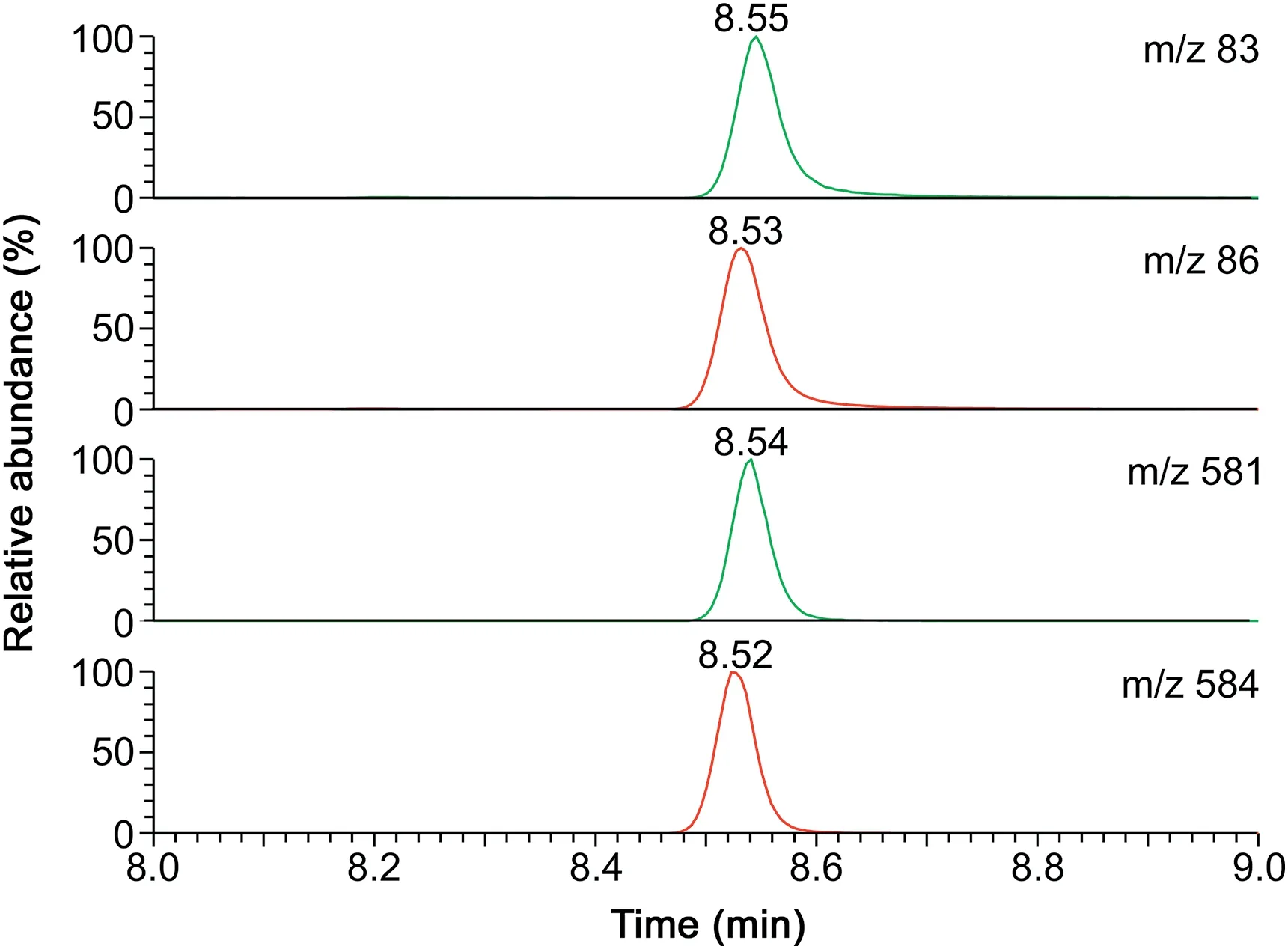
Fig.4.GC-MS chromatograms from the analysis of acetazolamide in urine(50μL)collected 2 h after ingestion of a 250 mg acetazolamide tablet(Acemit?).SIM of m/z 83,86,581 and 584 was performed.The concentration of the internal standard was 250μM.The retention time of 8.53 min is due to the use of a new GC column.
Nowadays,liquid chromatography-tandem mass spectrometry(LC-MS/MS)is one of the most efficient analytical techniques used to measure numerous analytes in biological samples,commonly with negligible labour and without analyte derivatization.This has been recently demonstrated for the measurement of acetazolamide in human plasma[6].Historically,GC-MS was the first instrumental technique combining chromatographic with MS separation and gained a wide application area in various disciplines,notably including pharmacology.Concerning acetazolamide and other sulfonamides,derivatization of the amino group is indispensable in GC-based methods.Alkylation(e.g.,with iodomethane)and silylation(e.g.,with MSTFA)of the amine group of sulfonamides including acetazolamide have been used for their analysis by GCMS and GC-MS/MS using electron ionization[14,21].In GC-MSbased methods using negative-ion chemical ionization(NICI),incorporation of fluorine(F)atoms in the analytes by using Fcontaining derivatization reagents such as PFB-Br,PFBoyl-Cl and PFPA,enhances analytical sensitivity by several orders of magnitude.In the present work,we tested these derivatizations reagents and the silylation reagent BSTFA for the GC-MS analysis of acetazolamide and other sulfonamides including methazolamide and dorzolamide.For this we used derivatization procedures that have been turned out to be very useful for some analytes including creatinine[24],dimethylamine[26]and amino acids[27].
In the present work,the most useful derivatization reagent for acetazolamide was found to be PFB-Br.PFB-Br is a versatile derivatization reagent[22].Derivatization reactions with PFB-Br can be performed directly in aqueous solutions including human plasma and urine,as has been demonstrated by us for carbonate[2],nitrite and nitrate[23],and creatinine[24].Derivatization reactions with PFB-Br can also be performed indirectly in non-aqueous solutions such as in anhydrous acetonitrile in the presence of a base serving as the catalyst.This derivatization approach is most useful for longchain carboxylic acids including the eicosanoids[28].In the present study,we found that acetazolamide can be derivatized with PFB-Br in anhydrous acetonitrile to form a di-PFB derivative[RSO2N(PFB)2]under very mild derivatization conditions(60 min,30 ℃).For the measurement of acetazolamide in urine,urine-water is completely removed under a stream of nitrogen.The solid residue is then reconstituted in anhydrous acetonitrile.After derivatization complete removal of acetonitrile,remaining base and PFB-Br reagent is performed under a stream of nitrogen gas;the remaining chargefree PFB derivative of acetazolamide is reconstituted in toluene,which is best suited as a solvent for PFB derivatives.We found that methazolamide and dorzolamide can also be converted to their di-PFB derivatives,suggesting the general use of PFB-Br for the derivatization of the sulfonamide group of organic sulfonamides.Using sulfonamides with larger molecular weights,we found that this derivatization reaction is limited by the size of the sulfonamide analyte rather than by the reactivity of the sulfonamide group towards PFB-Br.To our knowledge this is the first work to report on the derivatization of acetazolamide with PFB-Br.
Due to our interest in acetazolamide as a pharmacological potent carbonic anhydrase(CA)inhibitor[1],we developed and validated a straightforward GC-MS method for the precise and accurate quantitative measurement of acetazolamide in 50μL aliquots of human urine using a commercially available isotopically labelled internal standard,[acetylo-2H3]acetazolamide(d3-AZM).NICI of the PFB derivative of acetazolamide generates multiple ions,from which some are characteristic for acetazolamide.These anions are m/z 581,m/z 83 and m/z 58.NICI of the PFB derivative of the internal standard[acetylo-2H3]acetazolamide generates multiple ions including m/z 584,m/z 86 and m/z 58.The mass fragment at m/z 58 is obtained from the PFB derivatives of both,unlabeled acetazolamide and[acetylo-2H3]acetazolamide.Therefore,the ion m/z 58 cannot be used for quantitative measurements using[acetylo-2H3]acetazolamide as the internal standard.The method validation demonstrated that SIM of m/z 581,m/z 584,m/z 83 and m/z 86 is specific for acetazolamide and provides closely comparable results in human urine in a pharmacologically relevant concentration range of acetazolamide.
We applied the method to measure acetazolamide in urine samples collected before and for 5 h after ingestion of a 250 mg acetazolamide(1.11 mmol)tablet by a healthy young volunteer.Two hours after ingestion,the creatinine-corrected excretion rate reached a relative constant plateau at the value of 96μmol acetazolamide per mmol creatinine.This observation is in line with observations from other studies,which used HPLC with ultraviolet absorbance detection after solvent extraction of acetazolamide[5].A similar pharmacokinetic behaviour has been reported for acetazolamide in human plasma by other groups[31,32],suggesting a fairly constant long-term equilibrium between circulating and excretory acetazolamide.
In the urine samples of the present study,we also measured the concentration of nitrite and nitrate by GC-MS as PFB derivatives[23]and the pH value of the urine samples.In confirmation of previous findings of our group,we found that the creatininecorrected excretion rate of nitrite and nitrate reached temporary maximum values.This observation suggests that acetazolamide inhibited the CA-dependent reabsorption of nitrite and nitrate in the kidney[2-4].In the present study,urinary pH reached its maximum value approximately 1.5 h post-ingestion.Previously,we found that ingested acetazolamide increased the excretion of bicarbonate in the urine with a kinetics resembling that of nitrite and nitrate[2,3].All these observations suggest that excretion of nitrite and nitrate in the urine partly depends upon the CA activity in the proximal tubule of the kidney.Further,the different kinetics of urinary(and circulating)acetazolamide compared to that of nitrite,nitrate and bicarbonate suggests that acetazolamide inhibits renal CA activity in two phases.First,acetazolamide inhibits acutely and strongly the CA activity 1-2 h post-ingestion,resulting in temporary inhibition of the reabsorption of urinary nitrite,nitrate and bicarbonate in the kidney.Consecutively,acetazolamide inhibits weakly but sustainably the activity of CA;inhibition of the CA activity in this phase is presumably not associated with nitrite and nitrate reabsorption,but is apparently associated with inhibition of urinary bicarbonate reabsorption in the kidney.
Table 3 summarizes main characteristics of our GC-MS method in comparison with those of previously reported LC-and GC-based methods for the measurement of acetazolamide in human urine and other biological samples.For the quantification of acetazolamide in human urine by GC-MS,we used only 50μL urine volume,while others used up to 5 mL urine in the GC-MS methods(Table 3).
In the vast majority of reported GC-based methods,acetazolamide was analyzed after derivatization mostly using CH3I.In our GC-MS method we used PFB-Br for derivatization of the sulfonamide group.This allows for a highly sensitive measurement of acetazolamide with minimum urine volume and sample work up.The LOQ value of our method may be reduced by increasing the urine volume and by decreasing the toluene volume.For practical reasons we used 200μL aliquots of toluene for reconstituting the acetazolamide derivative.Reduction of the toluene volume is limited for technical reasons.In our GC-MS system the minimum solvent volume is about 50μL in commercially available autosampler vials including microinserts.In LC-based methods,acetazolamide is generally analyzed without any derivatization step as they utilize the UV absorbance of acetazolamide.In HPLC methods with UV absorbance detection,endogenous compounds may interfere with the measurement of acetazolamide in human urine,despite the use of extraction steps such as solvent extraction with ethyl acetate[7].By using an HPLC system previously reported for the analysis of reduced glutathione(GSH)as o-phthaldialdehyde(OPA)derivative[33],we observed co-elution of unknown endogenous compound(s)with acetazolamide which may become important in the lowerμM-range(Fig.S6).As the extent of such interferences is unpredictable,accurate measurement of low concentrations of acetazolamide in human urine may be seriously compromised.
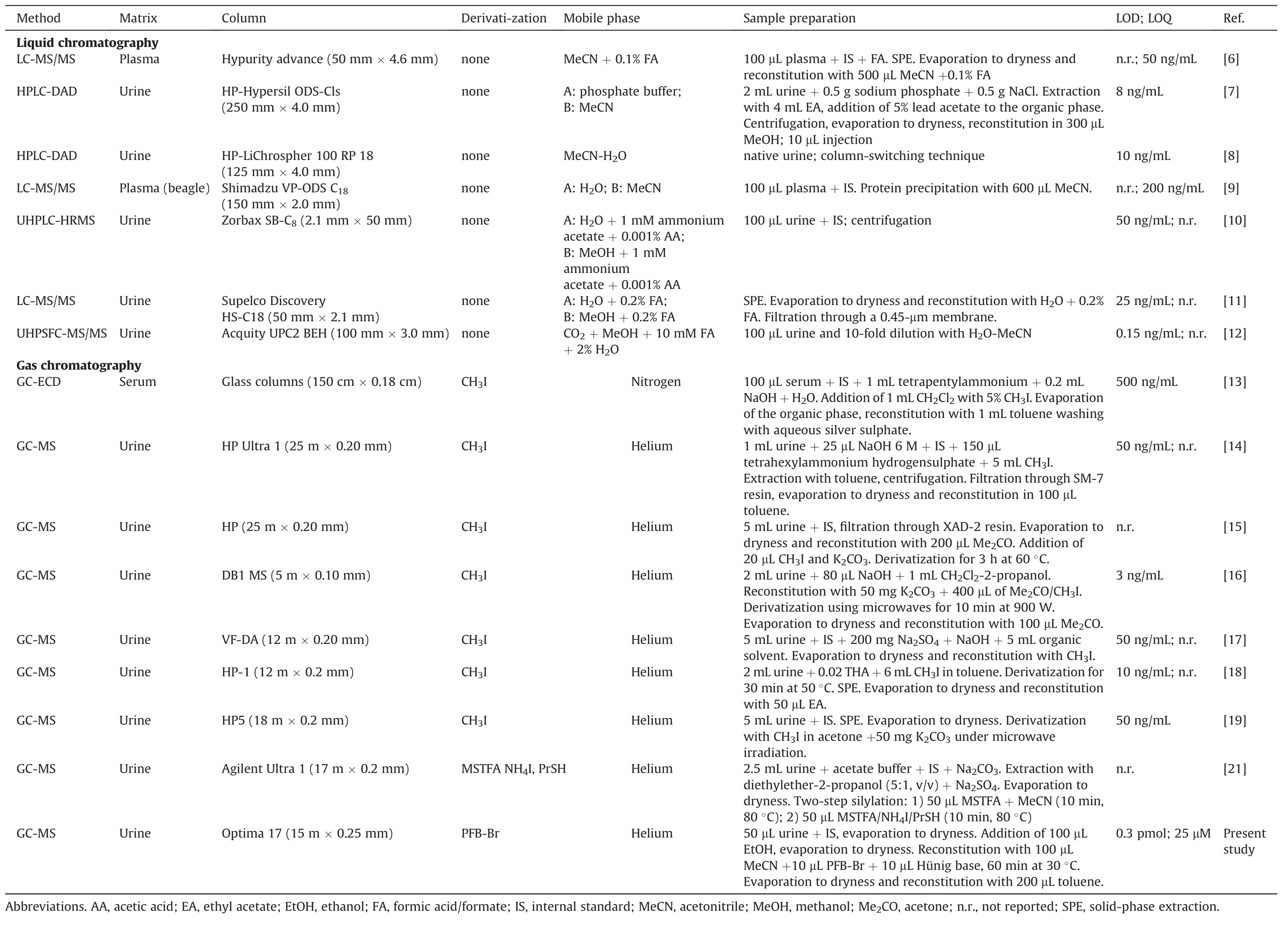
Table 3Reported liquid chromatographic(LC)and gas chromatographic(GC)methods for the quantification of acetazolamide in human urine,plasma or serum.
5.Conclusion
Acetazolamide,an organic sulfonamide drug(RSO2NH2)and a potent CA inhibitor,can be derivatized with PFB-Br in anhydrous acetonitrile using an organic base as the catalyst to form RSO2N(PFB)2.Derivatization of acetazolamide of urinary origin is performed under mild conditions(30 ℃,1 h)after evaporation of the urine water to dryness without prior extraction from the urine.In routine,acetazolamide can be analyzed quantitatively in urine(50μL urine;range,0-1000μM acetazolamide;200μL toluene)by GC-MS in NICI mode using[acetylo-2H3]acetazolamide as the internal standard(250μM).Ingestion of a 250 mg acetazolamide containing tablet by a healthy volunteer resulted in constant creatinine-corrected excretion rates of acetazolamide in the urine and in reversible inhibition of the reabsorption of urinary nitrite and nitrate after about 1.5 h.The PFB-Br derivatization procedure is applicable to the measurement of methazolamide,another sulfonamide.The size of the organic moiety of the sulfonamide drug rather than the reactivity of its sulfonamide group towards PFB-Br limits the application of the GC-MS method to other larger sulfonamides.
 Journal of Pharmaceutical Analysis2020年1期
Journal of Pharmaceutical Analysis2020年1期
- Journal of Pharmaceutical Analysis的其它文章
- Trace determination and characterization of ginsenosides in rat plasma through magnetic dispersive solid-phase extraction based on core-shell polydopamine-coated magnetic nanoparticles
- Thermodynamics of clay-drug complex dispersions:Isothermal titration calorimetry and high-performance liquid chromatography
- Determination of L-norvaline and L-tryptophan in dietary supplements by nano-LC using an O-[2-(methacryloyloxy)-ethylcarbamoyl]-10,11-dihydroquinidine-silica hybrid monolithic column
- Analysis of pesticide residues in commercially available chenpi using a modified QuEChERS method and GC-MS/MS determination
- Rapid identification of chemical profile in Gandou decoction by UPLCQ-TOF-MSE coupled with novel informatics UNIFI platform
- Comparing different domains of analysis for the characterisation of N-glycans on monoclonal antibodies
