Role of dynamic perfusion magnetic resonance imaging in patients with local advanced rectal cancer
Davide Ippolito, Silvia Girolama Drago, Anna Pecorelli, Cesare Maino, Giulia Querques, Ilaria Mariani,Cammillo Talei Franzesi, Sandro Sironi
Abstract
Key words: Rectal neoplasm; Chemotherapy; Radiotherapy; Tumor staging; Treatment response; Magnetic resonance imaging
INTRODUCTION
The incidence of rectal cancer in the European Union is 125000 per year, accounting for 35% of total colorectal cancer incidence, and it is predicted to increase further in both genders[1]. In the past 20 years, the management of this tumor has changed due to the introduction of magnetic resonance imaging (MRI) primarily as a diagnostic tool for staging and restaging, and secondarily as an implement in decision making after neoadjuvant therapies or surgical procedures (total mesorectal excision)[2]. Moreover,the possibility to achieve a complete response (CR) after the chemo-radiotherapy(CRT) in up to 30%-40% of patients and the results of non-surgical management of the disease (called “watch and wait” approach)[3]revival the discussion in the research field to obtain an accurate clinical assessment of CR before surgery. To date, the evaluation of CR is based on the histopathology assessment by using different tumor regression grade (TRG) features (e.g., Dworak or Mandard classifications)[4-6]. From the radiological point of view, the main attention for the prediction of a CR after CRT focuses on MRI and the potential role of diffusion-weighted images and perfusion imaging represented by dynamic-contrast enhanced MRI (DCE-MRI). The main aim is to find a reliable tool to predict tumor response in comparison to histopathologic findings (such as TNM stage and TRG scale). MRI with T2 weighted and diffusionweighted images is mandatory[7]and it is the first-choice examination for primary staging and restaging after CRT to decide the appropriate management of rectal cancer patients. When considering “watchful and waiting” as a treatment option after CRT, it is necessary to correlate the restaging MRI findings with clinical and endoscopic examination[3,7,8]. In this setting, DCE-MRI is considered a promising functional research implement[7]. This technique allows us to assess the vascularity of the tumor and can provide valuable information about tumor aggressiveness and the degree of angiogenesis in staging and restaging[9,10]. Since angiogenesis is a key factor in the growth and dissemination of colorectal cancer, the characterization of the angiogenic status of the tumor could allow for a more targeted approach to treatment[11]. The presence of hypoxic areas inside the tumor may influence the outcome of CRT[12]. due to the reduction of uptake and retention of chemotherapeutic agents within the pathological tissue[12]. These factors depend on tumor perfusion and chemotherapeutic agent extravasation through the vessel wall. Therefore, the ability to measure tumor vessels and tumor microenvironment before therapy may provide critical information on the selection of the most effective treatment strategy[13].Moreover, vascular changes in the tumor may occur during CRT, and they may be an expression of tumor response or indicate the persistence of viable tumor cells before surgery. All semi-quantitative parameters [e.g., area under the curve (AUC),maximum signal intensity or peak enhancement ratio, wash-in slope, mean transit time], are extracted directly from time-signal intensity curves created by specific software from the dynamic sequences. Semi-quantitative parameters are less timeconsuming in comparison with semi-qualitative ones[14], but are affected by the acquisition systems so that comparison and quantification of these parameters can be difficult[15,16]: The real concentration of contrast agent in tissues is not estimated[17]due to a non-linear relation between signal-intensity change and contrast-medium concentration[11]. Only one study[18]have been assessed the correlation between semiquantitative DCE-MRI parameters in rectal cancer and healthy rectal wall in the same group of patients. The purpose of this study is to investigate the value of DCE-MRI parameters in the evaluation of the response to CRT, by having histology as the reference standard, in patients with local advanced rectal cancer. In particular, we investigated whether a difference exists in DCE-parameters between the tumor tissue and in the healthy rectal wall, both before and after CRT, and a possible correlation with pathological findings expressed as Mandard’s TRG and TNM stage.
MATERIALS AND METHODS
Patients
The study was ethically approved by the San Gerardo Hospital Ethics Committee and informed consent was obtained from all patients.
One-hundred-ninety consecutive patients with a biopsy-proven diagnosis of rectal adenocarcinoma from 2006 to 2018, who underwent MRI examination of lower abdomen at our department, were enrolled.
The inclusion criteria were: (1) Biopsy-proven adenocarcinoma; (2) MRI of lower abdomen for rectal cancer staging (MR1) performed with standard protocol and DCEsequences; (3) Neoadjuvant preoperative CRT; (4) MRI of the lower abdomen after CRT (MR2) performed with standard protocol and DCE-sequences; and (5) Excision of the primary rectal cancer and following histopathological examination, resulting in the definition of the ypTNM grade and TRG sec. Mandard. Exclusion criteria were: (1)Surgery performed without previous neoadjuvant therapy; (2) No ypTNM or TRG assessment; (3) No MRI study performed at our radiology department/or MRI study performed without DCE sequences; and (4) Suboptimal DCE sequences not useful for software analysis.
MRI protocol
All MRI examinations were performed on a 1.5-T system (Achieva Plus; Philips, The Netherlands) with multi-channel phased-array body coil. After a planning scan, axial T2 weighted turbo spin-echo (T2WI-TSE) images covering the entire length of the rectum were acquired and used to plan high-resolution scans. Scan protocol included axial TSE T1 weighted sequences (slice thickness: 3 mm; slice: 20; gap: 3 mm; TR: 612 ms; TE: 14 ms; flip angle: 90°; FOV: 180; RFOV: 85; matrix: 272 × 320; NSA: 4; time:4.43 min); sagittal TSE T2 sequence (slice thickness: 3 mm; slice: 32; gap: 0 mm; TR:5501 ms; TE: 85 ms; flip angle: 90°; FOV: 220; RFOV: 105; matrix: 276 × 200; NSA: 4;time: 4.40 min); orientated axial TSE T2 sequence (slice thickness: 3.5 mm; slice: 18;gap: 3.5 mm; TR: 4750 ms; TE: 120 ms; flip angle: 90°; FOV: 180; RFOV: 85; matrix: 256× 256; NSA: 4; time: 3.05 min); orientated coronal TSE T2 sequence (slice thickness: 3 mm; slice: 20; gap: 0.5 mm; TR: 5058 ms; TE: 125 ms; flip angle: 90°; FOV: 180; RFOV:100; matrix: 256 × 256; NSA: 4; time: 3.47 min). The orientated axial and coronal oblique images were performed orthogonal and parallel, respectively, to the long axis of the rectal tumor. Diffusion-weighted images with background body-signal suppression using a Multi-slice Spin Echo Eco-planar Single Shot (SE-EPI-SSh)sequence were included in the standard protocol (b-value 0 and 1000 s/mm2; slice thickness: 6 mm; slice: 12; gap: 6 mm; TR: 3000 ms; TE: 74 ms; flip angle: 90°; b-value:0 and 700 s/mm2; FOV: 380; RFOV: 80; matrix: 240 × 256; NSA: 4; time: 1.30 min;SENSE factor: 1.5). Multiphase DCE-MRI was performed using ten consecutive acquisitions of 3D T1-weighted fat-suppressed spoiled recalled-echo sequences(THRIVE) on the axial plane (number of slices: 50; flip angle: 10°; slice thickness:1mm; TR: 4.1 ms; TE: 1.97 ms; NSA: 2; matrix: 272 × 320; FOV: 180; time: 4.43). The dynamic study was obtained before, during, and after the intravenous injection of 0.1 mL/kg gadoxetic acid (1.0 mol Gadovist, Bayer, Leverkusen, Germany), with a flow rate of 1.5 mL/s and followed by a 30-mL saline flush at the same rate. The volume of the contrast agent was calculated based on the patient’s body weight (0.1 mL per kg body weight). The arterial phase MRI was initiated immediately after the visual detection of contrast material at descending aorta by using a real-time bolus displayed method; the venous phase was performed with a fixed image delay of 80 s, followed by delayed coronal imaging at 4 min after contrast agent injection.
All sequences were obtained in free breathing. The total examination time was approximately 30 min. Luminal distention was achieved with the rectal administration of a small amount of sonography transmission gel (80 mL) to distend the rectal lumen. No intestinal cleansing nor spasmolytic drugs were administered.
MRI analysis
DCE raw data sets were uploaded on a dedicated workstation (Intellispace Portal;Philips Medical Systems), with a dedicated perfusion software (T1 Perfusion Package,Philips Medical Systems) that generated functional perfusion maps displayed in a color scale ranging from blue to red, considering blue the lowest range of the display.Functional color maps were analyzed in combination with contrast-enhanced images,both for staging (MR1) and restaging (MR2) images. A single radiologist, with more than 10 years of experience in the pelvis and rectal MRI, blinded to the histopathology results, manually drawn 3 different freehand regions of interest (ROI) on the rectal tumor on three consecutive slides, avoiding including the lumen and the mesorectal fat in the delineation. Other 3 different freehand ROIs were drawn on the normal rectal wall. The software also generated time-intensity curves and calculate perfusion parameters of tumor tissue and the healthy rectal wall (Figure 1). On the MR2 images,ROIs were manually drawn based on visual analysis of focal thickness within the location of the primary tumor bed; when no remaining thickness was observed the ROIs were drawn on MR2-T2W images considering the location of the tumor on MR1 images, to identify the presence of residual fibrotic tissue in the tumor bed. The size of the ROI of one section was not less than 10 voxels. For each ROI, located both in the tumor tissue and in the healthy rectal wall, the following quantitative parameters were calculated: Relative arterial enhancement (RAE, %), relative venous enhancement (RVE, %), relative late enhancement (RLE, %), maximum enhancement(ME, %), time to peak (TTP, s) and AUC (mm2). RAE, RVE, and RLE represent the highest values percentage of intensity signal of contrast material concentration in the three different enhancement phases (arterial, venous, and delayed phase). The relative enhancement (RE, %), derived from the signal enhancement of a pixel of certain dynamic relative to that same pixel in the reference dynamic. The reference dynamic is normally the first, pre-contrast dynamic. The reference dynamic can be set to another dynamic, where I(D) stands for pixel intensity of current dynamic and I(Dref)stands for pixel intensity of reference dynamic created with the following formula: RE= [ID /I(Dref)-1] × 100, where I(D) stands for pixel intensity of current dynamic and I(Dref) stands for pixel intensity of reference dynamic.
ME represents the highest absolute values of signal intensity at the analyzed tissue level, and TTP corresponds to the time need to reach the maximum value of contrast material concentration. The AUC represents the value of the area under the timeintensity curve generated for a selected ROI by the perfusion software.
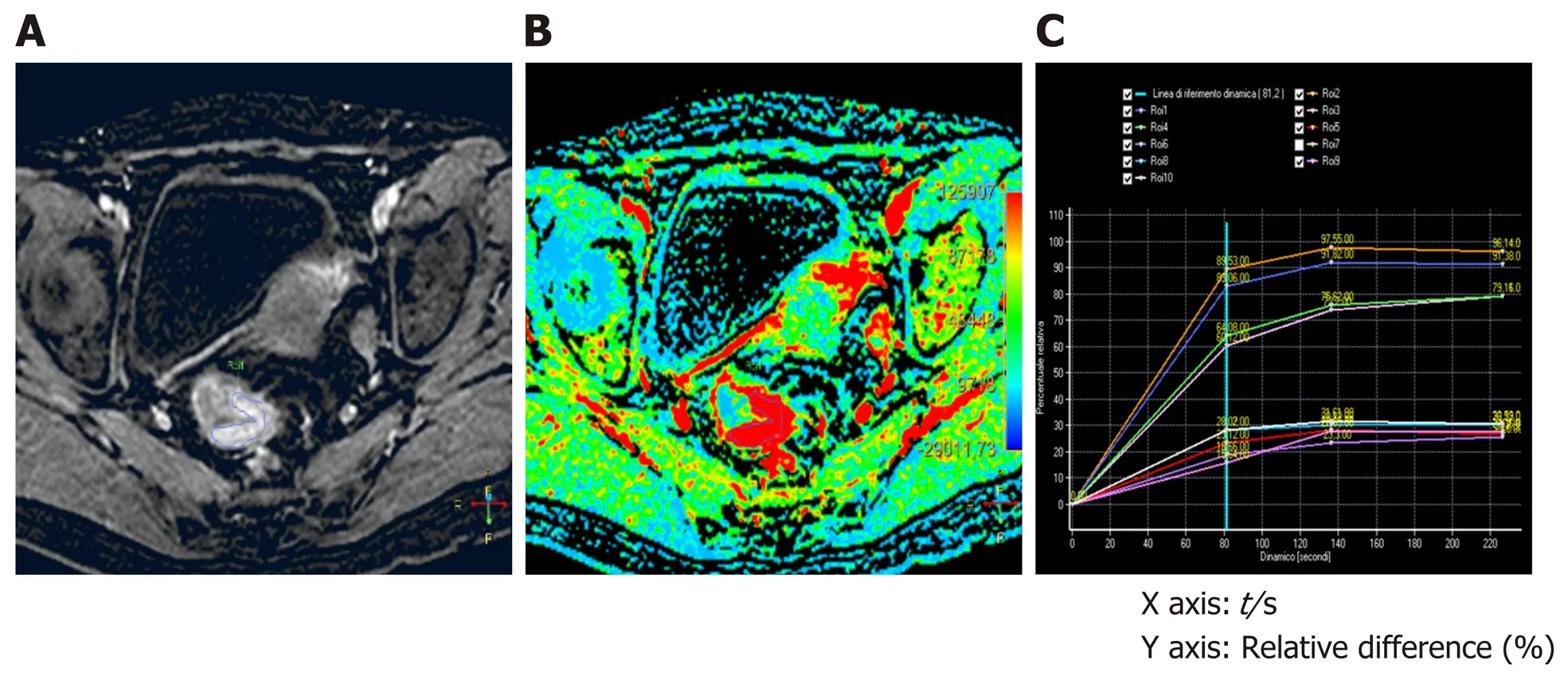
Figure 1 Staging dynamic-contrast enhanced magnetic resonance imaging (MR1) performed with 3D-T1 THRlVE images; the corresponding color map and time-intensity curves. A: The T1 THRIVE image shows an example of freehand delineation of the tumor during an MRI study performed for staging purpose (the tumor was a T3N0); B: The related color map was created by the software, and the delineation of the tumor on the T1 THRIVE was automatically reported also on the color map; C: The time-intensity curves table show the results of the delineations on the tumor (which correspond to the highest curves) and on the healthy rectal wall.
Statistical analysis
Imaging-derived data were statistically analyzed by using IBM SPSS 21 (SPSS Incorporated, Chicago, Illinois, United States) and R software (R Foundation for Statistical Computing, Vienna, Austria https://www.Rproject.org/). Linear regression curves have been used to assess the relation of pre- and post-treatment perfusion values to the TRG classes. Wilcoxon test was used to verify data correlation before and after CRT. Mann-Whitney test for unpaired data was used to verify statistically significant differences for each parameter among tumor tissue and the healthy rectal wall, before and after CRT.
RESULTS
One-hundred-sixty-two patients did not meet the inclusion criteria, mainly because of the absence of DCE-sequences and a final group of 28 patients was enrolled in this study (men: 14, women: 14; mean age: 69 ± 19 years). Four patients were staged as cT3N0, 16 patients as cT3N1, 2 patients as cT3N0, and 6 patients as cT3N2. All of them completed CRT protocol with a total radiation dose of 50.4 Gy, in 28 fractions and 5-fluorouracil or 5-fluorouracil plus oxaliplatinum. MRI restaging (MR2) was performed 8-10 wk after the completion of CRT and then all patients underwent surgery (10-12 wk after the completion of CRT). After surgery 6 patients were ypT0N0, 8 patients were ypT2N0, 8 patients were ypT3N0, 6 patients were ypT3N1.Moreover, according to Mandard's TRG[6]: 6 patients were TRG1 and 4 patients were TRG2 (these patients, 36%, were considered as responders). Eighteen patients (64%)were TRG3 and were classified as non-responders.
Results of perfusion parameters at baseline (MR1)
At the baseline (MR1) perfusion MRI parameters were significantly higher in the tumor tissue compared to the healthy tissue (P< 0.05) (Table 1). These results were also confirmed from the visual assessment of the time-intensity curves (Figure 1).When considering the healthy rectal wall, no differences in the perfusion parameters were observed between responders and non-responders’ group before CRT (MR1)(Table 2). When considering the tumor tissue at MR1, there was also no significant difference in the perfusion parameters between responders and non-responders(Table 3). All the perfusion parameters in the tumor tissue before CRT did not show any correlation with the ypT stage histology after surgery.
Results of perfusion parameters after chemoradiotherapy (MR2)
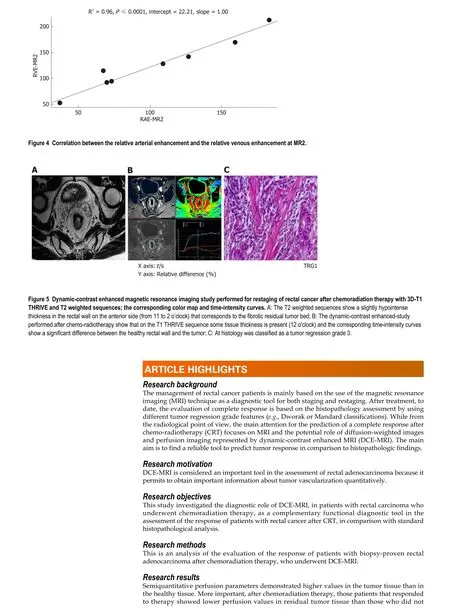
After CRT (MR2) no differences in the perfusion parameters were observed between responders and non-responders group concerning the healthy rectal wall (Table 2).Moreover, in the healthy rectal wall, perfusion parameters were not modified after CRT in comparison to corresponding values at the baseline (MR1) (Table 2). At MR2,perfusion values of the rectal residual tumor in responders were significantly lower (P< 0.05) [RAE (%): 54 ± 20; RVE (%) 73 ± 24; RLE (%): 82 ± 29; ME (%): 904 ± 429]compared to those ones of non-responders (RAE (%): 129 ± 45; RVE (%):154 ± 39; RLE(%): 164 ± 35; ME (%):1714 ± 427) (Table 4). Concerning the time-intensity curves, the AUC at MR2 showed a significant difference (P= 0.03) between responders and nonresponders [AUC (mm2× 10-3) 121 ± 50vs258 ± 86], with lower AUC values of the tumor in responders compared to non-responders. In the responders group, perfusion values in the tumor decreased significantly at MR2 [RAE (%): 54 ± 20; RVE (%): 73 ±24; RLE (%): 82 ± 29; ME (%): 904 ± 429] compared to the corresponding perfusion values at MR1 [RAE (%): 115 ± 21; RVE (%): 119 ± 21; RLE (%): 111 ± 74; ME(%): 1060 ±325); (P< 0.05) (Table 4). No significant differences in the TTP values were found between responders and non-responders at MR2 concerning the rectal tumor. After CRT, at MR2, differences in perfusion parameters between the residual tumor tissue and the healthy tissue were still present, but in responder patients, the perfusion parameters of the residual tumor tended to be closer to the perfusion parameters of the healthy rectal wall, with similar time-intensity curves (Figures 2 and 3). A strong correlation between RAE and RVE in the tumor tissue after CRT (R2 = 0.96;P=0.0001) was also found (Figure 4). Perfusion parameters of the residual tumor at MR2 in non-responders were higher in comparison to the corresponding healthy rectal wall at MR2 with an increasing difference in the time-intensity curve shape (Figures 3 and 5). Moreover, in non-responders, no reduction of the perfusion parameters in the tumor was observed at MR2 in comparison to the MR1 values. All perfusion parameters in the tumor tissue after CRT didn’t show any correlation with the ypT stage histology after surgery.
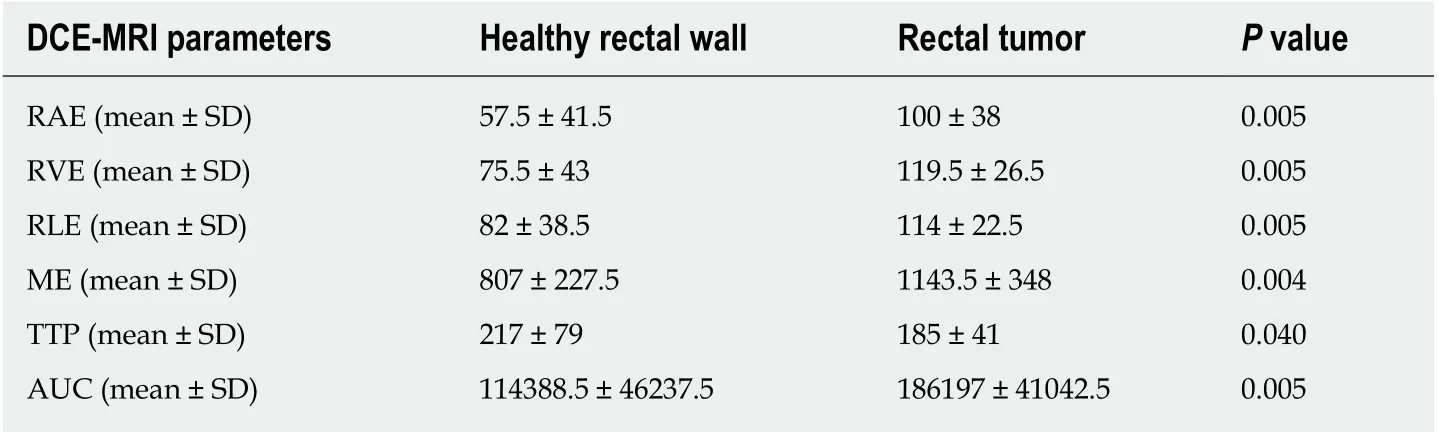
Table 1 Differences in terms of perfusion parameters in the healthy rectal wall and the tumor tissue, determines through the dynamic contrast enhanced magnetic resonance imaging study for staging rectal cancer (MR1)
DISCUSSION
DCE-MRI is one of the most recent functional implementations in the MR spectrum of imaging in breast imaging and prostate cancer to identify malignant tumors based on specific enhancement patterns[19,20]. In rectal cancer, it is considered a promising research tool[7]for the assessment of treatment response after CRT. With this technique, the vascularity of the tumor can be assessed, and it can provide valuable information about tumor aggressiveness and the degree of angiogenesis in both staging and restaging[9,10]. There is also evidence that DCE-MRI can help predict and assess response to neoadjuvant treatment[21,22]. DCE-MRI, using low-molecular-weight(< 1 kDa) gadolinium-based paramagnetic contrast media, is an imaging technique where T1-weighted sequences are rapidly repeated before, during and after intravenous contrast injection to study signal intensity changes induced by the path of the contrast bolus through tissues[9]. From a DCE-MRI study, time-intensity curves are usually created using specific software and they can be analyzed with three different approaches: Qualitative (by the visual analysis of the time-intensity curves), or with quantitative and semi-quantitative parameters[22,23]. All semi-quantitative parameters are extracted directly from time-signal intensity curves. These parameters, less timeconsuming[14]in comparison with a quantitative approach, require less complicated software algorithms, are easier to obtain and reproduce than quantitative parameters[9], and are more reliable to be used in clinical practice. Up to 8%-30% of patients with local advanced rectal cancer treated with CRT achieve a pathological CR confirmed at histology after surgery[3]. The possibility to assess a CR to neoadjuvantCRT before surgery could potentially modify the management of rectal cancer treatment and lead to the new treatment approach, which is the “wait and see” or“watchful and wait”[3]. Different studies addressed the potential role of quantitative and semi-quantitative DCE-MRI parameters in predicting the response to therapy in primary rectal cancer with conflicting results[18,23-25]. Unfortunately, only a few studies investigated the potential of semi-quantitative parameters in the assessment of tumor characteristic with controversial results, demonstrating that tumors that better respond to CRT have lower values of TTP, AUC[24,25]or have a different degree of correlation with angiogenetic markers or micro-vessel density[9,26]. Our results are in line with those achieved by Shenet al[27]and Krishanet al[18]that compared perfusion parameters obtained from DCE-MRI in patients with rectal cancer to a control group of healthy patients[27]or the healthy rectal wall in the same group of the patient[18].After CRT, there was a significant difference between responders and non-responders concerning all the RE parameters. In particular, patients that responded to treatment had lower absolute perfusion value after CRT than non-responders, in line with Petrilloet al[23,24]and Krishanet al[18], where maximum signal difference decreased in responders patients when compared to non-responders. Moreover, in line with the literature[9,18,25]the AUC was lower in responder patients in comparison to nonresponders. The relation of semi-quantitative parameters and vascular changes in the tumor tissue is confirmed by the fact that no changes in these parameters were found after CRT when we analyzed the healthy tissue. The delineation and the assessment of perfusion values in the healthy tissue allowed to indirectly assess if changes in the perfusion parameters were due to post-radiation inflammation after CRT or to real changes in vascular characteristics. Interestingly all the perfusion parameters of the tumor tended to increase at MR2 in non-responders in comparison to the baseline values at MR1, while no increase in the perfusion value was observed after CRT in the same group of non-responder patients when considering the healthy rectal wall.Krishanet al[18]also demonstrated that in responder patients, rectal perfusion became similar to the adjacent normal rectal wall; their results correlated with normalization of perfusion parameters in tumor tissue with the healthy rectal wall. No robust evidence has been found for the value of DCE parameters as a measure of tumor aggressiveness, measured as the TNM stage. Although we found a difference between perfusion parameters in benign tissue and malignant tissue, we did not find any correlation between pre and post-treatment semi-quantitative DCE-parameters and pathological T stage after CRT. This could be due to the small sample of patients since other studies[24,25]showed lower semi-quantitative value for more aggressive tumors.
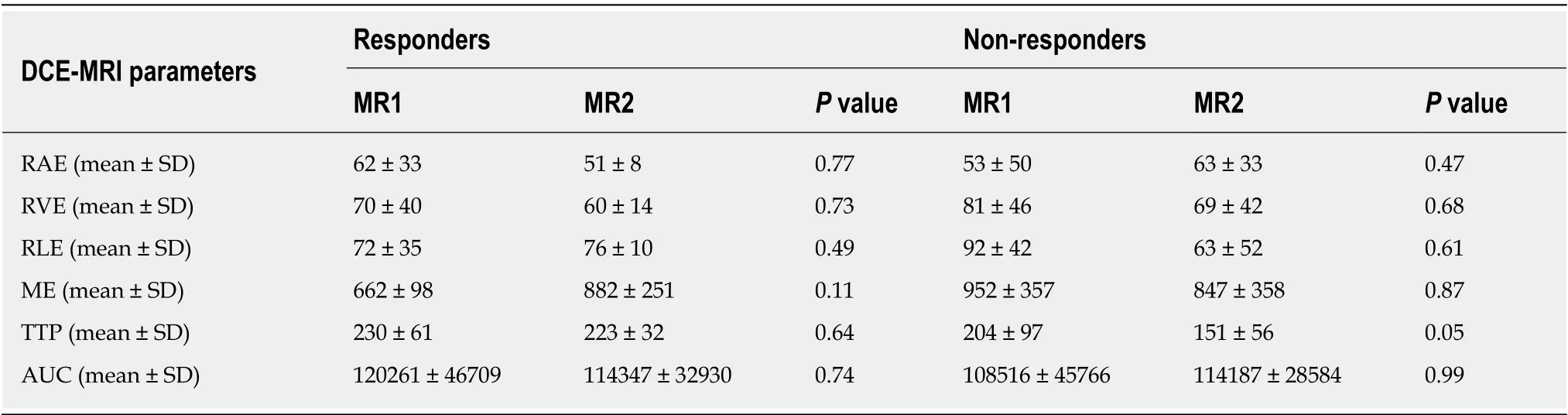
Table 2 Differences in terms of perfusion parameters between responders and non-responders, in the healthy rectal wall, determines through a dynamic contrast enhanced magnetic resonance imaging study during staging (MR1) and restaging (MR2) of rectal cancer after chemo-radiotherapy
This study has some limitations. The main one is due to the small number of patients. Second, we did not consider the wash-in slope as previous authors[9,10,25], due to the different acquisition phases and software employed. Third, is the lack of pixel correlation to histology so that the delineated parameters are not exactly related to the histological specimen. We tried to overcome these limitations by drawing multiple ROIs and including in the analysis also the healthy rectal wall; a volumetric evaluation or a prospective study should be performed to obtain more reliable data.
In conclusion, Dynamic contrast perfusion-MRI parameters represent a complementary diagnostic tool in identifying vascularity characteristics of local advanced rectal cancer since they have been demonstrated to be related to the vascular changes which occur in the tumor in comparison to the healthy tissue.Moreover, DCE-MRI parameters can express the changes occurred in the tumor thatrespond to the neoadjuvant treatment and are more reproducible than quantitative parameters.
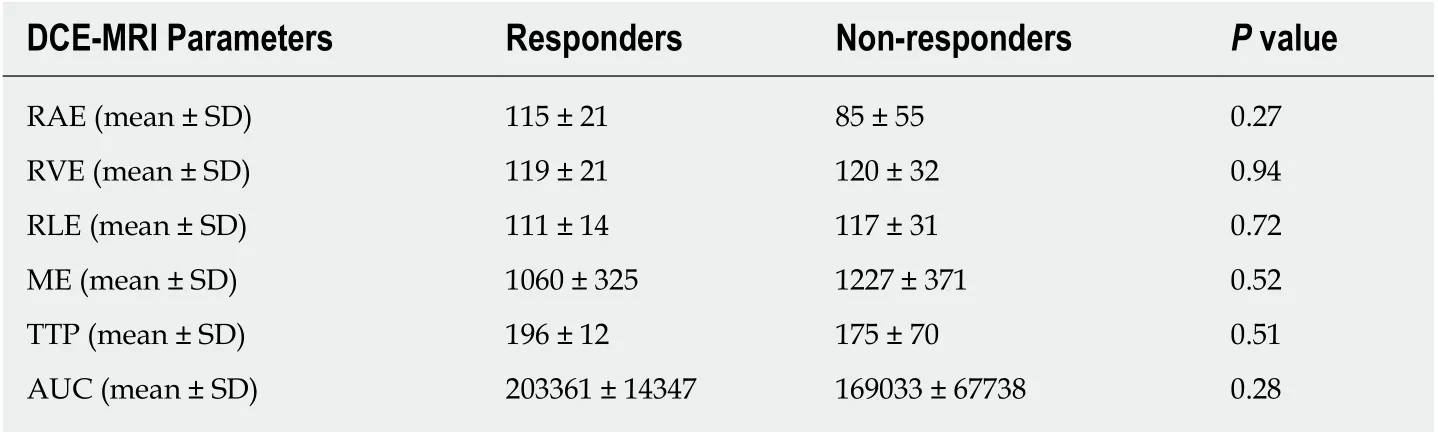
Table 3 Differences in terms of perfusion parameters between responders and non-responders,in the tumor tissue, determines through a dynamic contrast enhanced magnetic resonance imaging study for staging rectal cancer (MR1)
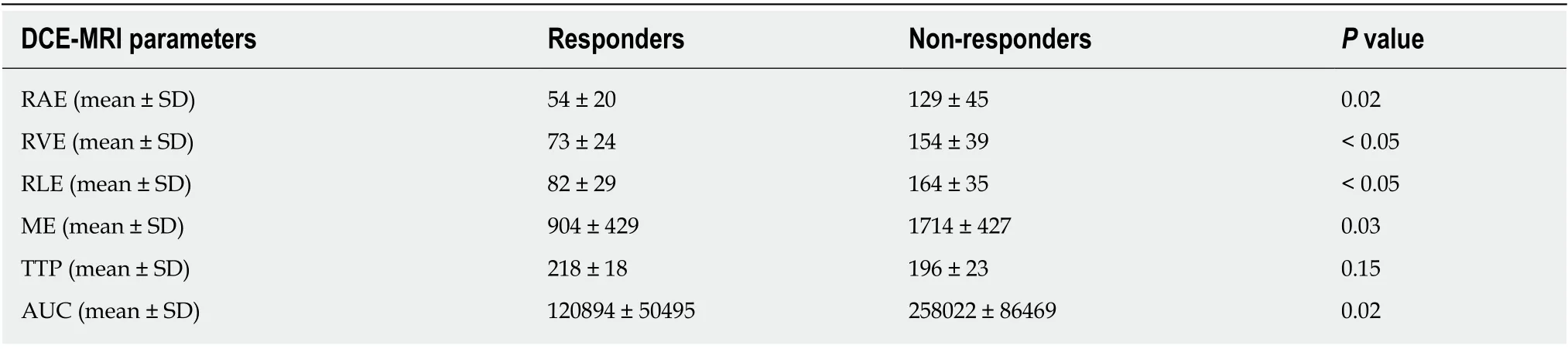
Table 4 Differences in terms of perfusion parameters between responders and non-responders in the tumor tissue, determines through a dynamic contrast enhanced magnetic resonance imaging study for rectal cancer restaging (MR2) after chemo-radiotherapy
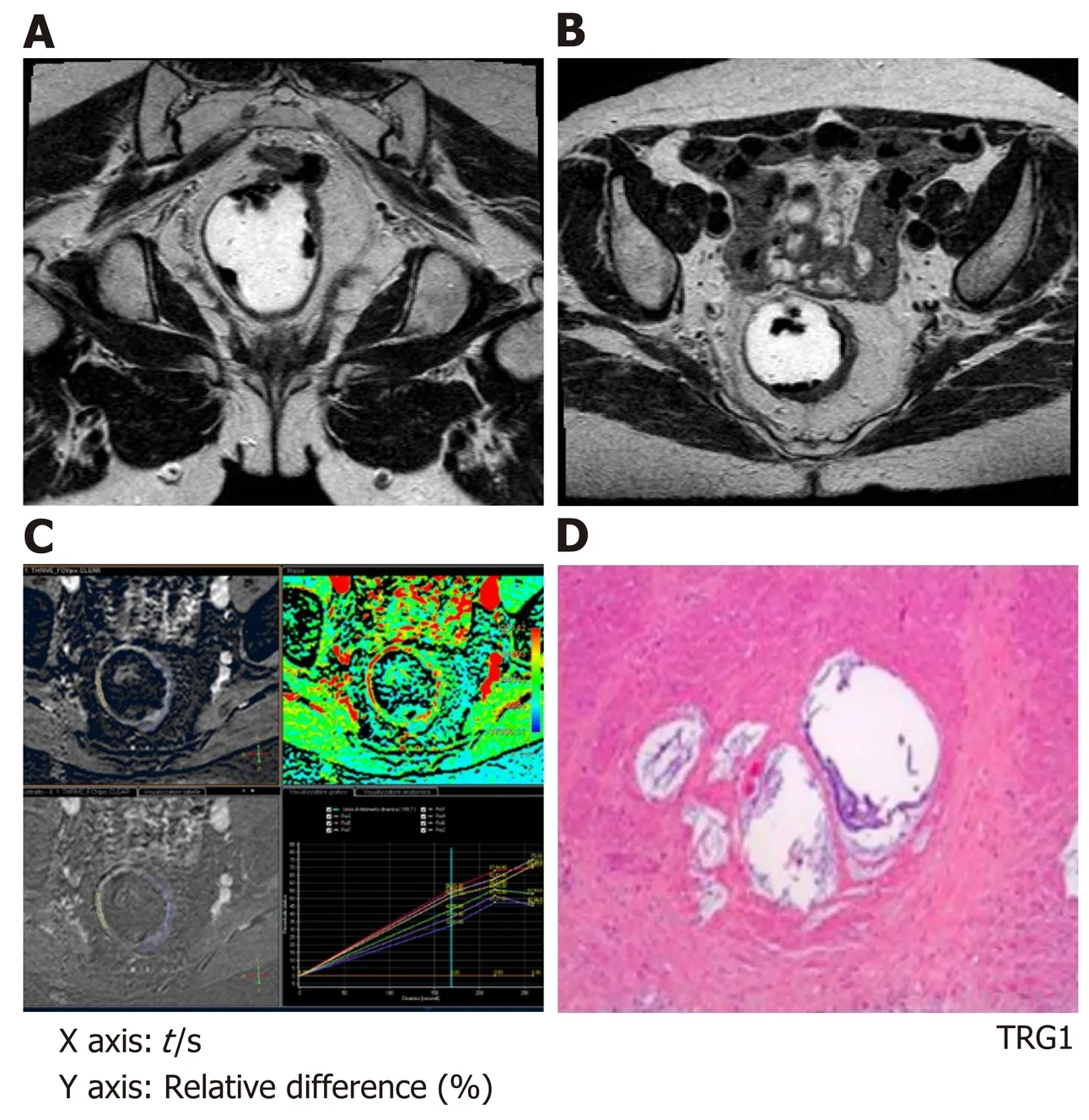
Figure 2 Magnetic resonance imaging study performed for rectal cancer restaging after chemo-radiotherapy with standard T2 weighted sequences and dynamic-contrast enhanced magnetic resonance imaging with 3D-T1 THRlVE images; the corresponding color map and time-intensity curves. A and B: The T2 weighted sequences show a slight thickness in the rectal wall on the left side (from 2 to 5 o'clock) that corresponds to the residual tumor bed; C: The dynamiccontrast enhanced-study show the delineation of the tumor and the time-intensity curves show, with similar curves for the tumor and the healthy rectal wall; D: At histology this patient was classified as a tumor regression grade 1.
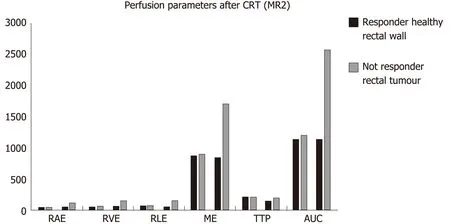
Figure 3 Differences in terms of perfusion parameters between responders and non-responders, in the tumor tissue and the healthy rectal wall,determines through a dynamic contrast enhanced magnetic resonance imaging study for rectal cancer restaging after chemo-radiotherapy. CRT: Chemoradiotherapy.
Research conclusions
Dynamic contrast MRI represents a complementary diagnostic tool in identifying vascularity characteristics of local advanced rectal cancer, before and after chemoradiation treatment.
Research perspectives
To strengthen our results, further studies should include a wider cohort of patients, by using pathological correlation as a gold standard.
 World Journal of Gastroenterology2020年20期
World Journal of Gastroenterology2020年20期
- World Journal of Gastroenterology的其它文章
- Percutaneous endoscopic gastrostomy – Too often? Too late? Who are the right patients for gastrostomy?
- Decreased of BAFF-R expression and B cells maturation in patients with hepatitis B virus-related hepatocellular carcinoma
- Anhedonia and functional dyspepsia in obese patients: Relationship with binge eating behaviour
- Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients
- Role of gut microbiota-immunity axis in patients undergoing surgery for colorectal cancer: Focus on short and long-term outcomes
- Diet in neurogenic bowel management: A viewpoint on spinal cord injury
