Secondary damage in trauma and limited access dressing: a review
Pramod Kumar, Akriti Gupta, Apoorva Gupta
1Consultant Plastic Surgeon, King Fahd Central Hospital, Jazan 82666, Saudi Arabia.
2Resident Physician (Pathology), University of Virginia, Charlottesville, VA 22903, USA.3Physician (Internal Medicine), Asante Rogue Regional Medical Center, Medford, OR 97504, USA.
Abstract Secondary damage in trauma may increase morbidity, mortality and the cost of treatment considerably. This article reviews the literature of 46 relevant articles on this topic. We hope to provide a better understanding of the various mechanisms that can lead to secondary damage following major trauma and aim to improve the management of such in trauma patients. We also explore the utility of limited access dressing and its ability to minimize and treat secondary musculoskeletal trauma. Four interdependent cellular mechanisms have been described that contribute and perpetuate secondary tissue damage - lysosomal, protein/enzyme denaturation,membrane permeability and mitochondrial. Systemic changes are mainly due to systemic hypoxia and the systemic inflammatory response syndrome. Limited access dressing appears to be an efficient and cost-effective method for the management of secondary damage, as evidenced by the reduced number of debridements, shorter wound coverage time, and reduction in total length of hospital stay while lowering treatment costs and improving quality of care.
Keywords: Trauma, secondary damage, limited access dressing
Cells are complex interconnected systems that work together to maintain a well-regulated microenvironment that is indispensable for their survival. Trauma to a single cell can affect overall homeostasis of the immediate environment. The extent of injury and tissue characteristics determine the extent of biochemical damage to adjacent cells, leading to cell death and tissue dysfunction. Secondary damage in trauma can increase patient morbidity, mortality and the cost of treatment considerably. A better understanding and control of secondary damage is thus essential for improved outcomes in trauma patients, which will, in turn, reduce morbidity and render management cost-efficient. In this article, we review the existing literature and analyze possible areas where standard medical and surgical intervention along with wound management using Limited Access Dressing (LAD) could lead to better outcomes.
METHOD
Secondary damage in trauma patients was de fined. We looked up the relevant literature by using “Secondary Damage in Trauma” and “Limited Access Dressing” on PubMed and Google search engines. Relevant articles from molecular biology, physiology and pathophysiology were also reviewed to explain the mechanism of secondary damage in trauma. A total of 46 relevant articles were reviewed analyzed to find areas where standard medical and surgical intervention, along with wound management using LAD, could lead to better outcomes.
Definition
secondary injury, in simple words “by-stander” damage, of cells result from a secondary insult with destructive and biochemical mechanisms triggered by the mechanical destruction of tissue following direct trauma (the primary insult). Secondary damage may develop immediately (within hours) following the primary insult, and last from a few days up to weeks after. For instance, necrosis of peripheral tissues surrounding the damaged tissue can develop late after crush injuries[1]. Cells or tissue with ultra-structural damage at the time of trauma may die within hours to a few days following trauma. It is still controversial however, whether such cell death should be included under primary or secondary damage[2]. Secondary damage may also involve local or distant cells/tissues. From a biomedical point of view, it can occur due to hemorrhagic shock, compartment syndrome and/or ischemic necrosis[1,2].
Mechanism of secondary damage
Secondary damage to local tissue: This was explained by Knight’s Sport Injury Model (1970)[2]that was later updated based on improved biochemical understanding of tissue damage.
Secondary damage to distant tissue: This mainly occurs due to systemic hypoxia following hemorrhage or due to pro-in flammatory cytokines following systemic in flammatory response syndrome (SIRS)[3].
Mechanism of local secondary tissue injury
Knight’s sport injury model (1970)
Knight’s Sport Injury Model (Secondary Injury Model) was first described in the mid 70’s to account for the series of events that occur following injury in athletes[2,4]. This model effectively explains the possible mechanism that is triggered due to the loss of cellular hemostasis occurring in adjacent, uninjured tissues following primary injury and cell death.
It could be speculated that the outcomes of a primary insult/trauma is immediate cell death or permanent ultra-structural changes that lead to death of injured cells over time. The physiological response to cell death, in theory, could affect the functionality of uninjured cells. The physiologic stress leading to tissue damage is called a secondary injury.
Knight hypothesized that secondary injuries are primarily caused by two mechanisms: (1) hypoxic injury causing oncosis (cell swelling) and acidosis; and (2) enzymatic (lysosomal enzymes) injury.
Hypoxic injury causing oncosis and acidosis
Secondary hypoxic injury occurs due to: hemorrhage, intravascular thrombosis, reduced blood flow from inflammation following increased viscosity, increased intravascular pressure following hematoma and muscle spasm.
Causes of secondary hypoxic injury are (1) anaerobic respiration - depending upon the susceptibility of the involved tissues to hypoxia, glycolic pathways of ATP production lasts from few minutes to 6 h. During aerobic respiration, ATP production is hampered, and acidosis occurs; (2) ATP dependent Na+K+ATPase pump failure[5]- Na+and Ca2+in flux due to ATP dependent Na+pump failure leads to cellular homeostatic mechanism failure, leading to oncosis, cell death and necrosis.
Hypoxia leads to reduced ATP production. Hence, initial cryotherapy in musculoskeletal trauma is bene ficial, as cold helps reduce ATP demand.
Enzymatic (lysosomal) injury
Enzymes released from lysosomes of the injured and dead tissues: (1) acid hydrolases and phospholipases:these enzymes lyse the cell membrane by cleaving the hydrocarbon chain of membrane phospholipids; and(2) proteases: proteases inactivate protein by cleaving peptide bonds.
Effect of enzyme on tissues: acid hydrolases, phospholipase and proteases can cause loss of cell membrane integrity and lead to increased hydropic swelling (oncosis) followed by cell death.
Also, trauma leads to inflammation and neutrophils are acute inflammatory cells and the first line of cellular defense. Human neutrophil proteins (defensins; HNP-1 to 3) are bactericidal agents and interfere with the function of smooth muscle cells in vessels, are prothrombotic, and may inhibit angiogenesis[6].
Updated secondary local tissue injury model
Mechanism of secondary ischemic injury to local tissue
The updated secondary injury model is based on improved biochemical understanding of tissue damage.It has now been proven that ischemia resulting from vascular injury, rather than hypoxia, plays a more significant role in driving secondary damage. Hypoxia poses only one problem of inadequate oxygen supply, whereas ischemia presents with three possible consequences[2]: (1) hypoxia - leads to anaerobic respiration and thus reduced ATP (adenosine triphosphate); (2) low glucose supply to tissues, leading to reduced ATP; and (3) acidosis that causes reduced cellular ability to produce ATP.
Hypoxia also affects mitochondrial function and leads to neural tissue damage as early as within 4 min[5],and musculoskeletal injury in approximately three hours[7].
Mechanism for secondary direct cell injury to local tissue
There are four possible mechanisms for secondary direct cell injuryto local uninjured tissues: (1) lysosomal mechanisms (due to the release of destructive enzymes that require low pHfor activation); (2) protein/enzyme denaturation mechanisms (due to low pH); (3) membrane permeability mechanisms (due to failure of sodium-potassium-ATPase pump) - this leads to cellular swelling; and (4) mitochondrial mechanisms(due to power house failure).
All these mechanisms are basically due to two types of changes - hypoxic and enzymatic.
Lysosomal mechanisms
Lysosomes, the main digestive organelle in a eukaryotic cell, is packed with catabolic enzymes maintained at an acidic intraluminal pH. Under physiological conditions, these highly destructive enzymes, such as lipases, proteases and cathepsins, are contained within the lysosomal complex by the lysosomal membrane.In theory, externalization of lysosomal enzymes to the neutral pH of the cytoplasm would render these enzymes inactive. However, it is now known that a few forms of cathepsins remain active even at neutral pH and can trigger a cascade of events that lead to cell death from the lysosomal pathway[8]. The acidity of the local microenvironment can further activate the lysosomal enzymes released due to lysosomal damage.
There are various stimuli that can lead to increased permeability of the lysosomal membrane and externalization of catalytic enzymes. Lysosomal membrane permeabilization may be caused by various stimuli but the most common mechanism is by destruction of the lipid organization and oxidative damage to membrane bound proteins. Ischemic-reperfusion injury following acute trauma may lead to oxidative stress and membrane damage and destabilization from reactive oxygen species. The hydroxyl radical can cause lipid peroxidation and damage to membrane bound proteins. In addition, reactive oxygen species may also contribute by altering the lysosomal mechanism and activating calcium channels[9].
By releasing highly destructive enzymes (e.g., phospholipase A, cathepsin B) following trauma, cell membranes and cellular proteins are damaged. Lysosomal enzymes cause intracellular cell death when released but when the enzymes become extracellular, secondary damage to adjacent tissue occurs. The normal extracellular pH (approximately 7.2) hampers the action of the majority of lysosomal enzymes but acidic environments (pH ≤ 5) provide optimal conditions for their functioning. Experimentally, it has also been shown that inhibitors of lysosomal proteolytic pathways produce beneficial effects on injured musculoskeletal tissue[2].
Protein/enzyme denaturation mechanisms
Metabolic failure leads to low pH that can cause denaturation of cellular proteins (enzymes)[5]. Denaturation of enzymes leads to loss of cellular functionality with the end-result of cell death. Although denaturation seems like an ultimate step in cell death, multiple factors can cause denaturation. Also, a low pH activates many lysosomal enzymes, leading to secondary damage by enzymatic mechanisms. Hence, there is an overlap of enzymatic and denaturation mechanisms that act together to produce secondary damage.
Membrane permeability mechanisms
Changes in membrane permeability cause: (1) oncosis (cellular swelling) leading to cells bursting; and (2)failure of ion pumps (sodium-potassium-ATPase pump)/changes in voltage gradient and the resulting uncontrolled in flux of ions (Na+and Ca2+) leads to cellular death. Increased intracellular Ca2+following Ca2+in flux causes activation of enzyme phospholipase, leading to disruption of the phospholipid membrane and cell death[10].
Mitochondrial mechanisms
Metabolic failure through mitochondrial (power plant of the cell) damage[11]is one of the leading causes of cell death. Mitochondrial failure resulting in insufficient ATP may trigger other lethal pathophysiological processes leading to cell death. This indicates overlap of various theories in this regard.
Causes of mitochondrial injury are: (1) hypoxic or ischemic injury, the oxidative production of ATP is reduced in hypoxia and becomes inadequate for mitochondrial or cellular homeostasis[2,5,7,11]. Hypoxia activates a number of phospholipases and proteases that cause progressive failure of mitochondrial ion pumps and damage to the mitochondrial membrane. Also, a number of (intracellular) proteins (stress proteins-heat shock protein 70 family; ubiquitin) that are expressed[12]induce protein catabolism within the injured cells; (2) oxidative or reperfusion injury, post-hypoxia reperfusion[5,11,13]produces enormous amounts of oxygen-derived free radicals that exceed normal antioxidant defenses and tissue damage results.Re-perfusion free radical damage of the mitochondrial membrane leads to cell powerhouse (mitochondria)failure. Vasodilatation and hyper perfusion caused by nitric oxide in the post-injury period may accentuate the reperfusion injury; and (3) calcium in flux injury, intra-cellular calcium levels increase due to energy dependent Na+K+pump failure and thus, mitochondria act as calcium sinks[11,14,15]. Increased mitochondrial Ca2+leads to activation of calcium-dependent proteases and phospholipases. Increased mitochondrial Ca2+leads to opening of the permeability transition pore, and inner mitochondrial membrane channel.This results in free passage of small molecules, osmotic swelling leading to outer membrane rupture, ATP depletion and apoptotic mitochondrial cell death.
Mechanism of systemic secondary tissue injury
Systemic hypoxia: systemic hypoxia can cause tissue hypoxia of various organs/tissues. Cellular and tissue hypoxia may lead to shock and various types of complications[16].
Delayed neurological changes in high voltage electric burns may be due to changes in the endothelium of small vessels supplying nervous tissues[17]. Kidney and heart muscle are damaged due to the release of various intracellular chemicals, hemoglobin and myoglobin[18].
SIRS: follows major trauma, leading to a phase of inflammatory response which involves the synthesis of acute phase proteins by the liver, complement activation and the release of pro- (IL-1, TNF alpha, IL- 6, IL-8)and anti-inflammatory cytokines (IL-10, IL-13). If the balance between the pro- and anti-inflammatory cytokines is disrupted, it leads to the development of SIRS and increases morbidity and mortality[19-22][Figure 1].The auto amplification of cytokine production following major trauma/surgery can occur due to overwhelming multiple organ dysfunction syndrome (MODS) and/or severe infection, leading to a cytokine storm[23][Figure 2]. Infection with exaggerated inflammatory responses induce more cells that produce cytokines and act as catalysts to the cytokine storm[24,25].
Very high level of circulatory pro-in flammatory cytokines, as in a cytokine storm, may cause uncontrolled,auto destruction that leads to life threatening MODS.
Immune depression: cytokines are known to cause immune depression in musculoskeletal trauma[26].
Microvascular dysfunction and increased tissue pressure can occur following musculoskeletal trauma and lead to secondary soft tissue damage and compromised skeletal muscle function[27].
Clinical implications of secondary injury
Repeated debridement and consequent exposure of vital structures - due to secondary injury following initial and surgical trauma, new necrosis occurs, requiring repeated debridement.
Complex reconstructive procedures are required on several occasions to cover vital exposed structures following repeated debridement.
SIRS - Pro-in flammatory cytokines are produced due to tissue damage that increase several times following infection, and if absorbed in large quantities, may produce SIRS, MODS and multiple organ failure.
Immobilization, edema and in flammation lead to functional loss and stiff joints[28].
Missed injuries like spinal injuries may lead to serious crippling injuries[29].
Clinical strategy against secondary damage in trauma
In the management of complex surgical problems, a surgeon has three options[30]: (1) avoidance (laissezfaire); (2) aggressive approach; or (3) temporizing maneuvers.
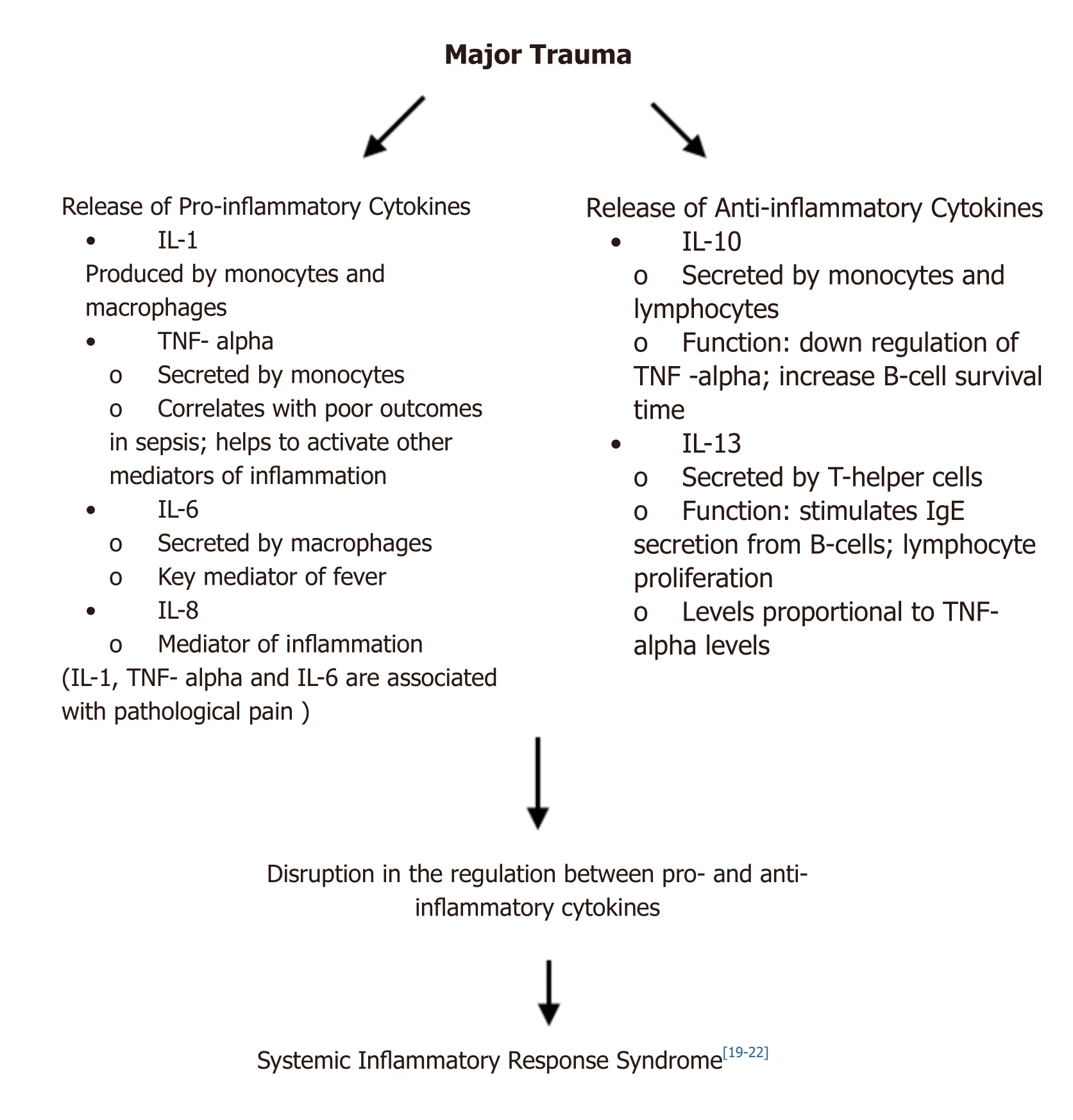
Figure 1. Pathogenesis of systemic inflammatory response syndrome
Avoidance leaves natural biochemical mechanism to overcome the damage but in contrast, the insult from an aggressive approach to counter secondary damage may aggravate the problem.
Hence, to counter secondary damage in trauma cases, the best clinical strategy is to employ temporizing maneuvers.
In temporizing maneuvers, damage control methodologies include: (1) quick action to stabilize a patient’s local and systemic conditions; (2) prevention of further secondary damage; and (3) after the acute recovery phase is over, secondary de finitive management is started.
Rapid and temporary stabilization is achieved by using LAD. Delaying the onset and progress of secondary damage has been attempted by using cryotherapy, but there is a lack of data related to the temperature and duration of cryotherapy required. Secondary, de finitive reconstruction may be performed under LAD.
Cryotherapy (exposure to extreme cold) has been used to delay or prevent secondary injury in trauma or sports injury, but optimal tissue temperatures, the duration of cryotherapy, and the need for application pressure to minimize secondary injury following musculoskeletal trauma have yet to be established[31].
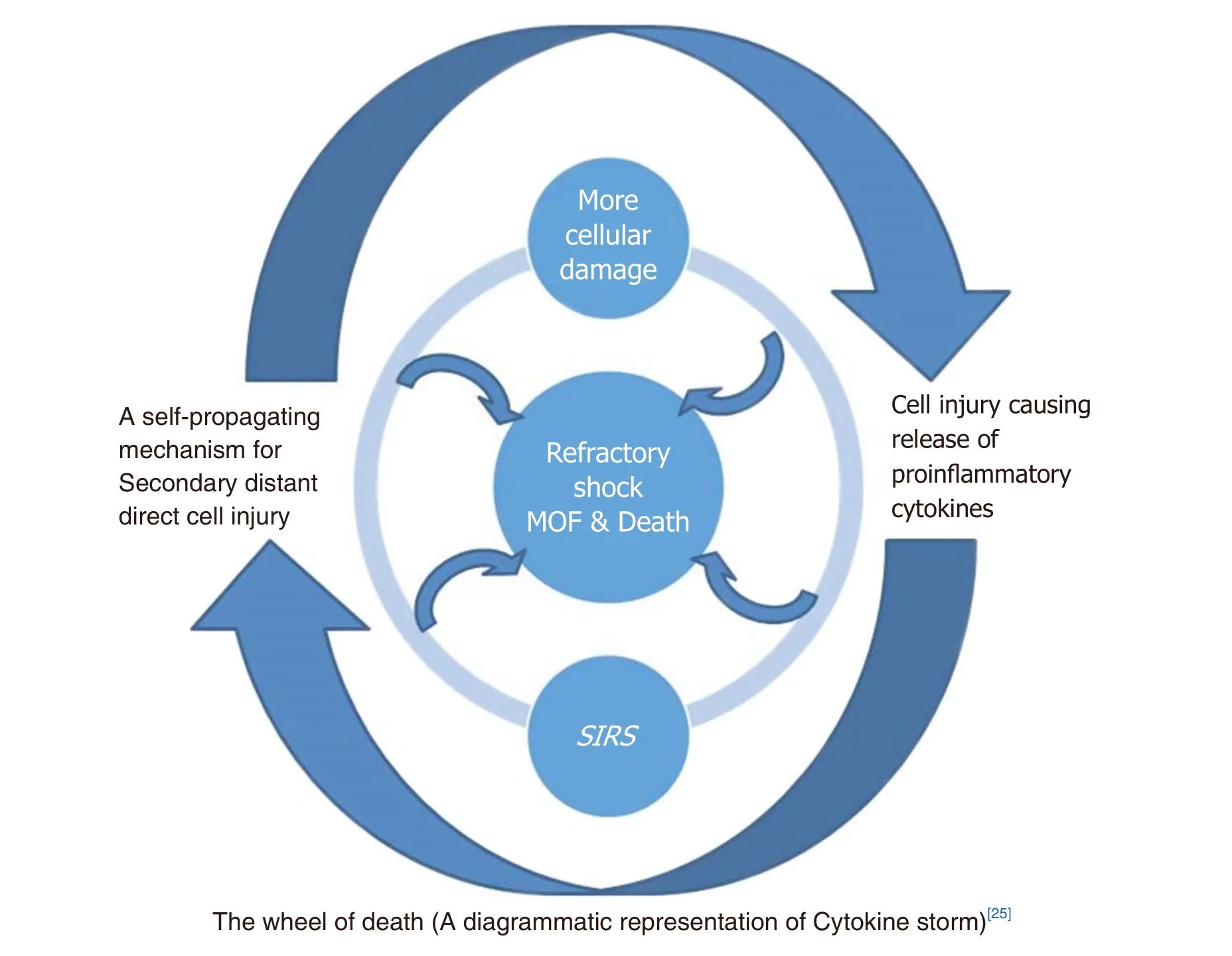
Figure 2. Diagrammatic representation of cytokine storm - the wheel of death. MOF: multiple organ failure; SIRS: systemic inflammatory response syndrome
Hyperbaric oxygen (HBO): in an experimental study on rats with contused calf muscle injury, following 7 days of treatment with 100% oxygen in a HBO chamber at 2.5 atmospheres pressure for 2 hours/day.HBO was found to reduce edema and in flammation, and accelerated myogenesis. This indicated bene ficial effects of HBO against secondary damage. The mechanisms of healing following HBO therapy for muscle injuries have yet to be explained however[32].
Anticytokine and antimediator therapy: has been tried in SIRS and MODS with limited success.
Forced diuresis and dialysis is recommended to prevent and treat renal dysfunction following myoglobinuria/hemoglobinuria[33].
Neurological/neurosurgical management and rehabilitation of neurological secondary injury has been advised[34,35].
Advanced wound management using special modern dressing signi ficantly reduces infection rates and the need for corrective surgeries[36].
LAD protocol in trauma
LAD is applied over the affected part after saline wash. After surgical removal of obviously dead tissue,application of antimicrobial agents, and complete hemostasis, LAD is applied. Pressure bandaging and appropriate splints are applied as required. Continuous or intermittent negative pressure is applied as per protocol or as required. The minimum required effective negative pressure produces folds of plastic of LAD that lie snuggly over the wound or skin under LAD; higher pressures are guided by pain complaints by the patient and bleeding from the wound. Physiotherapy and occupational therapy may be started at the earliest. Obviously dead tissues are removed surgically and a new LAD is reapplied every 5-7 days. The LAD may be changed if signi ficant leakage occurs. Daily LAD wash with saline and antimicrobial (Betadine)solution is performed. A secondary, elective procedure is performed (e.g., split skin graft/ flapetc.) as and when required. Physiotherapy may be required after satisfactory healing[25,30,37].
Role of LAD
LAD provides limited access of wound pathogens to the hospital environment and vice versa. LAD is helpful in preventing and treating secondary damage in trauma by reducing edema and removing harmful enzymes, toxins and other chemicals from the open wound[37].
Effects of LAD on oncosis (cellular swelling)
Cellular swelling due to sodium and potassium pump failure obstructs the microcvasculature, leading to cell death and necrosis of the affected tissue. Intermittent negative pressure of LAD produces intermittent compression of the part under LAD leading to reduction in edema. This intra LAD compression, if combined with early intra LAD physiotherapy, effectively controls edema. Edema reduction thus improves the circulation of adjacent tissues[37].
Effects of LAD on pH
In a RCT (randomized control trial)[38], 42 patients with chronic wounds in each group (LAD, conventional dressing) were studied for wound surface pH. On the 10th post-operative day, the LAD group showed a signi ficant (P =0.048) reduction in pH as compared to the conventional dressing group (LAD group 0.41 ±0.26vs.conventional group 0.83 ± 0.52) with the mean wound surface pH (± SD) in the LAD group 7.5 ± 0.43.
Following trauma, the release of lysosomal enzymes is responsible for secondary damage. Lysosomal enzyme activity (lysosomal digestion) is optimal in acidic pH (< 5), reduced at near neutral pH, and is nearly de-activated at a pH of 7.2[39]. Lysosomal enzymes are not only removed by LAD but also deactivated, or its activity reduced by a change in pH under LAD. Hence, LAD may prove to be an effective tool to control secondary damage following trauma.
LAD provides a safe environment during waiting or temporization
during the waiting period, infection and SIRS may pose a difficult problem but is controlled effectively with LAD.
1. Control of infection. LAD controls infection in the following ways[37,40]: wound isolation and safe disposal of drainage. The important feature of LAD design helps to control infection.
Prevention of wound invasion: negative pressure of LAD provides an alternate channel for the movement of microorganisms. Intermittent or continuous negative pressure reduces bacterial concentrations (< 105/g tissue) to a level that prevents invasion.
Mechanical disruption of quorum sensing by negative pressure occurs as negative pressure prevents the desired concentration of bacterial chemicals through intermittent/continuous removal.
Mechanical disruption of bio film: Higher intra-LAD negative pressure can cause disruption of the bio film and expose bacteria in the niche environment to negative pressure.
MDR (multi drug resistant) organisms can be effectively treated: Multi-drug resistant organisms are not resistant to the negative pressure of LAD.
2. Control of SIRS
LAD reduces systemic symptoms and signs of toxicity related to traumatized tissues, burns and gangrene.In a study of two groups comprising 54 burn patients (27 in each of LAD and control groups; at the time of induction, both groups showed no signi ficant difference), there was no statistical difference in SIRS on day 1,but SIRS and organ dysfunction on day 5 was signi ficantly lower (P-values of 0.029 and 0.017 respectively)in the LAD treated group[30,37].
Protection of ischemia induced oxidative damage in LAD treated wounds
Ischemia induced anaerobic respiration leads to reduced ATP production, and reduced antioxidant protection[41]. Studies on LAD treated burn wounds[42,43], diabetic wounds[44]and chronic wounds[45,46]have shown signi ficant reduction in oxidative stress (malondialdehyde level), and signi ficant increase in antioxidants and nitric oxide levels.
Clinical study to find role of LAD in trauma
In a case series[30]of 20 consecutive cases of musculoskeletal extremity trauma treated with LAD without specific controls, 14 cases had exposed, problematic structures with exposed bone in 8 cases, exposed tendons in 3 cases, exposure of both bone and tendon in 2 cases, and an exposed injured brachial artery in 1. Results were quite encouraging. Edema under LAD in these cases was minimal. There was a reduction in the number of debridements: total number of debridement procedures was 23 in 20 patients (average 1.15/patient; range 0-3). Wound bed preparation time was excellent in 5/18 cases, fair in 11/18 cases and poor in 2/18 cases. Excellent (> 99% of grafted area) graft take was seen in 18/20 cases. Conversion rates from cases that required complex reconstructive procedures (e.g., flap) for exposed vital structures to simple reconstructive procedures (SSG) was 11/13 × 100 = 84.6%. Functional recovery of the hand was excellent in 4/10 cases, fair in 2/10 cases, and poor in 4/10 cases.
The average cost of treatment was less than one-third of the treatment cost for similar procedures using wet-to-dry dressing (cost calculation was done based on reduced number of debridements, reduced anesthetic requirement, excellent graft take, reduced post-treatment physiotherapy, and rehabilitation costs).
From an administrative point of view, the quality of care was improved due to the reduction in required resources in emergency.
CONCLUSION
It was concluded that in addition to available medical and surgical interventions, substituting conventional closed dressings with LAD in cases of musculoskeletal trauma helps in reducing secondary damage as evidenced by the reduced number of debridements, reduced wound coverage time, and reduction in total length of hospital stay while lowering treatment costs and improving quality of care.
DECLARATIONS
Authors’ contributions
Made substantial contributions to the conception and design of the study and performed data analysis and interpretation: Kumar P
Made substantial contributions to data acquisition and performed data analysis and interpretation: Gupta A,Gupta A
Availability of data and materials
Not applicable.
Financial support and sponsorship
None.
Conflicts of interest
All authors declared that there are no con flicts of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Copyright
? The Author(s) 2020.
 Plastic and Aesthetic Research2020年6期
Plastic and Aesthetic Research2020年6期
- Plastic and Aesthetic Research的其它文章
- Stem cells and tissue engineering: an alternative treatment for craniofacial congenital malformations and articular degenerative diseases
- AUTHOR INSTRUCTIONS
- Established and experimental techniques to improve phalloplasty outcomes/optimization of a hypercomplex surgery
- Nerve transfers in distal forearm and in the hand
- Lympho-SPECT/CT as a tool to evaluate postoperative outcomes after LVA for lymphedema repair
