Development of innovative tools for investigation of nutrient-gut interaction
Wei-Kun Huang, Cong Xie, Richard L Young, Jiang-Bo Zhao, Heike Ebendorff-Heidepriem, Karen L Jones,Christopher K Rayner, Tong-Zhi Wu
Abstract The gastrointestinal tract is the key interface between the ingesta and the human body. There is wide recognition that the gastrointestinal response to nutrients or bioactive compounds, particularly the secretion of numerous hormones, is critical to the regulation of appetite, body weight and blood glucose. This concept has led to an increasing focus on “gut-based” strategies for the management of metabolic disorders, including type 2 diabetes and obesity. Understanding the underlying mechanisms and downstream effects of nutrient-gut interactions is fundamental to effective translation of this knowledge to clinical practice. To this end, an array of research tools and platforms have been developed to better understand the mechanisms of gut hormone secretion from enteroendocrine cells. This review discusses the evolution of in vitro and in vivo models and the integration of innovative techniques that will ultimately enable the development of novel therapies for metabolic diseases.
Key words: Nutrient-gut interaction; Metabolic disorders; Incretin hormones; Enteroendocrine cells; Enteroids; Intestinal intubation; Intestine-on-a-chip
INTRODUCTION
It is now widely appreciated that the gastrointestinal (GI) tract not only serves to process food, but also represents the largest endocrine organ in the body, releasing a wide array of peptide hormones to orchestrate metabolic homeostasis[1]. Ghrelin, for example, is released from gastric Gr-cells into the circulation during fasting or periods of negative energy balance and triggers hunger to drive food intake[2]; ghrelin levels in circulation are subsequently suppressed upon feeding[3]. The interaction of nutrients and digestive juices with the intestinal mucosa triggers the secretion of a number of postprandial hormones, including cholecystokinin (CCK) from enteroendocrine (EE) I-cells[4]and glucose-dependent insulinotropic polypeptide (GIP) from K-cells in the upper small intestine, and glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) from L-cells located predominantly in the distal small and large intestine[5,6]. A subset of EE cells in the proximal small intestine have also been shown to secrete both GLP-1 and GIP[7]. GLP-1 and GIP are known as the incretin hormones; both stimulate insulin secretion in a glucose-dependent manner[8,9]. GLP-1 also suppresses glucagon and acts with CCK and PYY to inhibit appetite, slow the delivery of nutrients from the stomach into the small intestine and retard their subsequent absorption[10]. Accordingly, the integrated responses of GI hormones to meal ingestion is a critical determinant of energy balance and postprandial glycaemia.
That plasma concentrations of GI hormones are typically increased after enteral, but not intravenous, nutrient administration attests to the importance of nutrient-gut interactions to the release of these hormones[11]. Accordingly, improved understanding of the sensor and actuator mechanisms through which nutrients or bioactive compounds interact with EE cells, has the potential to yield novel “gut-based” approaches for the management of metabolic diseases. In the last few decades, a broad range of preclinical and clinical models have been developed to study nutrient-gut interactions, with increasing efforts to achieve clinically relevant outcomes. To this end,ex vivostudies have extended from the use of EE cell lines towards primary intestinal tissues and organoids, and have increasingly incorporated sophisticated culture conditions to mimic normal physiology. Clinical studies employing customised intestinal perfusion catheters for targeted delivery of nutrients or therapeutic compounds, or novel ingestible sensors, have attempted to better characterise the regional specificity of GI responses. In this review, we summarise the research tools and models used to investigate nutrient-gut interactions, and discuss their advantages and limitations for clinical translation of findings (Table 1).
CELLULAR MODELS
The GI mucosa incorporates a monolayer of columnar epithelium with region-specific architecture and EE cell composition that is uniquely tuned to secrete specific gut hormones and absorb nutrients to fulfil specific metabolic functions. EE cells account for less than 1% of all epithelial cells, and their distribution varies substantially along the GI tract (Figure 1)[12]. Immortalised cell lines derived from murine and human intestinal tumours have been developed forin vitrostudies, and retain the capacity to secrete GI hormones in response to nutrient stimuli (Table 2).
STC-1 cells are a heterogeneous and poorly differentiated EE cell line derived from intestinal secretin-producing tumours in mice. They have a high immunoreactivity to anti-proglucagon sera and are capable of releasing glucagon-like immuno-reactants[13]. STC-1 cells were subsequently shown to secrete multiple gut hormones, including CCK[14], GLP-1[15,16], GIP[17], and PYY[18,19], in a similar manner to native murine EE cells, when stimulated by glucose[20], amino acids and fatty acids. As a result, STC-1 cells have been a popular model to screen for gut hormone-releasing stimuli. However, the clinical relevance of this model has been frequently questioned. For example, treatment with potato protease inhibitor concentrate (PPIC) or whey protein does not induce CCK secretion from STC-1 cells[21,22]. By contrast, oral administration of PPIC (100 mg/kg per day) stimulates CCK secretion in rodents[21], while ingestion of whey protein (55 g) increases plasma CCK levels in humans[23].
GLUTag cell line is a subcloned homogeneous EE cell model developed by the Drucker group from an endocrine carcinoma of the large bowel in transgenic mice[24]. These cells express both proglucagon and CCK genes[25]but produce primarily GLP1(7-36)-amide. GLUTag cells are equipped with a wide repertoire of nutrient sensors and transporters, including G-protein coupled receptors (GPCRs)[26], glucokinase[27]and the sodium-glucose linked transporter 1 (SGLT1)[28]involved in nutrient-induced GLP-1 secretion. In agreement within vivofindings, GLUTag cells exhibit robust release of GLP-1 in response to glucose[29], bile acids[30], fatty acids[31]and amino acids[32]. These observations have promoted GLUTag cells as a frontline model of L cells, leading to a wide application for studying the mechanisms underlying GLP-1 secretion and for screening potential GLP-1 secretagogues. However, clinical studies are still required to validatein vitrofindings. For example, the treatment of glutamine (10 mmol/L) was shown to markedly increase GLP-1 secretion (7-fold) from GLUTag cells[32]. However, oral administration of encapsulated ileal-release glutamine (6 g) or intra-duodenal glutamine infusion (7.5-15 g) evoked only modest increases in plasma GLP-1 levels in healthy subjects and patients with type 2 diabetes[33,34].
The human cell lines NCI-H716 and HuTu-80 have also been used widely to characterise nutrient-evoked GLP-1 release. The NCI-H716 cell line was first reported by Parket al[35]from human colorectal carcinoma. It contains dense-core granules, expresses chromogranin A, and secretes GLP-1 in response to glucose, fatty acids and protein hydrolysates[36]. Studies incorporating the NCI-H716 cell line have revealed critical roles of amino acid transporters[37], type 1 taste receptors[38]and monoacylglycerol-sensing GPCR[31]in GLP-1 secretion. However, the secretory profile of NCI-H716 cells is more limited compared to murine STC-1 or GLUTag cells. For example, NCI-H716 cells secrete GLP-1 and GLP-2 but not GIP, PYY or CCK in response to 50 mmol/L KCl, or combined glucose (10 mmol/L), forskolin and phosphodiesterase inhibitor (10 μmol/L)[39]. That NCI-H716 cells do not secrete PYY reflects their limited resemblance to native L-cells.
The HuTu-80 cell line is an alternative EE cell model of human origin that secretes GLP-1, GIP, PYY and CCK[40]and was developed initially to study the biology of GI cancers[41]. Sweet and bitter taste receptors are abundantly expressed in HuTo-80 cells as in native human L-cells, making them a potential model to investigate tastantinduced gut hormone secretion[42,43]. However, unlike native L-cells, bitter tastants, including quinine, denatonium benzoate and phenylthiocarbamide fail to trigger GLP-1 secretion from HuTu-80 cells[44]. Relative to the three aforementioned cell lines, HuTu-80 cells have been less frequently employed to study nutrient-gut interactions.
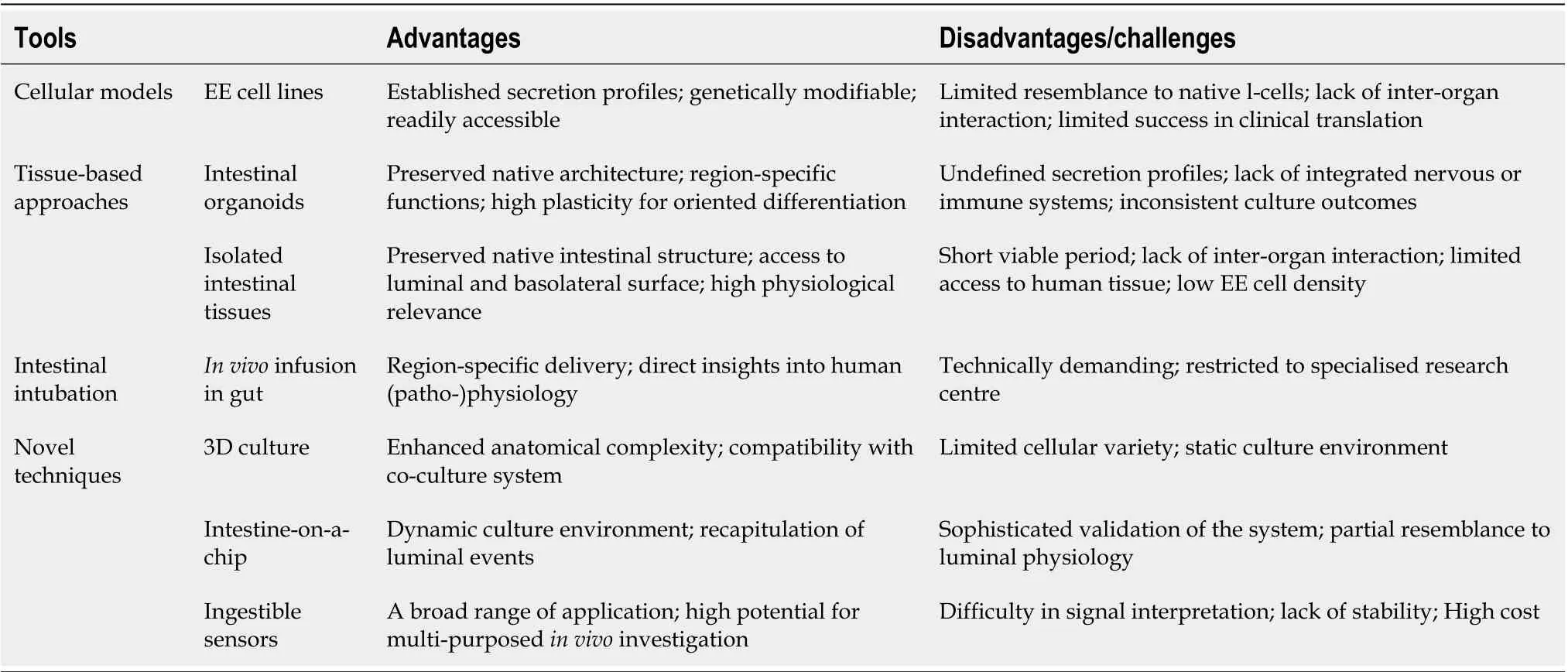
Table 1 Available tools used for investigation of nutrient-gut interactions
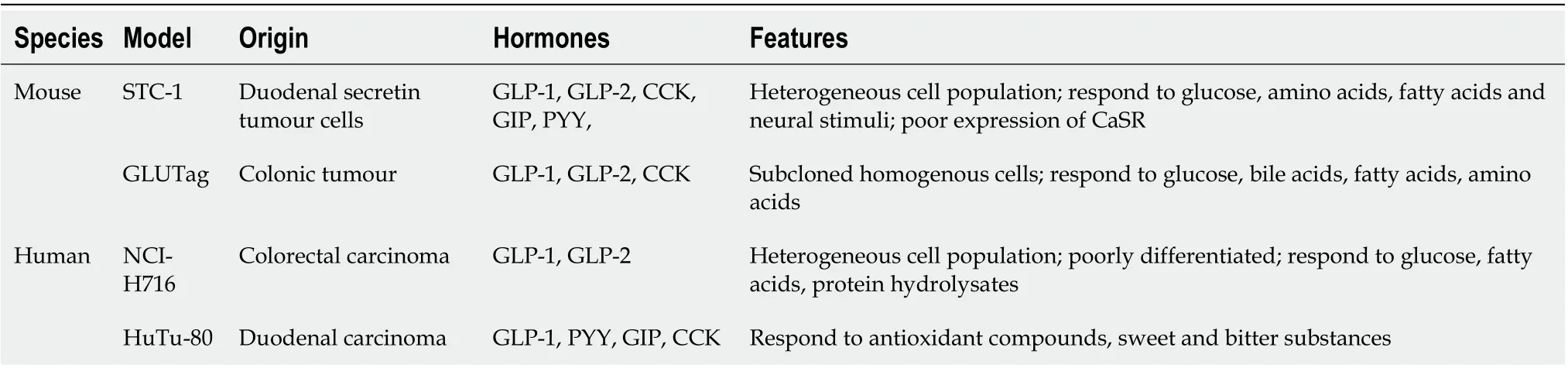
Table 2 Enteroendocrine cell models
TISSUE-BASED GUT HORMONE RELEASE EX VIVO
The major functional differences between immortalised intestinal cell lines and primary EE cells have led to an increased research focus on primary intestinal models to study the endocrine function of the gut. These have included the isolation and use of primary EE cells[45-47]and use ofex vivointestinal tissue preparations from animals[48-51]and humans[52,53]. These tissue-based approaches maintain native cell-cell connections and polarity, and have hitherto yielded a deep understanding of the mechanisms governing nutrient and drug-evoked 5-hydroxytryptamine and GLP-1 release[54,55]. However, clinical access to gut endoscopic, colonoscopic or surgical tissues, tissue viability and potentially low EE cell density can limit these primary models. The purification of primary EE cells is also technically demanding. The recent development of intestinal organoids holds the promise to overcome some of these limitations.
INTESTINAL ORGANOIDS
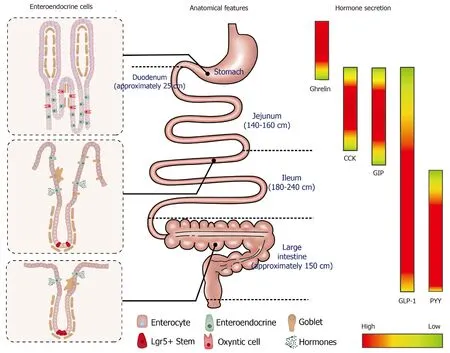
Figure 1 The composition of intestinal epithelial cells along the gastrointestinal tract (left); anatomical features and typical length of different sections of gastrointestinal tract (middle); regionally specific secretion profile of different gut hormones, including ghrelin, cholecystokinin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1 and peptide YY (right). CCK: Cholecystokinin; GLP-1: Glucagon-like peptide 1; GIP: Glucose-dependent insulinotropic polypeptide; PYY: Peptide YY.
Intestinal organoids, also known as “mini-guts”, are miniaturised intestinal units that display many features of gut tissue architecture and function. In 2007, Barker and colleagues identified leucine-rich repeat-containing GPCRG-5 (Lgr5) -positive cells as stem cells in the small intestine and colon via genetic lineage tracing experiments[56]. Subsequently, a single Lgr5-positive stem cell was shown to differentiate into cryptvillus organoids, namely enteroids, that are inclusive of all cell types present in the native intestinal epithelium[57]. Of note, enteroids can be developed from Lgr5-positive cells originating from any section of the gut.Ex vivocharacterisation has shown that these enteroids display the basal-apical polarity of mature epithelial cells[58,59]. Moreover, they retain many region-specific functions of the original location from which the stem cells were taken[60].
Intestinal organoids can also be developed from human pluripotent stem cells, which are referred to as human intestinal organoids (HIOs)[61,62]. HIOs have similar morphology as enteroids and display crypt-villus structures inclusive of all intestinal cell types. By contrast, HIOs contain a mesenchyme layer that is composed of myofibroblasts, endothelial cells and smooth muscle[63]. Moreover, HIOs do not show region-specific features and eventually grow into an unselective population of EE cells[61]. The differentiation process has been shown to be enhanced by the Happy Cell Advanced Suspension Medium[64]and by activation of the bone morphogenetic protein signalling pathway[65].
In contrast to primary intestinal epithelium, intestinal organoids remain viable for over 1-yearex vivoand show plasticity in cellular composition in response to changes in the culture environment or modified gene expression. Accumulating evidence suggests that the density of EE cells in organoids is subject to the expression of several translational factors, including Neurogenin 3 andAristaless-related homobox[61,66,67], raising the prospect that EE cells can be customised in an organoid. Indeed, exposure of mouse or human enteroids to short-chained fatty acids (SCFAs) increases the number of L cells, and hence GLP-1 secretion, over 48 h of SCFA treatment[68]. Similar trends in differentiation have also been observed with enteroids treated with dibenzazepine or bile acids[69,70]. However, the secretory profile of intestinal organoids in response to nutrients or non-nutritive compounds has not been well characterised. It should also be noted that delivery of stimuli to the lumen of the organoids requires individual microinjection, which is both labour-intensive and technically demanding due to their small size[71]. Moreover, the culture of organoids in conventional platforms makes it difficult to mimic the continuous movement of luminal contents and constantly changing nature of the extracellular fluid. Finally, it is not yet possible to recreate the architectural complexity of the GI tract, including its vascular, nervous, immune, mucous elements and the microbiome, in any organoid preparation.
INTESTINAL PERFUSION IN VIVO
The development of intestinal perfusion techniques and analytic methods capable of measuring GI hormones released into the peripheral circulation has allowed the evaluation of nutrient-gut interactionsin vivo. In rodents, dietary effects on gut hormone secretion have been investigated in models of isolated intestinal perfusion[72,73]. In humans, it is also possible to characterise the responses of various regions of the gut to intraluminal stimuli, and to examine the underlying mechanisms.
A rubber feeding tube was initially designed to deliver medication to the intestine and to examine luminal contents in paediatric patients[74]. This early design incorporated 1-2 cm wide lateral window(s) for infusion/aspiration of liquids and a weighted terminal bulb to facilitate passage of the catheter by peristalsis. Subsequently, intestinal catheters have been increasingly customised to study gut function. For example, the integration of an inflatable balloon at the distal end of the catheter was employed to evaluate the perception of distension or control the position of the catheter[75]. Use of a multi-lumen catheter has allowed for multiple inflatable balloons, making it possible to isolate segments of the lumen[76], within which nutrient absorption can be carefully characterised[77-79]. Incorporation of manometry and impedance sensors into the catheter design has further facilitated concurrent recording of gut motility[34]and flow events[80]. Positioning these catheters has relied on fluoroscopy, for which radiation exposure represents a major limitation. To overcome this, Andersson and Grossman established an alternative method of monitoring catheter position by measuring transmucosal potential difference (TMPD) between skin or blood and the intestinal lumen[81]. Corresponding to the differences in pH between the stomach and the duodenum, TMPD in the distal antral channel and the proximal duodenal channel record around -40 mV and 0 mV, respectively[82,83]. Accordingly, a change of TMPD from -40 mV to 0 mV reflects passage of channels through the transpyloric area (Figure 2).
Relative to oral administration, intestinal perfusion of nutrients or investigational compounds circumvents the impact of inter-individual variations in the rate of gastric emptying – which can be substantial[84-86]– such that the exposure of the small intestine to nutrients can be standardised. Studies employing intraduodenal infusion of nutrients spanning the normal range of gastric emptying (1-4 kcal/min) have established that the stimulation of gut hormones, including CCK, GIP, GLP-1 and PYY, is dependent on the rate of nutrient entry into the small intestine. In line with the biological distribution of respective EE cells, the secretion of CCK and GIP appears to be proportional to the load of glucose, lipid or protein, whereas GLP-1 and PPY responses are non-linear, being modest at 1-2 kcal/min and substantially greater at 3-4 kcal/min[87]. Moreover, when glucose and fat are infused intraduodenally at an identical rate of 2 kcal/min, it is observed that fat is significantly more potent than glucose at stimulating GLP-1 and GIP secretion[88].
A multi-lumen catheter of adequate length can also be positioned over a long length of small intestine to allow targeted delivery of nutrients or investigational compounds into proximal or distal sites, to determine the regional specificity of nutrient-gut interactions. In this way, infusion of glucose (2 kcal/min) into jejunum (50 cm distal to pylorus) was shown to elicit more GLP-1 and GIP release compared to equivalent duodenal infusion (12 cm distal to pylorus) in healthy men[89]. Furthermore, ileal glucose infusion (2 kcal/min, 190 cm distal to pylorus) resulted in markedly greater GLP-1 and lower (but more sustained) GIP responses compared to intraduodenal infusion, and was associated with a greater incretin effect and GI-mediated glucose disposal in both healthy subjects and patients with type 2 diabetes (Figure 3)[90]. Administration of compounds into the rectum can similarly be undertaken using a soft tube with minimal discomfort[91,92]. Characterisation of the region-specific profile of gut hormone release has shed light on the mechanisms by which Roux-en-Y gastric bypass surgery improves blood glucose control in type 2 diabetes[93]. In addition, this knowledge has directed the precise delivery of stimuli to optimise gut hormone response for therapeutic gain. For example, enteric coating of a small dose of lauric acid to allow targeted release in the ileum and colon was shown to be effective at stimulating GLP-1 secretion and lowering blood glucose in patients with type 2 diabetes[94].
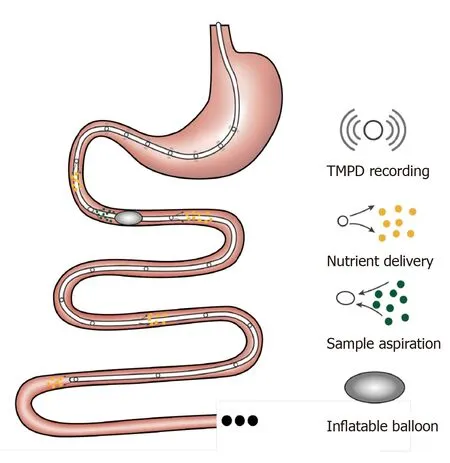
Figure 2 Schematic of a multichannel intestinal catheter to study regional specificity of nutrient-gut interactions. Multiple channels are opened on the catheter to record the transmucosal potential difference and monitor its position. These channels can also deliver investigational compounds of aspirate luminal samples in a specific region of intestine. The balloon is generally designed to create physical restriction to prevent the fluid flow or the movement of the catheter. TMPD: Transmucosal potential difference.
Access to the intestines via endoscopy and colonoscopy has provided an additional means for targeting intestinal perfusion to a specific region, while also allowing for the collection of mucosal biopsies to study anatomical and molecular mechanisms underlying nutrient-gut interactions (discussed in earlier section). In this way, sweet taste receptors (STRs) (heterodimeric T1R2 and T1R3) were found to be involved in intestinal glucose sensing and linked to regulation of glucose absorption in both health and type 2 diabetes; in patients with type 2 diabetes, a defect in the downregulation of STRs in the face of hyperglycaemia was shown to contribute to excessive postprandial glycaemic excursions[95]. Moreover,ex vivostudies using human intestinal biopsies have revealed a critical role for both SGLT1 and the facilitative glucose transporter 2 in mediating glucose-induced GLP-1 secretion[55].
NOVEL TECHNIQUES TO STUDY GUT HORMONE SECRETION
Several novel techniques are emerging to evaluate nutrient-gut interactions with improved physiological or therapeutic relevance, while overcoming limitations of clinical studies.
Recent development of culture engineering techniques has allowed integration of advanced culture interfaces into the conventional 2D culture platforms of intestinal organoids and primary epithelial cells. This has enabled the provision of culture frameworks that support the growth of intestinal cells and facilitate the assessment of tissue function in a more physiologically relevant environment[96,97]. For example, culturing intestinal cells on a porous polyester membrane provides access to both basolateral and apical sides of the polarised epithelial cells, which is of particular importance for the investigation of the intestinal function in response to luminal stimuli (Figure 4A). In addition, the membrane can be coated with an extracellular matrix containing growth factors to induce growth and differentiation of organoids. This experimental platform is being increasingly used to study intestinal barrier function[98,99], immune responses[100], and drug metabolism[101,102], with a handful of studies focusing on nutrient-gut interactions. Kozukaet al[103]developed an intestinal monolayer culture platform utilising Transwell (a culture plate with an inserted membrane) with a 0.4 μm or 1 μm pore membrane and successfully cultured murine and human intestinal enteroids. Treatment with forskolin (100 μM) in the apical chamber stimulated GLP-1 release into the basolateral chamber, consistent with the presence of functional L-cells and GLP-1 deployment mechanisms. With this compartmental culture system, it is possible to model the interaction between the intestinal epithelium and luminal content and monitor the hormonal response in the downstream chamber.
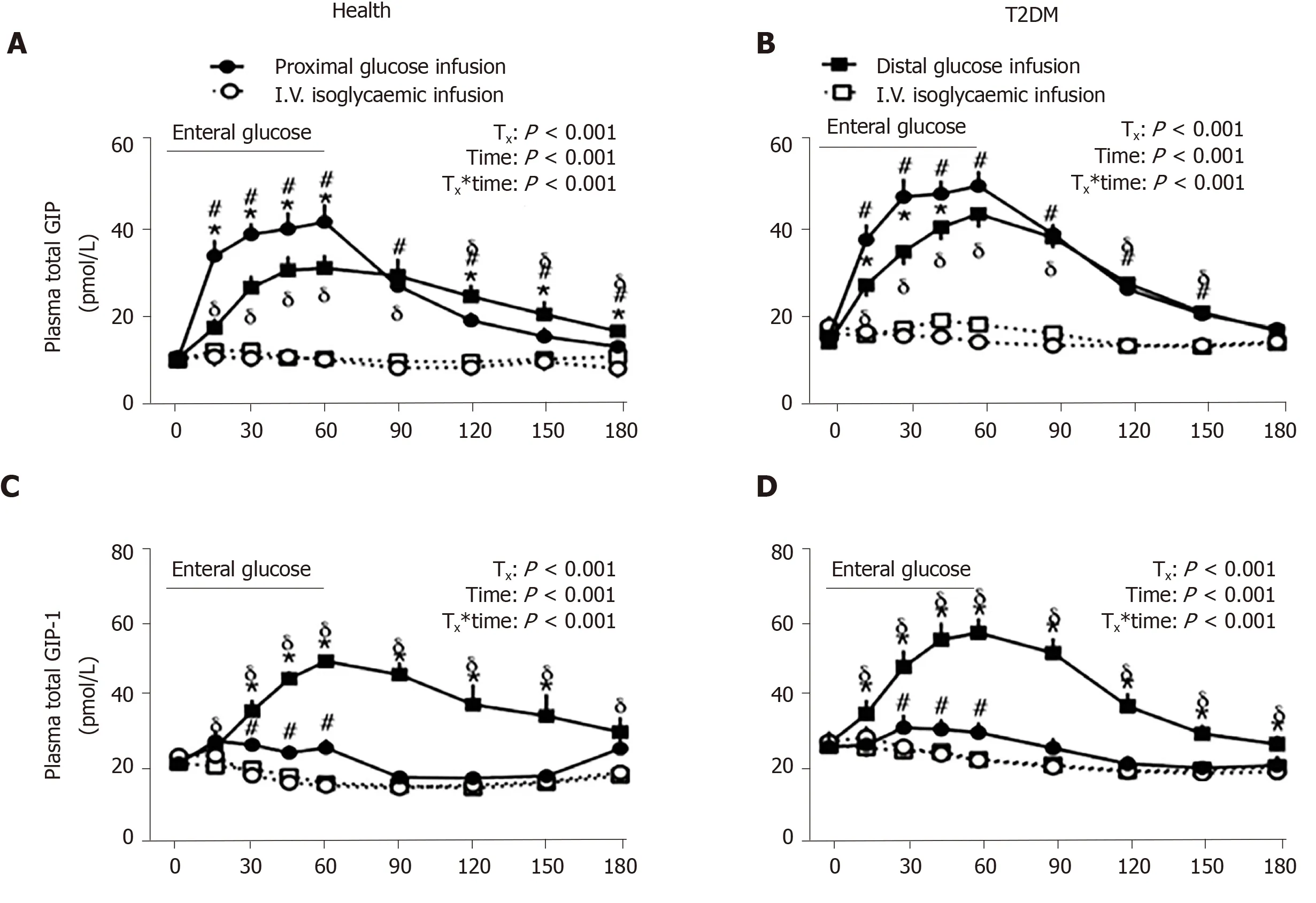
Figure 3 Comparison of the effect of enteral (proximal or distal) and intravenous (i.v.) isoglycemic glucose administrations on plasma incretin hormone, glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 secretions in healthy subjects and subjects with type 2 diabetes mellitus. A and B: Glucose-dependent insulinotropic polypeptide; C and D: Glucagon-like peptide-1. Asterisk represents P < 0.05 for proximal vs distal enteral glucose infusion; Numbersign represents P < 0.05 for proximal enteral vs corresponding i.v. glycemic glucose infusion; Delta represents P < 0.05 for distal enteral vs corresponding i.v. glycemic glucose infusion. Data are presented as mean ± SEM. GLP-1: Glucagon-like peptide 1; GIP: Glucosedependent insulinotropic polypeptide; T2DM: Type 2 diabetes mellitus. Citation: Zhang X, Young RL, Bound M, Hu S, Jones KL, Horowitz M, Rayner CK, Wu T. Comparative Effects of Proximal and Distal Small Intestinal Glucose Exposure on Glycemia, Incretin Hormone Secretion, and the Incretin Effect in Health and Type 2 Diabetes. Diabetes Care 2019; 42: 520-528. Copyright? The Authors 2019. Published by American Diabetes Association.
More advanced and complex intestine models have been achieved by applying microfluidic devices in gut function studies, also known as “intestine-on-a-chip”. These microfluidic devices have the capacity to provide a dynamic culture environment, including continuously refreshed culture media and biomimetic mechanical strain, to more accurately resemble physiological conditions (Figure 4B). Currentin vitrogut models on microfluidic devices have mainly been used to investigate drug metabolism[104]and gut-liver interactions[105]. The application of the “intestine-on-a-chip” model for gut hormone secretion study is in its infancy. In 2016, Hsiaoet al[106]developed a high-throughput automated microfluidic platform to assess the response of NCL-H716 cells to sweet and bitter stimuli. Although gut hormones were not measured in the study, the microfluidic system recorded the dynamic changes in intracellular Ca2+in over 500 single NCI-H716 cells trapped in each microwell. In another study, Park and his colleagues established a co-culture of GLUTag cells and the β cell line INS-1 to screen compounds of anti-diabetic potential[107]. Relative to the use of intestinal cancer cell line, intestinal organoids cultured on a microfluidic device display a high resemblance to the native intestine transcriptome, including the expression of genes related to cell proliferation, digestion and responses to nutrients[108], and may prove to be a usefulex vivomodel for studying GI hormone secretion.

Figure 4 Emerging advanced techniques to study nutrient-gut interaction. A: 2D culture of intestinal epithelium on a porous membrane; B: intestineon-a-chip model with intestinal organoids cultured in a microfluidic device, where constant perfusion and periodic mechanical strain can be applied on the system; C: ingestible sensors for measuring various parameters relevant to gut functions.
Ingestible sensors are under rapid development in clinical settings. These are typically capsule devices of up to 11 mm in diameter and 28 mm in length, to allow easy transit through the gut while measuring biomedical parameters (Figure 4C). To date, ingestible sensors have been developed for imaging[109-112]and measurements of gases[113], pH, temperature[114-117], pressure[118]and luminal contents[119-121]. The pH sensors have been used to assess gastric emptying and small intestinal transit, marked by abrupt pH changes between the stomach and duodenum (> 3 units) and between the ileum and colon (> 1 unit)[122,123]. The wide application of ingestible sensors will require further technical development to improve stability, signal interpretation and reduce costs, but offer an exciting glimpse into the future of GI surveillance.
CONCLUSION
A better understanding of the mechanisms underlying nutrient-gut interactions is fundamental to the development of gut-based therapies for major metabolic disorders. For this purpose, the development ofin vitroEE cell models, and techniques suitable forin vivostudies, particularly in humans, is of critical importance. EE cell lines of both murine (STC-1 and GLUTag) and human (NCI-H716 and HuTo-80) origin are useful for early studies on gut hormone secretion, but have had limited translational success. This necessitates the development of more physiologically relevantin vitrogut models. The emergence of intestinal organoids and novel co-culture systems represents a major advance in this area. In particular, the combination of intestinal organoids and microfluidics will provide an unprecedented opportunity to study the dynamic hormonal response to stimuli under various conditions.In vivovalidation of research outcomes derived from these models remains critical. In clinical studies, intestinal intubation and the application of novel ingestible sensors, have provided deep knowledge of the region-specific nature of nutrient-gut interactions, and ensuing hormonal and metabolic responses. Further development of non-invasive techniques suitable for use in humans will expand opportunities to translate research findings from the bench to bedside.
 World Journal of Gastroenterology2020年25期
World Journal of Gastroenterology2020年25期
- World Journal of Gastroenterology的其它文章
- Chinese expert consensus and practice guideline of totally implantable access port for digestive tract carcinomas
- Monoacylglycerol lipase reprograms lipid precursors signaling in liver disease
- Type I and type II Helicobacter pylori infection status and their impact on gastrin and pepsinogen level in a gastric cancer prevalent area
- Retrievable puncture anchor traction method for endoscopic ultrasound-guided gastroenterostomy: A porcine study
- Predictors of irreversible intestinal resection in patients with acute mesenteric venous thrombosis
- Multiphase convolutional dense network for the classification of focal liver lesions on dynamic contrastenhanced computed tomography
