Nonalcoholic fatty liver disease in lean subjects: Prognosis, outcomes and management
Lampros Chrysavgis, Eleftheria Ztriva, Adonis Protopapas, Konstantinos Tziomalos, Evangelos Cholongitas
Abstract Nonalcoholic fatty liver disease (NAFLD) accounts for most cases of chronic liver disease worldwide, with an estimated global prevalence of approximately 25% and ranges from simple steatosis to nonalcoholic steatohepatitis and cirrhosis. NAFLD is strongly connected to metabolic syndrome, and for many years, fatty liver was considered to be an exclusive feature of obese patients. However, recent studies have highlighted the presence of NAFLD in non-obese subjects, with or without increased visceral fat or even in lean subjects without increased waist circumference. “Lean NAFLD” is a relatively new concept and there is significant scientific interest in understanding the differences in pathophysiology, prognosis and management compared with NAFLD in overweight/obese patients. In the present editorial, we discuss the clinical and metabolic profiles and outcomes of lean NAFLD compared with both obese NAFLD and lean healthy individuals from Asian and Western countries. Moreover, we shed light to the challenging topic of management of NAFLD in lean subjects since there are no specific guidelines for this population. Finally, we discuss open questions and issues to be addressed in the future in order to categorize NAFLD patients into lean and nonlean cohorts.
Key Words: Lean nonalcoholic fatty liver disease; Non-obese nonalcoholic fatty liver disease; Clinical outcomes; Metabolic outcomes; Disease management; Lifestyle interventions
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) has been recognized as the predominant cause of chronic liver disease in the industrialized world[1]. It encompasses a wide spectrum of clinical and histological entities, ranging from simple steatosis, defined as triglyceride (TG) accumulation > 5% within the hepatic parenchyma, to nonalcoholic steatohepatitis (NASH), which is characterized by inflammation and fibrosis and can lead to cirrhosis and even hepatocellular carcinoma (HCC)[2,3]. The prevalence of NAFLD is increased in patients with type 2 diabetes mellitus (T2DM), metabolic syndrome (MetS) and obesity[4]. Although the latter is not only a risk factor for NAFLD but is also associated with more severe forms of the disease, a significant proportion of subjects develop NAFLD despite having a relatively normal body mass index (BMI), a condition referred to as non-obese or lean NAFLD[5]. Non-obese/lean NAFLD is divided into 2 major categories[5]: The first and more prevalent includes non-obese patients who may be overweight (BMI between the 85th-95th percentile for age) with or without increased waist circumference and adipose tissue, while the second category includes lean subjects with no excess visceral fat mass[5]. In the latter category, several secondary causes have been implicated, such as high fructose intake, protein malnutrition (Kwashiorkor) as well as administration of steatogenic drugs (amiodarone, tamoxifen, methotrexate, prednisolone,etc.) and genetic predisposition[5,6]. Regarding the latter, Romeoet al[7]have emphasized the involvement of the rs738409 single nucleotide polymorphism in patatin-like phospholipase domain-containing protein 3 (PNPLA 3) gene in NAFLD onset and progression. Yet, a plethora of other gene variants have been also associated with increased susceptibility to NAFLD/NASH and progression to liver fibrosis and even HCC, such as the transmembrane 6 superfamily member 2 (TM6SF2)[8-10], glucokinase regulatory gene (GCKR)[11,12]and membrane bound O-acyltransferase domain containing 7 (MBOAT7) genes[13]. In addition, a variant of interferon-λ3 (IFN-λ3) gene has been related with increased liver inflammation and fibrosis among NAFLD patients[14], while the rs72613567 polymorphism in hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) gene was recently shown to reduce the risk of liver fibrosis, NASH and HCC[15,16]. Of note, both dietary composition and socioeconomic factors have been correlated with NAFLD development. Adherence to Mediterranean diet has been demonstrated to ameliorate hepatic insulin sensitivity and reduce hepatic fat accumulation while the Western dietary pattern, which mainly consists of high fructose and saturated fats intake, has been involved in NAFLD development[17,18]. Moreover, prolonged sitting time, usually related with high calorie intake and unhealthy dietary composition, and decreased physical activity are independent risk factors for NAFLD, even in lean subjects[19].
Current data on the prevalence of non-obese/lean NAFLD worldwide is characterized by wide variability. In a recent systematic review including 84 studies with 10530308 individuals, Yeet al[20]demonstrated that among the general population, the prevalence of lean and non-obese NAFLD was 5.1% and 12.1%, respectively. In addition, the overall prevalence of NAFLD among the lean general population was 10.6%, while the prevalence of NAFLD in the non-obese population was 18.3%. Interestingly, the prevalence of non-obese NAFLD among the total NAFLD population was highest in Europe (51.3%) and lowest in eastern Asia (37.8%)[20]. Of note, NAFLD patients were categorized according to the World Health Organization (WHO) and Asian Pacific recommendations as overweight and lean when their BMI was 25 to 30 kg/m2and < 25 kg/m2, respectively, in non-Asian populations and 23 kg/m2to 27.5 kg/m2and < 23 kg/m2, respectively, in Asian populations[21-23]. However, it is wellestablished that individuals with similar BMI may have different degrees of visceral obesity, which is closely associated with the development of NAFLD[24-26]. Waist circumference is considered a more accurate marker of visceral obesity than BMI, but is not available in the majority of the relevant studies[27]. The present editorial will discuss the metabolic profile, prognosis and related clinical outcomes, as well as the management of non-obese or lean patients suffering from NAFLD.
CLINICAL IMPACT OF NON-OBESE/LEAN NAFLD
Literature search
PubMed database was systematically searched from the date of inception of this editorial until April 2020, to identify studies focusing on non-obese/lean NAFLD. The terms used were “Lean non-alcoholic fatty liver disease” OR “Lean nonalcoholic fatty liver disease” OR “Lean NAFLD” OR “Non-obese non-alcoholic fatty liver disease” OR “Non-obese nonalcoholic fatty liver disease” OR “Non-obese NAFLD” OR “Nonoverweight fatty liver disease” OR “Non-overweight NAFLD”. Since we aimed to emphasize the metabolic, hepatic and cardiovascular outcomes in obesevsnonobese/lean NAFLD patients as well as non-obese/lean individuals with or without NAFLD, studies evaluating the histological aspects of NAFLD were excluded.
Non obese/lean NAFLD vs controls: Metabolic and clinical outcomes (Table 1)
Younossiet al[28]in a study performed in the United States reported that lean (BMI < 25 kg/m2) NAFLD patients compared to lean healthy subjects had higher prevalence of insulin resistance (IR), T2DM, hypercholesterolemia and hypertension,i.e., the components of MetS. In the cross-sectional NHANES III study, Golabiet al[29]reported that lean (BMI < 25 kg/m2) NAFLD patients, had higher risk of all-cause [Hazard Ratio (HR): 1.54] and cardiovascular-related mortality (HR: 2.38) than lean non-NAFLD subjects after adjustment for potential confounding variables[29]. Interestingly, in another study from the United States, Zouet al[30]showed that in a non-obese population (BMI < 30 kg/m2for non-Asians and < 27 kg/m2for Asians), patients with NAFLD had higher blood pressure, fasting plasma glucose (FPG), insulin, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C) and TG levels and higher Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), a marker of IR, than subjects without NAFLD. In addition, the former group had increased overall, cardiovascular and cancer-related mortality during a 15-year follow-up, but these findings were not confirmed in multivariate analysis[30].
In a post hoc analysis in Japanese subjects, Yoshitakaet al[31]reported that lean (BMI < 23 kg/m2) NAFLD patients had higher blood pressure, increased FPG and TG serum levels, as well as greater risk (HR: 10.4) for cardiovascular events than to lean non-NAFLD individuals, independently of potential confounders. In a retrospective cohort study of 4629 lean Japanese participants (BMI < 23 kg/m2) who were enrolled in a regular health checkup program, Fukudaet al[32]showed that patients with NAFLD had more than 3 times higher incidence of T2DM than subjects without NAFLD. Regarding non-obese subjects, Nishiojiet al[33]found that non-obese (BMI < 25 kg/m2) Japanese NAFLD patients had a higher prevalence of MetS components compared with healthy individuals. Both retrospective and prospective studies from South Korea also showed that non-obese NAFLD patients have an increased risk for T2DM than non-NAFLD, non-obese individuals, independently of other risk factors[34,35]. Moreover, Sunget al[36]in a large cohort of non-obese (BMI < 27 kg/m2) South Korean individuals, reported that non-obese NAFLD patients have higher estimated cardiovascular risk based on the Framingham risk score than healthy controls, whereas in another South Korean cross-sectional study, non-obese (BMI < 25 kg/m2) subjects without NAFLD had better metabolic profile than non-obese patients with NAFLD[37]. Accordingly, Kwonet al[38], in another retrospective study from South Korea, showed that non-obese (BMI < 25 kg/m2) NAFLD patients had higher prevalence of MetS components than non-obese controls and had.
In lean (BMI < 23 kg/m2) Chinese individuals, the presence of NAFLD was associated with increased odds for T2DM and MetS, independently of demographic and lifestyle parameters[39]. Regarding non-obese populations, 2 independent studies inChina confirmed that non-obese (BMI < 25 kg/m2) patients with NAFLD suffered more frequently from hypertension and MetS than healthy non-obese subjects[40,41], whereas a cross-sectional study in China reported that non-obese (BMI < 27.5 kg/m2), normotensive and non-diabetic NAFLD patients had increased arterial stiffness, higher serum levels of FPG, TC, LDL-C, TG and greater HOMA-IR than non-obese, healthy subjects[42]. Similar findings were observed in Chinese women[43]. Moreover, a cohort study in India also showed that NAFLD patients are at higher risk for metabolic disorders irrespectively of the presence of obesity[44].
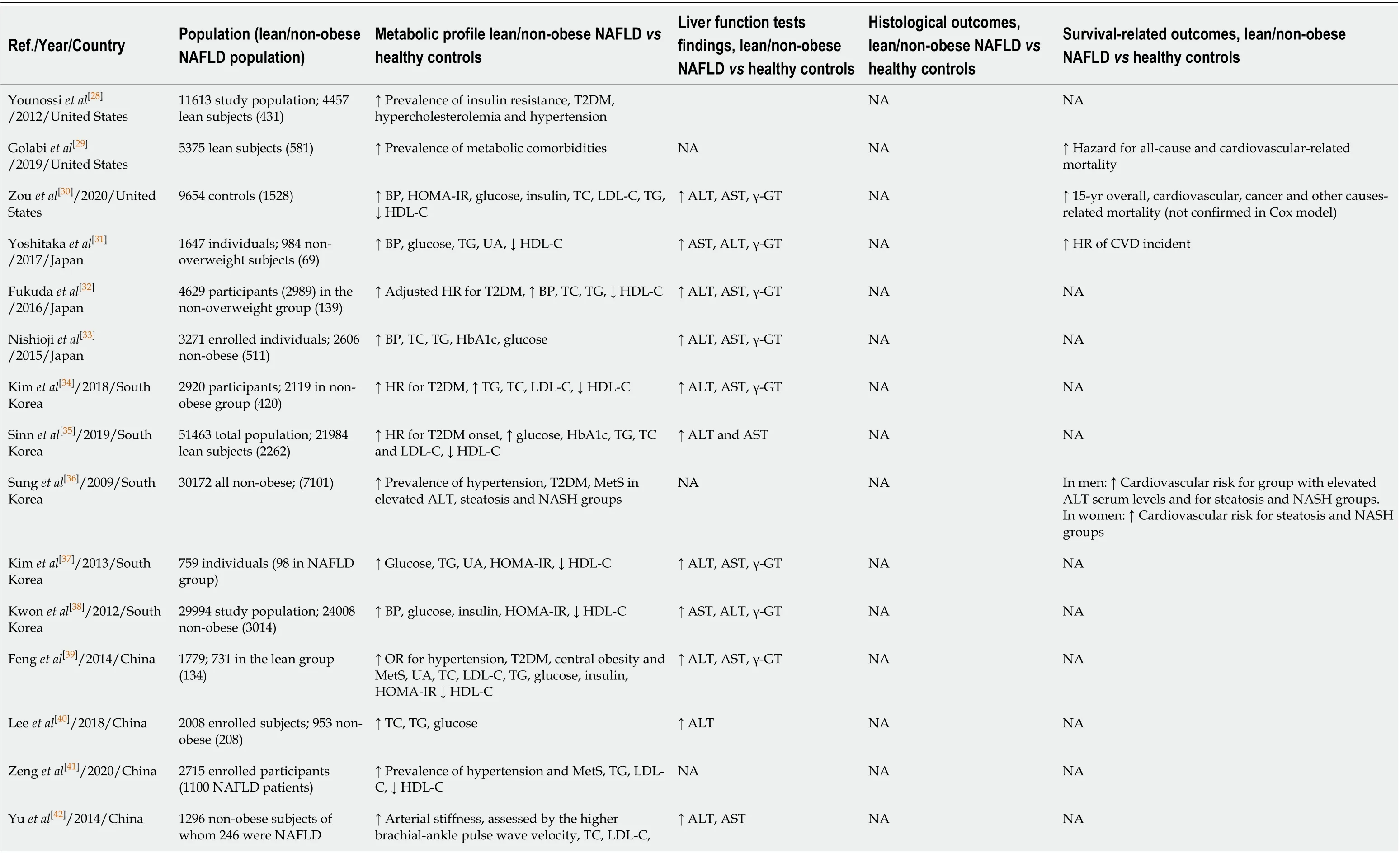
Table 1 Main findings and outcomes of lean (or non-obese) non-alcoholic fatty liver disease patients’ vs lean (or non-obese) healthy individuals
NAFLD: Non-alcoholic fatty liver disease; ALT: Alanine aminotransferase; T2DM: Type 2 diabetes mellitus; NASH: Non-alcoholic steatohepatitis; NAS: NAFLD activity score; BP: Arterial blood pressure; TC: Total cholesterol; LDL-C: Low density lipoprotein-cholesterol; HDL-C: High density lipoprotein-cholesterol; TG: Triglycerides; HR: Hazard ratio; HbA1c: Glycosylated hemoglobin, type A1C; HOMA-IR: Homeostasis Model Assessment Insulin Resistance; CVD: Cardiovascular disease; HCC: Hepatocellular carcinoma; NA: Not applicable; UA: Uric acid; AST: Aspartate aminotransferase; γ-GT: γ-Glutamyl transferase; MetS: Metabolic syndrome.
In accordance to the aforementioned studies, Oralet al[45]reported that non-obese (BMI < 30 kg/m2) NAFLD patients from Turkey were more frequently glucose intolerant and had higher TG and TC levels than non-obese controls. Similar findings were also reported in another Turkish study, where lean (BMI < 25 kg/m2) NAFLD patients had higher prevalence of hypertension and MetS as well as higher HOMAIR[46]. Finally, in Europe, Feldmanet al[47]reported that Austrian lean (BMI < 25 kg/m2) healthy subjects were more frequently glucose tolerant and had lower prevalence of T2DM than lean NAFLD patients and these findings were confirmed by Gonzalez-Canteroet al[48]in a non-obese (BMI < 30 kg/m2) Spanish cohort.
Obese vs non-obese/lean NAFLD (Table 2)
Studies with metabolic outcomes:Regarding metabolic outcomes in NAFLD obese and NAFLD non-obese/lean patients, in a retrospective study of 669 NAFLD patients in Italy, lean (BMI < 25 kg/m2) NAFLD patients had lower prevalence of hypertension, T2DM and MetS than overweight (25 kg/m2≤ BMI < 30 kg/m2) and obese (BMI ≥ 30 kg/m2) NAFLD patients[49]. Notably, the former group had significantly thinner carotid intima-media, indicating less atherosclerotic burden[49]. Although this result was not confirmed in a study by Shaoet al[50]in obese (BMI > 25 kg/m2) and non-obese Chinese NAFLD patients, the authors showed that the latter group had lower FPG and serum TC and TG levels as well as a lower prevalence of hypertension. Moreover, Liet al[51]demonstrated that the proportion of Chinese patients with elevated FPG and serum TG levels was higher among obese (BMI > 25 kg/m2) compared with non-obese NAFLD patients, while another cross-sectional study from China confirmed that obese NAFLD women (BMI > 28 kg/m2) had higher FPG than non-obese women[43]. In addition, 2 studies from China showed a higher prevalence of MetS and hypertension in obese (BMI > 25 kg/m2) compared to non-obese patients with NAFLD[41,52]. In the Indian population, Kumaret al[44]reported that among NAFLD patients, lean (BMI < 23 kg/m2) patients had had lower serum insulin levels and HOMA-IR, as well as lower prevalence of T2DM and MetS than obese (BMI > 25 kg/m2) patients. In a case control study from Sri Lanka, Niriellaet al[53]reported a higher prevalence of hypertension in non-lean (BMI > 23 kg/m2) patients with NAFLD compared with lean NAFLD patients. In studies performed in Japan, Yoshitakaet al[31]reported lower blood pressure and FPG and higher serum high density cholesterol (HDL-C) levels in lean (BMI < 23 kg/m2) than in overweight NAFLD patients[44], while Hondaet al[54]reported that FPG, insulin, TG and HOMA-IR were increased among Japanese obese (BMI > 25 kg/m2) NAFLD patients compared with non-obese NAFLD patients. In a study form Hong-Kong, Weiet al[55]reported that non-obese (BMI < 25 kg/m2) NAFLD patients had lower IR than obese NAFLD patients. Moreover, the prevalence of MetS and hypertension was increased in obese patients. A study performed in Bangladesh also showed that non-obese (BMI < 25 kg/m2) NAFLD patients had lower TC, FPG, HOMA-IR and higher HDL-C levels than obese NAFLD patients[56].
Similar findings were observed in the cross-sectional NHANES III study, in which lean (BMI < 25 kg/m2) NAFLD patients had less frequently hypertension, T2DM and hypercholesteremia as well as lower levels of FPG and HOMA-IR than overweight /obese NAFLD patients[28]. In a prospective study from Turkey, Akyuzet al[57]reported that lean (BMI < 25 kg/m2) NAFLD patients had a less prevalence of MetS and hypertension than overweight NAFLD patients, while in a study from Spain, Gonzalez-Canteroet al[48]also confirmed that overweight (BMI: 25-29 kg/m2) patients with NAFLD had higher HOMA-IR and TG and lower HDL-C serum levels than lean NAFLD patients. In a study from Austria, Feldmanet al[47]also reported that lean (BMI < 25 kg/m2) NAFLD patients had lower FPG, insulin and HOMA-IR and higher HDLC levels than obese NAFLD patients.
In contrast to these findings, a retrospective study from South Korea reported a higher prevalence of MetS components in non-obese (BMI < 25 kg/m2) NAFLD patients compared with obese NALFD patients, even after adjusting for confounders[38]. It is possible that unrecorded differences in dietary patterns, physical activity and smoking status between the 2 groups might explain this paradoxical might[38]. Leeet al[40]also reported that non-obese (BMI < 25 kg/m2) NAFLD patients had higher prevalence of MetS and hypertension as well as lower serum HDL-C levels than obese NAFLD patients. However, this study was hospital- and not communitybased suggesting the presence of selection bias as an explanation for these unexpected findings[40].
Studies with both metabolic and clinical outcomes:In a prospective cohort study in 307 NAFLD patients from Hong-Kong, Leunget al[52]reported that non-obese patients (23.5% patients of the total cohort) had lower prevalence of MetS and hypertension as well as lower NAFLD activity score, serum cytokeratin-18 fragments and decreased liver stiffness based on transient elastography than obese patients. Of note, deaths, HCC and liver failure occurred only in obese patients during a follow-up period of 49 mo[52].
In contrast, a United States study in 483 biopsy-confirmed NAFLD patients showed that lean (BMI < 25 kg/m2) patients had higher all-cause mortality than non-lean patients during a follow-up of 133 mo, although they had lower prevalence of T2DM, MetS, hypertriglyceridemia and hypertension, and less advanced fibrosis. Notably, even after adjustment for potential confounders, lean NAFLD was an independent risk factor (HR: 11.8) for higher all-cause mortality[58]. In the NHANES study, Zouet al[30]also reported that non-obese NAFLD (BMI < 30 kg/m2for non-Asians and < 27 kg/m2for Asians) patients had a higher prevalence of metabolic comorbidities, more advanced fibrosis and higher mortality due to cardiovascular disease and cancer and higher all-cause mortality than obese NALFD. However, these findings were not confirmed in a multivariate analysis, where only T2DM and fibrosis stage were independent risk factors for mortality[30]. Finally, in a retrospective cohort study in 646 NAFLD patients in Sweden, Hagstr?met al[59]reported that lean (BMI < 25 kg/m2) NAFLD patients had higher risk for cirrhosis, decompensated cirrhosis and HCC than overweight (25 kg/m2≤ BMI < 30 kg/m2) patients, independently of confounders; allcause mortality did not differ between the 2 groups. Of note, lean patients had lower serum TG and FPG levels as well as lower prevalence of NASH and lower fibrosis stage[59].
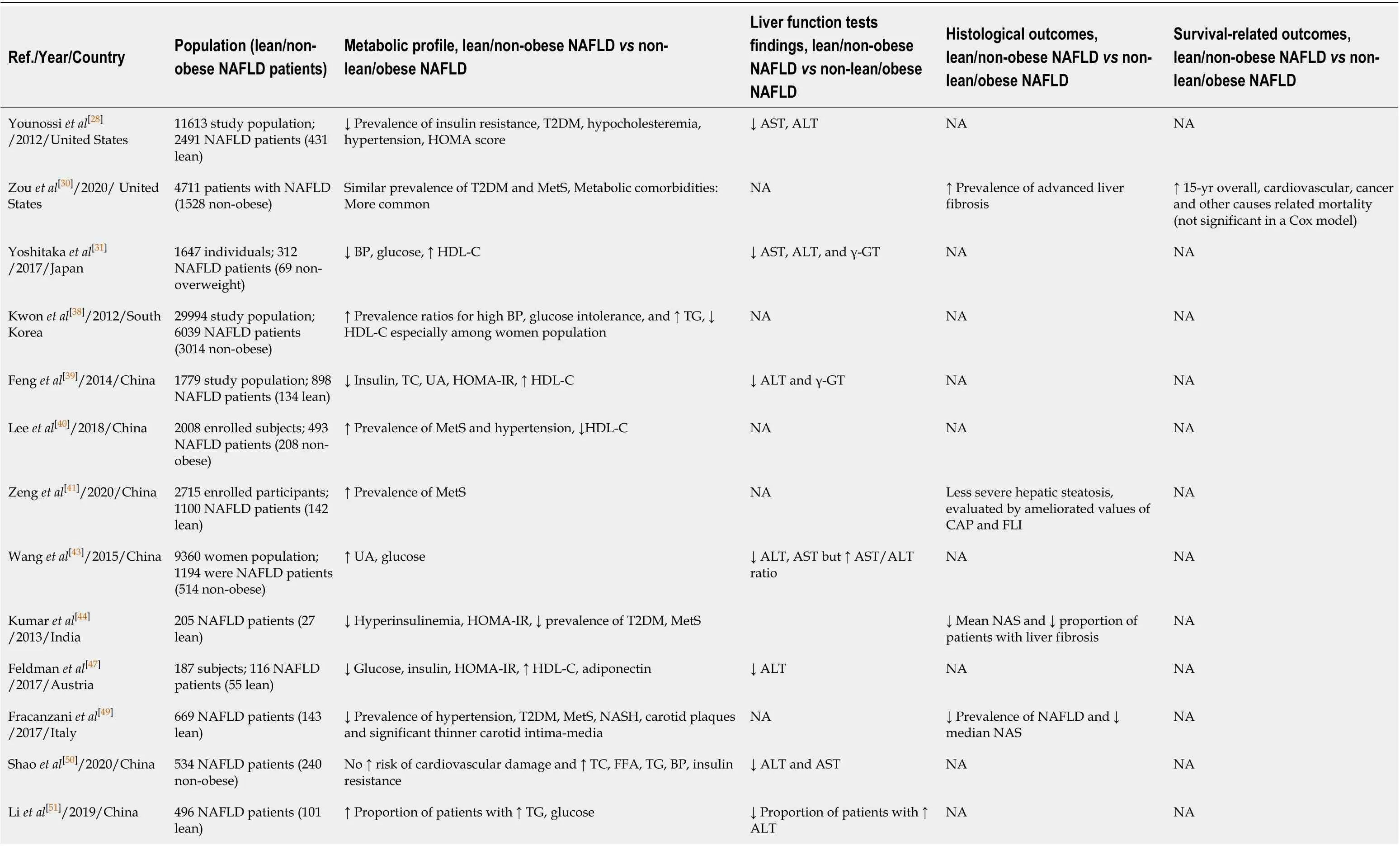
Table 2 Main findings and outcomes of lean (or non-obese) non-alcoholic fatty liver disease patients’ vs obese ones
NAFLD: Non-alcoholic fatty liver disease; ALT: Alanine aminotransferase; T2DM: Type 2 diabetes mellitus; BP: Blood pressure; MetS: Metabolic syndrome; TC: Total cholesterol; HDL-C: High density lipoprotein cholesterol; LDL-C: Low density lipoprotein cholesterol; TG: Triglycerides; UA: Uric acid; NASH: Non-alcoholic steatohepatitis; NAS: NAFLD activity score; HOMA-IR: Homeostasis Model Assessment Insulin Resistance; HCC: Hepatocellular carcinoma; CAP: Controlled attenuation parameter; FLI: Fatty liver index; HR: Hazard ratio; PNPLA 3: Patatin-like phospholipase domain-containing protein 3; NA: Not applicable; AST: Aspartate aminotransferase; γ-GT: γ-Glutamyl transferase; MetS: Metabolic syndrome.
It may seem paradoxical that most studies[30,58,59], although not all[52], reported a worse prognosis in non-obese/lean patients with NAFLD compared with obese NAFLD patients. Zouet al[30]attributed the worse outcome of non-obese NAFLD patients to the advanced fibrosis stage and the higher frequency of metabolic comorbidities in this group. Hagstr?met al[59]speculated that genetic predisposition and unhealthy lifestyle were associated with the worse liver-related outcomes of lean NAFLD patients. Another explanation could be that in all studies, BMI was used as a surrogate marker to define the thresholds for leanness or obesity. However, BMI is not a specific marker of abdominal obesity; waist circumference reflects more accurately the visceral adiposity fat fraction[60]. Nonetheless, even waist circumference cannot distinguish visceral from subcutaneous fat and cannot allow quantification of adipose tissue parts. Accordingly, more accurate markers of abdominal obesity, such as magnetic resonance imaging (MRI), might be useful in distinguishing between obese and lean patients with NAFLD. Indeed, MRI is considered a more accurate and quantitative tool for evaluation of visceral adipose tissue[61]. Thus, both the definition of lean/non-obese NAFLD and the categorization of patients into lean/non-obese or obese should be based in the near future on MRI to overcome the limitation of current, BMI-based definitions.
MANAGEMENT OF NON-OBESE/LEAN NAFLD
Management of NAFLD in lean patients is particularly challenging, since the cornerstone of NAFLD treatment is weight loss, which might not apply in these patients. In addition, there are no specific guidelines for the management of NAFLD in lean subjects. However, accumulating data suggest that several interventions might be useful in this population. A summary of the key elements of management of lean NAFLD is given in Table 3.
Initial workup and assessment of disease severity
To select the most appropriate management, a thorough diagnostic workup should be performed. The initial workup of a lean patient with suspected NAFLD may include a variety of modalities. Usually, ultrasound is the screening imaging method of choice and can provide information regarding the presence and severity of steatosis and the presence of cirrhosis. The Fibrosis-4 (FIB-4) and NAFLD fibrosis score can be useful for assessing the severity of liver fibrosis in patients with NAFLD[62,63]. In patients with inconclusive findings, elastography (transient, shear wave, or magnetic resonance) is the most widely used method to assess the severity of hepatic fibrosis, otherwise liver biopsy is recommended[64,65].
Management of NAFLD in lean subjects
Weight reduction:Similar with obese patients with NAFLD, weight reduction appears effective in lean subjects with NAFLD. In a study in 333 patients with NAFLD, weight change was an independent predictor of disease progression or resolution after a mean follow-up of 28.7 mo. Interestingly, among patients who also experienced NAFLD progression, non-obese subjects had greater weight gain than obese patients whereas among patients who experienced NAFLD resolution, non-obese patients showed smaller weight loss than obese subjects[66]. Moreover, 2 studies showed that 5% of body weight reduction led to significant decrease in steatosis in lean patients with NAFLD[67,68]. In the first study (n= 120 patients with NAFLD), a 10-wk program including diet modification and exercise resulted in improvement in steatosis in the repeated liver biopsy[67], while in the second one (n= 14 Lean NAFLD patients), an 8-wk intervention consisting of intensive dietary and lifestyle measures induced a decrease in both steatosis and stiffness assessed with transient elastography[68].
Dietary modifications and physical activity:Diet appears to improve NAFLD in lean patients independently of weight loss. It has been reported that lean patients with NAFLD have comparable total caloric intake with obese patients with NAFLD[69,70]. However, the former have higher intake of cholesterol and lower intake of polyunsaturated fatty acids (PUFAs)[70]. In a study in 120 patients with NAFLD who followed a 10-wk program including diet modification and exercise, most patients achieved a reduction of steatosis without weight reduction; instead, a reduction in fat intake and in overall body fat was observed and might have contributed to the improvement in steatosis[67]. Therefore, low-fat diet appears more appropriate for lean patients with NAFLD.
There are also reports highlighting physical exercise as a contributing factor to NAFLD amelioration irrespectively of its effect on body weight. In a largeretrospective study (n= 3718), lack of physical activity was independently associated with the presence of NAFLD, after adjusting for visceral adiposity and IR. These results might be particularly relevant for lean patients with NAFLD, who have lower prevalence of IR and visceral adiposity compared with obese patients[28,58].

Table 3 Key elements of management of lean non-alcoholic fatty liver disease
Management of comorbidities:As already mentioned, lean patients with NAFLD appear to have higher incidence of T2DM compared with overweight patients without NAFLD[32]. Given that T2DM is a major risk factor for NAFLD progression[71-73], these findings highlight the importance of T2DM prevention in this population. Furthermore, it has been reported that elevated TG levels are independently associated with development or resolution of NAFLD, especially in non-obese patients[66]. In another study, a ≥ 10% reduction in TC levels was independently associated with ≥ 20% reduction of steatosis in biopsy after a 10-wk exercise and diet modification program[67]. Given the increased cardiovascular risk of lean NAFLD patients, screening for and management of cardiometabolic comorbidities are essential to reduce cardiovascular morbidity in this population.
Sleep patterns:Short duration and poor quality of sleep have been associated with increased incidence of NAFLD[74-77]. Considering that a substantial proportion of lean patients with NAFLD have disturbed sleep[69], recommendations for more rest and efforts to improve sleep quality should be considered in this population.
Pharmacological interventions
Treatment options for NAFLD include pioglitazone and vitamin E but are limited to non-diabetic patients with biopsy-proven NASH. However, in both European and American guidelines, these agents are recommended to be used with caution and in carefully selected patients[4,64]. In addition, only ezetimibe has been evaluated in lean patients with NAFLD. In a pilot study (n= 8 non-obese patients), treatment with ezetimibe for 12 mo resulted in a decrease in aminotransferase levels but had no effect on hepatic steatosis assessed with ultrasound[78]. Interestingly, BMI did not change during the study. A larger, placebo-controlled, randomized study evaluated a symbiotic supplement consisting of seven bacterial strains in 50 lean NAFLD patients who also received lifestyle recommendations[79]. The supplement resulted in a greater reduction in liver stiffness and steatosis, in serum TG and TC levels and in inflammatory markers including high-sensitivity C-reactive protein and nuclear factor-κB activity than placebo. This study supports the findings of a previous report in obese patients with NAFLD[80]and suggests a role of gut microbiota manipulation in the management of NAFLD.
CONCLUSION
Even though NAFLD is strongly associated with obesity and related comorbidities, a substantial proportion of lean subjects can also develop NAFLD. Visceral obesity as opposed to general obesity, genetic predisposition, unhealthy dietary pattern consisting of high cholesterol and fructose intake may be associated with lean NAFLD. Although lean patients appear to have a worse prognosis but a healthier metabolic profile than obese patients with NAFLD, we should bear in mind that the current categorization into lean or obese cohorts was mostly based on BMI and not on visceral fat mass evaluation. Thus, the use of MRI as a reliable and quantitative diagnostic tool for evaluating the presence and severity of abdominal obesity in NAFLD patients might be useful. Currently, lifestyle interventions including weight loss, physical activity and a healthier dietary pattern seem to have beneficial impact on lean NAFLD. Beyond that, sleep interventions and pharmacotherapy along with strict management of comorbidities should also be incorporated in the management of this disease. Without a doubt, lean NAFLD raises many challenges since the pathophysiology and the natural history of the disease has not been widely studied and physicians should have high clinical suspicion in order to identify individuals at risk of lean NAFLD who lack the common, easily recognizable phenotype of obesity.
 World Journal of Gastroenterology2020年42期
World Journal of Gastroenterology2020年42期
- World Journal of Gastroenterology的其它文章
- Modern surgical strategies for perianal Crohn's disease
- Simultaneous colorectal and parenchymal-sparing liver resection for advanced colorectal carcinoma with synchronous liver metastases: Between conventional and mini-invasive approaches
- Estimation of visceral fat is useful for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease
- Nomograms and risk score models for predicting survival in rectal cancer patients with neoadjuvant therapy
- Attention deficit hyperactivity disorder and gastrointestinal morbidity in a large cohort of young adults
- Reactive oxygen species-induced activation of Yes-associated protein-1 through the c-Myc pathway is a therapeutic target in hepatocellular carcinom
