Top ten tips for perfect corona-2 prophylaxis
Ahmad Abul-Ainine, Ali A Sadek
Ahmad Abul-Ainine, Consultant Paediatrician, Paediatric Department, Thuwal, Jeddah 23955,Saudi Arabia
Ali A Sadek, Consultant Public Health and Medical Statistics, MOH, Kuwait
Abstract The current corona-2 pandemic has stimulated wide research for hydroxychloroquine (Quine) therapy and lately, prophylaxis. To optimize prophylaxis proper methods of use are explained. The focus is on tools of assessment and robust comparison; defining infection objectively; loading and maintenance dose designing based on pharmaco-viro-kinetics; confirming Quine threshold-levels and its sufficiency; and Quine side-effects vigilance/amelioration. Attention to statistics to study valid endpoints of goals in appropriately-sized population is essential. Mass interactive quine dose auto designer software is built to simplify, optimize and help collaboration of complex Quine dosing system. A similar chloroquine software can be built.
Key Words: Corona-2; COVID-19; Hydroxychloroquine; Prophylaxis; Dose; Mass interactive quine dose auto designer
TO THE EDITOR
A good effort to study post-exposure hydroxychloroquine (Quine) prophylaxis was recently published[1]. Despite that it has decent statistical design, it has salient issues that need to be addressed to perfect the outcome of further Quine prophylaxis: (1) The primary outcome should involve viro-conversion from positive at entry to negative on exit (little testing in this study); (2) Primary outcome involves clinical symptoms reported by patients (was its percent reliability factored in sample-size calculations?)and was rated by 4 Infectious Disease doctors (without mention of inter-rater training or kappa of agreement reliability)[2]; (3) No data on further exposure to corona-2; other flu virus or having nasal allergy during the 2-wk study; (4) Using primary outcome as clinical symptoms is subjective, neither sensitive (as 80% are asymptomatic) nor specific to COVID19, (is it another flu virus or hay-fever?); (5) The Quine antimalarial dose is smaller than its antiviral loading doses (LD) calculated from the pharmacokinetics data held by FDA[3](Table 1). Low LD will produce sub-inhibitory levels, so that patients are not protected for 1-4 d pre-enrollment and 4-5 d post-enrollment(treatment start 1 d after enrollment and might take 4 d to reach the level required for protection-threshold); (6) although measuring drug levels by using finger-prick to selfcollect 10 μm blood samples (VAMS) is well-known, in-vivoQuine IC50(= 50%Inhibitory Concentration) is never assured; pharmacokinetics from one study proposed VeroE6 cell IC50of 4.5 μmol/L in 48 h of post-infection (mcM/hpi)[4,5], and another 6.3-5.9 in 24-48 mcM/hpi[6]requiring higher LD (15 and 20 tablets x 200 mg each, respectively); plus 2-3-wk maintenance doses (MD) or until patients develop their own immuno-protection; (7) Finding of safety-thresholds (10% Toxic Concentration = TC10) for liver enzymes elevation (= TCLiver10), for heart QTprolongation (= TCHeart10), clinical hepatitis and dysrhythmia issues; (8) Since Quine is virostatic, its prophylactic-level must be maintained for at least 2-3 wk to build immunity that can clear virion particles (not possible in VeroE6 cell-kinetic cultures).So, dosing for 5 of 14 d is inadequate; (9) the folate-placebo helps one-carbon atom transfer to thymine to produce uracil, the rate-limiting substrate for RNA synthesis–undesired confounder; and (10) Although using sophisticated statistics to end the study early at a priori statistical power outcome is good, extending Quine prophylaxis(following correct LD) to achieve and define human IC50, is a missed historical landmark in the human/corona-2 contest. Sadly, statistical passion forced ending at only 2.4% incidence reduction rather than a 7% reduction –glorifying statisticalsignificance sacrificed nearby finding/measuring the more clinically important IC50–cf.McNamara fallacy.
CONCLUSION
Quine's role in corona-2 pandemic prophylaxis can be assuredviadesigning correct LD/MD, therapy duration, and VAMS concentrations, assuring human IC50and Liver and Heart safety thresholds of TCLiver10 and TCHeart10. Surly, good care will translate VeroE6 Viro-kinetics into human Viro-kinetics and help human-tailored dosing; not misguided by improper models, malaria, or rheumatology doses.
Mass interactive quine dose auto designer, viral count and VAMS test help initial Quine LD/MD designing and human-tailored LD/MD dosing.
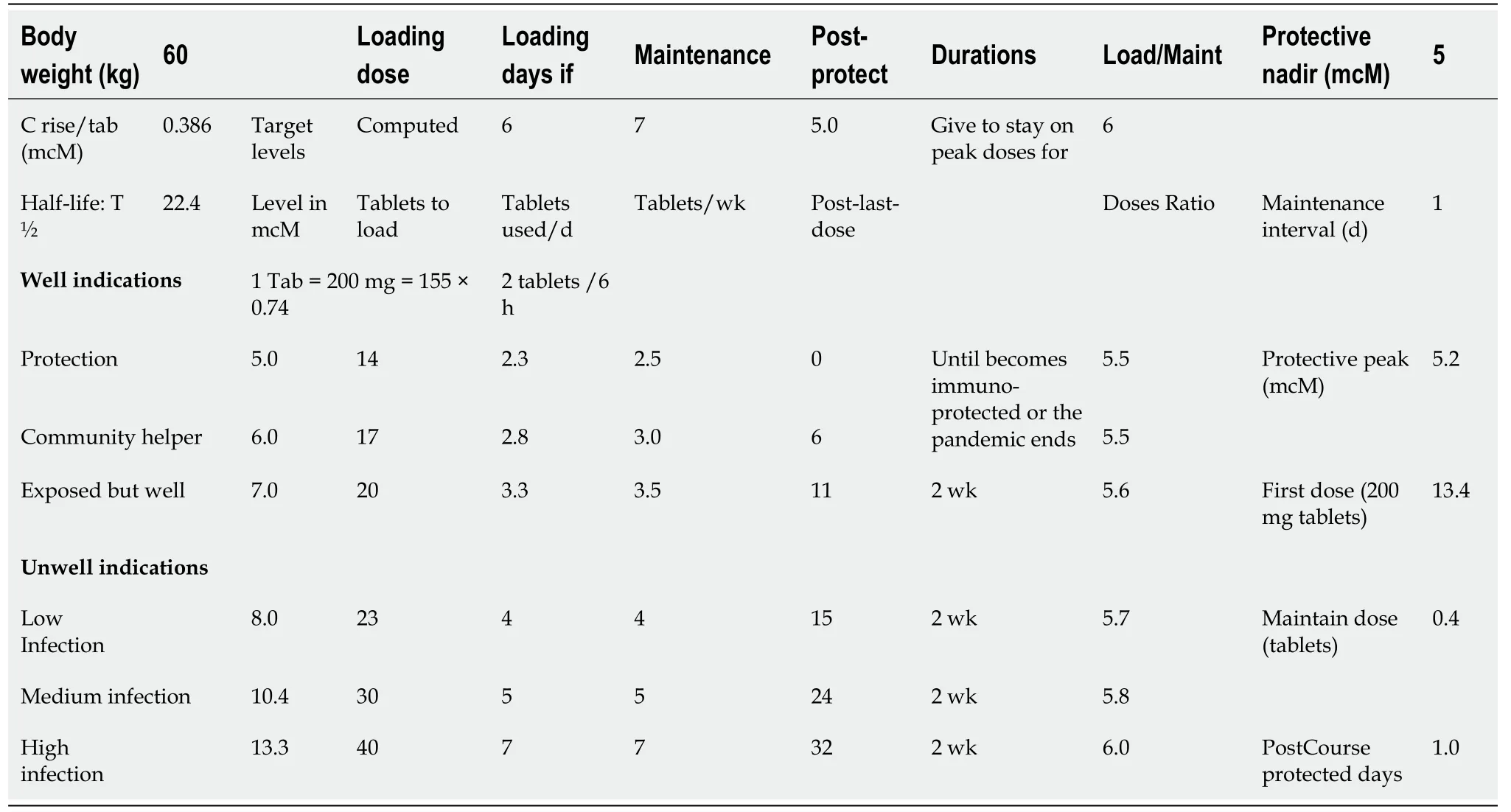
Table 1 The mass interactive quine dose auto designer (MIQDAD,Download)
ACKNOWLEDGEMENTS
Dr. Osman Akhtar, Pharmacist in Thuwal Clinic for reviewing and testing the dosing software mass interactive quine dose auto designer.
 World Journal of Clinical Infectious Diseases2020年4期
World Journal of Clinical Infectious Diseases2020年4期
- World Journal of Clinical Infectious Diseases的其它文章
- COVID-19 and dengue coinfection in Brazil
- COVID-19 risk comorbidities: Time to reappraise our physical inactivity habits (again!)
