P-glycoprotein mediated interactions between Chinese materia medica and pharmaceutical drugs
YANG Xi, PENG Yuzhong, HE Yufei, HUANG Xuejun, XU Aili, BI Xioli, XIE Ying,*
a.State Key Laboratory of Quality Research in Chinese Medicines, Macau University of Science and Technology, Macau 999078, China
b.School of Pharmacy, Macau University of Science and Technology, Macau 999078, China
c.Guangdong Province Engineering Technology Research Institute of Traditional Chinese Medicine, Guangzhou, Guangdong 510095, China
Keywords P-glycoprotein (P-gp)Chinese materia medica (CMM)Drug interactions Pharmacokinetic-pharmacodynamic effects Inducers Inhibitors
ABSTRACT P-glycoprotein (P-gp) is an important transmembrane ATP-binding cassette (ABC) drug efflux transporter expressed in various human tissues such as the intestines, liver, kidneys, and bloodbrain barrier.It limits the intracellular concentration of xenobiotics by pumping them out of the cells, affecting drug pharmacokinetics and therapeutic effects.With its broad substrate specificity, it has the potential to remove a wide range of drugs from Chinese materia medica (CMM), including conventional medicines and active compounds.Increasing evidence has confirmed the superior therapeutic effectiveness of CMM in treating a wide range of diseases worldwide, as well as in conjunction with western drugs.As a result, herbal medicine-drug compounds have prompted widespread concern, with the majority of these interactions involving transporters such as P-gp.This review systematically summarizes the inhibition or induction of P-gp expression/function by active CMM compounds and the underlying regulatory mechanisms.It will aid in improving understanding of the synergistic or inhibiting effects associated with transporter P-gp as well as rational safety concerns for using CMM,particularly in combination with drugs.
1 Introduction
With prominent therapeutic effects, Chinese materia medica (CMM) has been extensively used as a traditional medicine for the prevention and treatment of a variety of diseases in China, as well as in other countries in East and Southeast Asia.In the absence of specific medicines and vaccines, traditional Chinese medicine (TCM) plays an essential role in preventing and treating COVID-19[1].Furthermore, herbal medicines, also known as complementary medicines, are often used as supplements with prescription drugs in western countries.According to the World Health Organization’s drug strategy, over the last 20 years,approximately 70% of the global population has used medicinal herbs as a complementary or alternative medicine[2].Moreover, CMM has the potential to treat severe and even life-threatening diseases while also reducing the side effects of co-administered pharmaceuticals[3].For these reasons, it is crucial to understand CMM-related drug interactions from the bench to the clinic to improve CMM use, avoid severe adverse events, and have detrimental clinical outcomes.
As a member of the ATP family, P-gp is widely distributed in intestinal epithelial, hepatic, and other tissues and plays a role in molecular efflux.Efflux transporters protect the body by preventing the entry of xenobiotics, particularly toxic substances; thus, their functions are to regulate drug absorption, distribution, excretion, and toxicity caused by drug-drug interactions[4].For a P-gp substance with a low therapeutic index, such as digoxin, P-gp inhibitors such as quinidine, cyclosporine, valspodar, and amiodarone,have been reported to have a 2-fold increase at the highest concentration (Cmax) or area under the curve(AUC) of digoxin.Moreover, as P-gp protects the brain by transporting xenobiotics, recommendations have been made to restrict the use of loperamide because the interaction of loperamide and a P-gp inhibitor could cause respiratory depression, which has a dangerous effect on the central nervous system[5].In addition, P-gp inhibitors with high efficiency and low toxicity have been extensively researched to overcome the multiple drug resistance induced by Pgp overexpression in tumors via co-administration with chemotherapeutics[6].As a result, any effect on P-gp expression and function may result in changes in the pharmacokinetics and pharmacodynamics of their specific substrates, resulting in side effects as well as safety and efficacy considerations.However,growing evidence suggests that active compounds from CMM have modulatory effects on ATP-binding cassette (ABC) transporters, particularly P-gp, leading to herb-drug interactions[7].
In this review, we focus on the inhibition or induction of P-gp by CMM active compounds and the potential regulatory mechanisms of P-gp to improve our understanding of the synergistic or inhibiting effects of CMM, as well as safety concerns when using CMM, in combination with drugs.
2 P-glycoprotein
P-gp is a primary transporter of the ABC transporter family and is composed of 1 280 amino acids.It is also known as multi-drug resistance protein 1(MDR1) or ATP-binding cassette sub-family B member 1 (ABCB1)[8].In the 1970s, it was first discovered in a colchicine-resistant mutant cell line, but it is widely expressed in various organs, including the apical domain of canalicular membranes of hepatocytes, brain, and kidney endothelial cells, jejunum,duodenum, and stomach[9].
Given the importance of drug transporters, the International Drug Transporter Consortium (ITC)published a white paper in Nature in 2010 that regulated the research significance and methods of studying various drug transporters[10].In recent years, the number of compounds related to drug transporters has increased significantly among new compounds approved for marketing by the United States Food and Drug Administration (FDA), accounting for approximately 50% of all listed compounds.Drugs related to drug transporters account for approximately one-quarter of the total number of delisted drugs[11].Therefore, assessing the affinity relationship between drugs and drug transporters and predicting potential safety risks in clinical treatment is crucial for guiding rational drug use, reducing side effects, and improving drug efficacy.
As a transmembrane protein, the primary function of P-gp is to pump foreign substances out of cells by using ATP as the energy source.Substances transported by P-gp are defined as “substrate” of P-gp, and they can be identified using the cell-based bidirectional permeability assay[8].P-gp has more than five substrate binding sites, which can transport broad substrates such as cardiac glycosides (digoxin), vincristine alkaloids (vincristine and vinblastine), HIV protease inhibitors (saquinavir and ritonavir), corticosteroids (dexamethasone and hydrocortisone),immunosuppressants (cyclosporine A), and antibiotics (erythromycin, validamycin)[12].It participates in drug absorption, distribution, metabolism, and elimination (ADME), leading to changes in safety and effectiveness.Therefore, P-gp is also known to be a“pharmacokinetic barrier”.For example, a randomized clinical trial with 12 healthy humans indicated that co-administration of a P-gp inhibitor, ritonavir,significantly decreased digoxin’s renal and total clearance and consequently increased the blood levels of digoxin by 86%in vivo[13].Figure 1 summarizes the P-gp mediated pharmacokinetic interactions and outcomes.
3 CMM-drug interactions
As shown in Figure 2, there are different types of pharmacodynamic and pharmacokinetic interactions between CMM-pharmaceutical drugs.To date,herb-drug pharmacokinetic interactions have been studied more in-depthin vivoandin vitroand are reviewed in detail elsewhere[2].
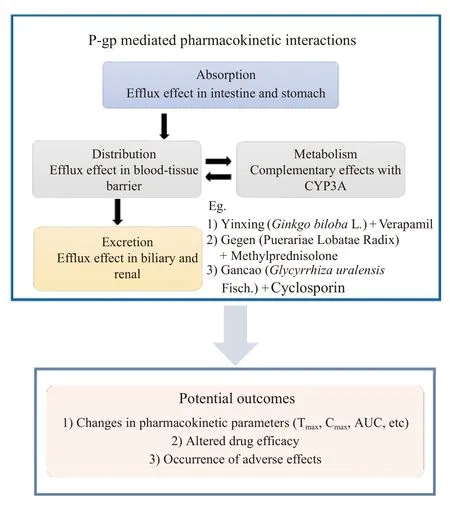
Figure 1 Illustration of P-gp mediated pharmacokinetic interactions and their outcomes
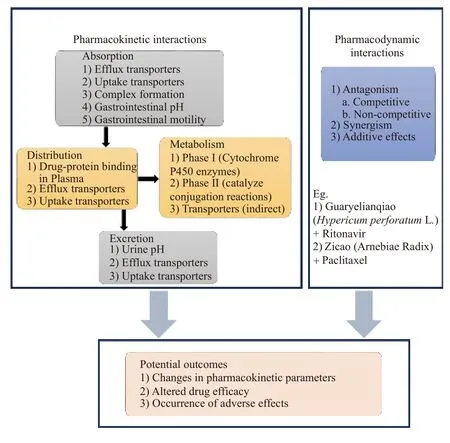
Figure 2 Different types of pharmacodynamic and pharmacokinetic interactions between CMM-pharmaceutical drugs and their potential outcomes
Previously, studies of CMM-pharmaceutical drug interactions have mainly focused on drug-metabolizing enzymes (in particular, cytochrome P450)[14,15].With advancements in the understanding of transporter function, an increasing number of studies are focusing on drug transporters that play a central role in controlling the ADME, resulting in a change in pharmacokinetics that is accompanied by pharmacodynamic interactions, including antagonistic and/or synergistic effects[5].
A large number of compounds involving pharmaceutical drugs and CMM can reduce or induce the expression of P-gp, thereby influencing the ADME of Pgp substances.
3.1 Induction or inhibition of P-gp by CMM
Several P-gp modulators have been discovered in the past three decades, either for drug safety issues or drug development.However, the selectivity and validity of known P-gp modulators remain restricted to the most clinically prescribed drugs and the primary active compounds of CMM.Recent evidence suggests that natural compounds derived from CMM act as efficacious and safe P-gp modulators[16].To help understand the CMM-drug interactions mediated by P-gp, the active compounds of the CMM that act as inhibitors or inducers of P-gp are summarized and presented in Table 1 (inhibitor) and Table 2(inducer).The following section describes a few wellstudied CMMs with similar mechanisms as typical examples to illustrate the CMM-drug interactions mediated by P-gp.
3.2 Herbs those act as P-gp inhibitors
3.2.1 Yinxing (Ginkgo biloba L.)Yinxing (Ginkgo bilobaL.) has cognition-improving, neuroprotective,antioxidant, and memory-enhancing properties[31].It contains a variety of active constituents, including terpenoids (ginkgolides and bilobalide), flavonoids(kaempferol, isorhamnetin, p-coumaric acid), and organic acids (ginkgolic acids, alkylphenols)[32].Yinxing (Ginkgo bilobaL.) has been shown to modulate P-gp, which may affect co-administered drugs'pharmacokinetics[34].A clinical study with 10 healthy volunteers revealed that Yinxing (Ginkgo bilobaL.)extracts significantly altered the pharmacokinetic behavior of talinolol by increasing Cmax(36%) and AUC0-24(26%)[39], suggesting that Yinxing (Ginkgo bilobaL.) affects the metabolism of talinololin vivoas a P-gp inhibitor.
3.2.2 Danshen (Salvia miltiorrhiza Bge.)Danshen(Salvia miltiorrhizaBge.) is a popular medicinal herb used to treat many diseases such as hemorrhage,menstrual disorders, insomnia, miscarriage, and cardiovascular disease[45].Active compounds include salvianolic acid A, salvianolic acid B, tanshinone I,miltirone,cryptotanshinone,and dihydrotanshinone.These components inhibit drug intake by inactivating P-gp[35].Tanshinone I has also been identified as a substrate for P-gp[46].For example, tanshinone I exhibited inhibitory effects on the transport of digoxin, a classical P-gp substrate drug.Danshen (Salvia miltiorrhizaBge.) combined with digoxinas an herb pair can improve digoxin bioavailability.
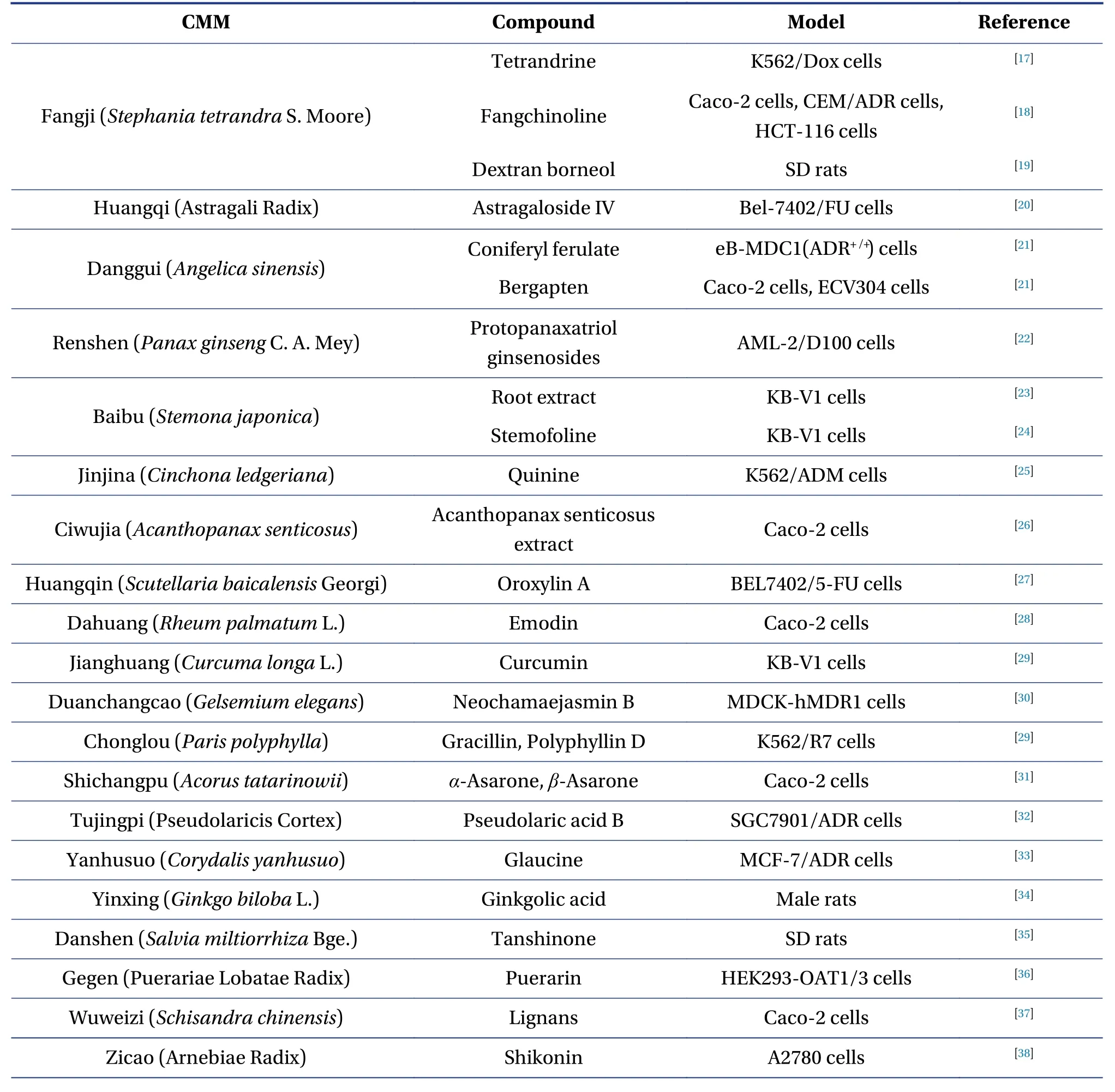
Table 1 Active compounds of CMM those act as inhibitors of P-gp
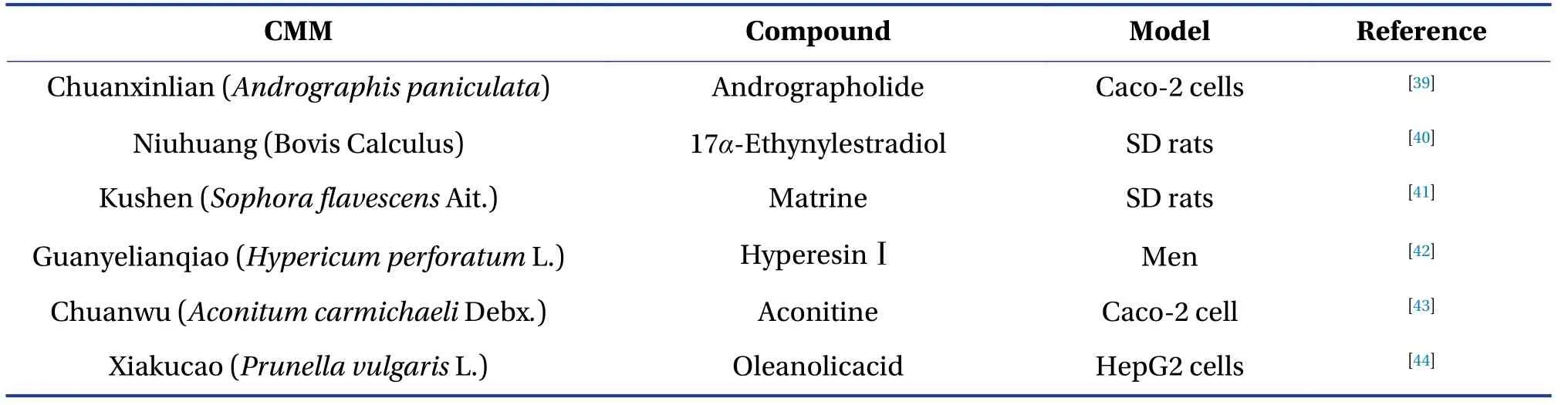
Table 2 Active compounds of CMM those act as inducers of P-gp
3.2.3 Gegen (Puerariae Lobatae Radix)Gegen (Puerariae Lobatae Radix) is used to treat cardiovascular diseases and has various therapeutic effects, including anti-fever and anti-diarrhea[47].Gegen (Puerariae Lobatae Radix) contains over 70 components, including isoflavones, isoflavone glycosides, coumarins,and puerarins.LIU et al.[36]found that co-administration of Gegen(Puerariae Lobatae Radix)with methotrexate increased the AUC of methotrexate by 2.24-fold via competitive inhibition of intestinal P-gp because puerarin has an inhibitory effect on P-gp.This suggested that a combination with Gegen (Puerariae Lobatae Radix) may improve the results of methotrexate treatment.
3.2.4 Wuweizi (Schisandra chinensis)The pharmacological effects of Wuweizi (Schisandra chinensis)include sedative and hypnotic effects, liver-protective properties, anti-cancer effects, and prevention of cardiovascular disease[37].The main active compounds in Wuweizi (Schisandra chinensis) are lignans.YOO et al.[48]found that deoxyschizandrin, one of the active compounds in Wuweizi (Schisandra chinensis) , effectively inhibited P-gp and increased digoxin transport ratios in Caco-2 cells from 2.2% to 15.2% when compared to the control.Similar to the effects of deoxyschizandrin, another ligand, schisandrin B, also inhibited P-gp and improved daunorubicin accumulation in drug-resistant tumor cell lines in a dose-dependent manner confirming the inhibitory effect of P-gp[49], indicating that they may act as resistance sensitizers.
3.2.5 Gancao (Glycyrrhiza uralensis Fisch.)Gancao(Glycyrrhiza uralensisFisch.), also known as licorice,sweetwood, or mulhatti, is a herbal remedy with immense potential in treating orofacial diseases.Its antiviral, glucocorticoid, anti-inflammatory, antioxidant, anti-ulcerative, and anti-carcinogenic properties are well-known[50].It is frequently used with aconite as a herb-pair, resulting in toxicity reduction and/or efficacy enhancement of aconite via P-gp inhibition[43].As a result, combining drugs with CMM provides a new approach for reversing toxicity by increasing the excretion of P-gp substances[51].
Moreover, its major constituents, such as glycyrrhetinic acid and glabridin, have been shown to suppress P-gp expression and increase doxorubicin accumulation in P-gp-overexpressing human carcinoma cells, suggesting that they may help improve the efficacy of cancer chemotherapy.
However, there are contradictory results regarding the inhibitory effects of Gancao (Glycyrrhiza uralensisFisch.).HOU et al.[52]reported that it reduced the oral bioavailability of cyclosporine because glycyrrhetinic acid-activated P-gp and CYP3A.These different phenomena observed may be due to the dosing time, as short-term administration of Gancao (Glycyrrhiza uralensisFisch.) increased the function of P-gp, but more research is needed.
3.2.6 Fangji (Stephania tetrandra S.Moore)The root of Fangji (Stephania tetrandraS.Moore) is an effective analgesic for swelling and dispelling wind.It has a complex chemical composition that includes alkaloids, flavonoids, volatile oils, and organic acids.The quality evaluation index components stipulated in theChinese Pharmacopoeiaare dibenzyl isoquinoline alkaloids, such as tetrandrine and fangchinoline,which are considered the main chemical components of its efficacy[53].
Tetrandrine has been shown in clinical trials to act as a P-gp inhibitor in reversing multidrug-resistant tumors by increasing the intracellular concentration of anticancer drugs and their efficacy.Moreover, it did not affect the pharmacokinetic parameters of the chemotherapy drugs[54].In addition, fangchinoline,another active compound from Fangji (Stephania tetrandraS.Moore), is also a P-gp inhibitor.
3.2.7 Zicao (Arnebiae Radix)Zicao (Arnebiae Radix)is a traditional medicinal plant in China, and its therapeutic value has been documented in numerous traditional Chinese medicine books, includingBencao Jingshu(《本草經(jīng)疏》).It has a wide range of biological activities, including antiviral and anticancer properties[55].Recently, Zicao (Arnebiae Radix)was co-treated with paclitaxel (PTX), which synergistically enhanced cytotoxicity and apoptosis in PTX-resistant ovarian cancer cells, which was dependent on inhibiting the activity of P-gp[56].Moreover, the active compounds in Zicao (Arnebiae Radix), shikonin derivatives (acetylshikonin and acetoxyisovalerylshikonin), downregulated P-gp expression[38], suggesting that they may contribute to the inhibitory effects of Zicao (Arnebiae Radix) on P-gp.
3.3 Herbs those act as P-gp inducers
3.3.1 Kushen (Sophora flavescens Ait.)Kushen (Sophora flavescensAit.) is also known as kuh-seng and has been used to treat fever, dysentery, and pain.It has also been reported to affect injury recovery, antiinfection, and anti-rotavirus[57].In rats, oral administration of Kushen (Sophora flavescensAit.) extract or sophora-derived products with indinavir twice a day for a week caused a dose-dependent decrease in indinavir plasma concentrations.As inducers, Kushen(Sophora flavescensAit.)and sophora-derived products could elevate P-gp mRNA and protein levels in the small intestine and liver[41].This indicates that the use of Kushen (Sophora flavescensAit.) extract or sophora-derived products in patients receiving indinavir should be done with caution due to the possibility of P-gp mediated herb-drug interactions.
3.3.2 Guanyelianqiao (Hypericum perforatum L.)Guanyelianqiao (Hypericum perforatumL.), also known as St John’s wort, has been shown to induce herb-drug interactions.St John’s wort contains active compounds such as hypericin, hyperforin, adhyperforin, and some flavonoids, including rutin[58].The induction of P-gp by St John’s wort can significantly reduce the bioavailability of many prescription drugs after co-administration.It was reported that 12 patients with depression who were treated with amitriptyline received co-medication with St John’s wort extracts, and the concomitant administration led to a significant decrease in the AUC and Cmaxof amitriptyline by 22% and 23%, respectively, without affecting the drug’s elimination half-life[59].These findings suggest that Guanyelianqiao (Hypericum perforatumL.)reduced the bioavailability of amitriptyline by increasing P-gp levels in the duodenal mucosa.
3.3.3 Chuanwu(Aconitum carmichaeli Debx.)Chuanwu (Aconitum carmichaeliDebx.) is an aconitum species that is high in bioactive aconitum alkaloids.Aconitine, the primary active and toxic compound in Chuanwu (Aconitum carmichaeliDebx.),is an effective aconitum alkaloid in treating rheumatoid arthritis, cardiovascular diseases, and tumors[60].Interestingly, aconitine, benzoylaconine, and aconine increased P-gp expression and activity.Aconitine significantly increased P-gp expression in the ileum,jejunum, and small intestine.WU et al.[61]found that co-administration of aconitine reduced the cytotoxicity of vincristine and doxorubicin, indicating that co-administration of aconitine reduced the absorption of P-gp substrate drugs.In a Caco-2 cell model,mesaconitine, aconitine, and hypaconitine served as P-gp inhibitors and substrates[62].These contradictory results may be attributed to the experimental design (in vitro/in vivo) and dosing time (short-term or long-term).
3.3.4 Xiakucao (Prunella vulgaris L.)Several studies have shown that Xiakucao (Prunella vulgarisL.)has anti-hypertensive, anti-tumor, anti-hyperglycemic, anti-viral, and anti-inflammatory properties[63,64].Moreover, Xiakucao (Prunella vulgarisL.) has been identified as a strong P-gp inducer.Compared to treatment with Rh123 alone, co-administration of Xiakucao (Prunella vulgarisL.) decreased the intracellular content of Rh123.In contrast, treatment with water extracts of Xiakucao (Prunella vulgarisL.) at 1,2, and 4 mg/mL significantly increased P-gp expression levels 2.28-, 2.96-, and 4.17-fold, respectively[65].Furthermore, rosmarinic acid, an active compound derived from Xiakucao (Prunella vulgarisL.), was demonstrated to be the component in inducing the expression of P-gp[65].
4 P-gp inhibition or induction mechanisms
In comparison with pharmaceutical drugs, there have been few studies on the regulatory mechanisms of P-gp by CMMs.These are essential to promote understanding and prevent complicated drug interactions.
4.1 Mechanisms for P-gp inhibition
P-gp inhibitors exert their effects through several pathways, including (1) occupying the substratebinding sites; (2) downregulating the expression of Pgp; (3) interfering with ATP hydrolysis; and (4) modifying the lipid content of the cell membrane(Figure 3).
One of the most common strategies for inhibiting P-gp function is to occupy P-gp-binding sites.These inhibitors may be competitive, uncompetitive, noncompetitive, or allosteric.For example, nobiletin, a flavonoid isolated from citrus peels, has been shown to be a competitive P-gp inhibitor by competing for drug binding sites rather than changing P-gp expression[6].
Many P-gp inhibitors have been tested for their effects in modifying P-gp expression.Several transcription factors and signaling pathways have been demonstrated to regulate P-gp expression, including the nuclear factor-κB (NF-κB) pathway, phosphoinositide-3-kinase (PI3K)/Akt pathway, p38/mitogenactivated protein kinase (MAPK) pathway, c-Jun NH2-terminal protein kinase (JNK) pathway, protein kinase C (PKC) pathway, Y-box binding protein 1(YB-1), activator protein 1 (AP-1), p38 MAPK-p53 pathway, and other kinases, as shown in Figure 3.Based on these findings, the bioactive chemicals puerarin and sinomenine derived from Gegen (Puerariae Lobate Radix) and Qingfengteng (Sinomenium acutum) are potent P-gp inhibitors that inhibit nuclear factor-κB activity.
Because the efflux effect of P-gp is heavily reliant on ATP as an energy source, it is conceivable that blocking the activity of ATPase disrupts P-gp function.Quercetin, a PI3K inhibitor, inhibits P-gp by inhibiting ATP hydrolysis[66].Furthermore, cryptotanshinone and dihydrotanshinone, isolated from Danshen (Salvia miltiorrhizaBge.), inhibit P-gp ATPase activity, resulting in P-gp substrate accumulation.
4.2 Mechanisms for P-gp induction
A P-gp inducer may modulate the expression of P-gp via the following mechanisms: (1) transcriptionally upregulates the expression of P-gp; and (2) increases P-gp activity (Figure 4).
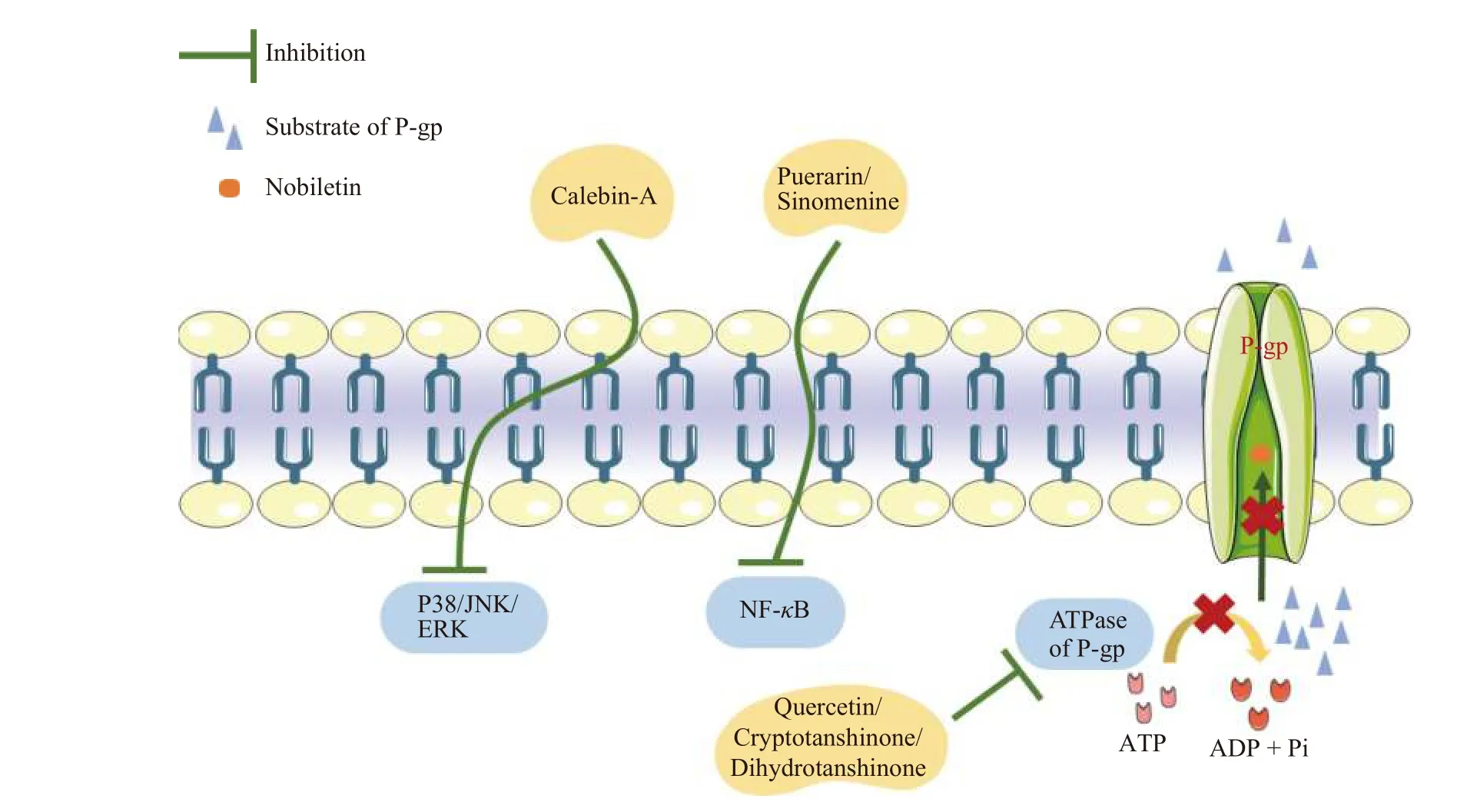
Figure 3 Mechanisms of P-gp inhibitors from CMM
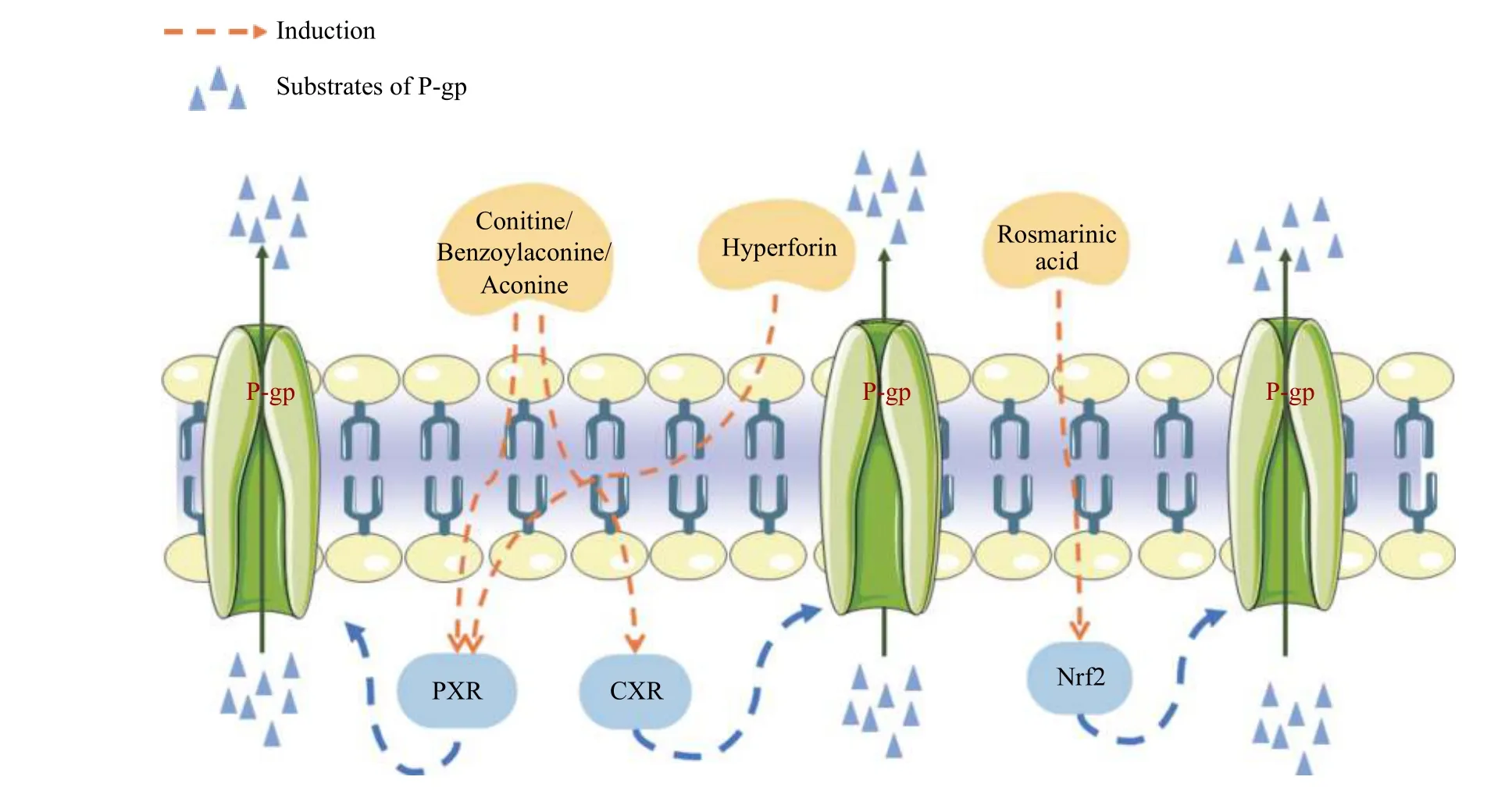
Figure 4 Mechanisms of P-gp inducers from CMM including transcriptional up-regulation of P-gp or increased P-gp function
The expression of P-gp is regulated by transcription factors and signaling pathways, including H-Ras,c-Raf, MEK1/2, ERK1/2, and p90RSK1/2.Anin vitrostudy found that aconitine, benzoylaconine, and aconine, three common Aconitum alkaloids, induced P-gp expression via activation of the pregnane X receptor (PXR) and constitutive androstane receptor(CAR) pathways[62].Furthermore, rosmarinic acid, a marker component(for quality)of Xiakucao(Prunella vulgarisL.), acts on nuclear factor E2-related factor-2 (Nrf2) to induce P-gp[65].
The transport of a substrate can be stimulated through P-gp activator-induced conformational changes without directly affecting protein expression.Cross-linking with a short cross-linker to bring the central region of the nucleotide-binding domain NBD1 (LSGGQ site) close to the N-terminal region of NBD2 (the Walker A site) leads to highly activated ATPase activity.This may mimic the structural rearrangements and stimulation of ATPase activity by binding potent stimulators such as verapamil and tariquidar[67].For example, Hoechst-33342 can promote transformation of P-gp[68].However, there have been no reports on the cross-linking of natural compounds.
5 Approaches to predict P-gp mediated CMMpharmaceutical drug interactions
In vitrotransport studies have included the utilization of Caco-2 and MDCKII cell lines, and cancer cells overexpressing P-gp.There are manyin silicomethods that have been used for high-throughput screening of P-gp inhibitors, accompanied by simple rule-based modeling and other assays[69].These methods have been utilized to investigate P-gp mediated drug interactions.
Recently, a physiologically based pharmacokinetic (PBPK) model has been widely used as an elaborate method for predicting drug interactions.The PBPK model is a mathematical modeling technique that uses a wide range of physiological properties of the human body and ADME parameters to extrapolate the potential drug interactionsin vivo.These approaches are classified into three categories: mechanistic dynamic, simple static, and mechanistic static models.The ADME outcomes data can be used as a reference for future clinical studies.For example,PBPK methods have been used to predict P-gp modulated drug interactions between P-gp substrates and inhibitors.In addition, a variety of professional modeling software such as GNU Octave, SAAM II, and acslX has been developed for the application of PBPK modeling, requiring users to input their model equations and physiological parameters.There is also well-designed software that includes physiological databases of predefined species and/or populations and is designed specifically for PBPK modeling, such as Simcyp, GastroPlus, PK-Sim, MOBI, and CloePK[11].
“Cocktail” approaches have been used extensively in clinical research to predict drug interactions.These use simultaneous administration of several substrates as probes to evaluate and characterize a drug’s potential effects upon multiple CYP enzymes and/or transporters (such as P-gp)[56].This method has been adopted by FDA, which is included in the clinical drug interaction study guide.The predictions are considered to be accurate and successful if the following criteria are satisfied: (1) the substrate is specific for individual enzymes or transporters;(2) the subjects are scaled appropriately; and (3) the substrates do not interact with one another[56].Today, validated drug “cocktail” probes include the“Pittsburgh cocktail” “Geneva cocktail” and “Karolinska cocktail”, each with different advantages and limitations.The “cocktail” approach was used in clinical research to assess the effect of fermented red ginseng on P-gp and CYP450 enzymes.The results revealed that the fermented red ginseng had no significant interaction with the CYP probe substrates but P-gp.
6 Conclusion
With the worldwide use of CMM, researchers are delving deeper into the interactions between CMM and pharmaceutical drugs, discovering that the complex effects caused by these interactions range from improving drug effects to causing adverse reactions.This study presents a clearer understanding of the inhibition or induction of P-gp by the active compounds of CMM and the regulatory mechanisms of P-gp.It has updated the knowledge of the synergistic and/or antagonistic effects associated with transporter P-gp and safety concerns for using CMM, particularly in combination with drugs.
Because CMM is characterized by complex components and multiple pharmacological targets, it is challenging to sort out all the pharmacokinetic and pharmacodynamic interactions between CMM and drugs.Meanwhile, we are still in the early stages of studying CMM-drug interactions due to a lack of information on the ADME process of multiple compounds in CMM.Moreover, well-designed clinical studies investigating herbal-drug interactions are limited.Therefore, it is necessary to collect all information on natural compounds that are inhibitors/inducers/substrates of metabolism enzymes and/or transporters such as P-gp.Guidelines for comprehensive, systematic, and mechanistic approaches to evaluating possible CMM-drug interactions must be established, which will aid in the clinical use of CMM and co-administration with other drugs.
Acknowledgements
We thank for the Macau Science and Technology Development Fund (No.0067/2019/A2 and No.0075/2019/AMJ) from the Macau Special Administrative Region.
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年4期
Digital Chinese Medicine2021年4期
- Digital Chinese Medicine的其它文章
- Systematic review of robust experimental models of rheumatoid arthritis for basic research
- Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
- Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
- Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
- Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
- Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
