Using injectabIe fiIIers for midface rejuvenation
Beatrice C. Go, ArieI S. Frost, Oren Friedman
1Department of Otorhinolaryngology, Hospital of University of Pennsylvania, Pennsylvania, PA 19104, USA.
2Division of Facial Plastic & Reconstructive Surgery, Department of Otorhinolaryngology, Hospital of University of Pennsylvania,Pennsylvania, PA 19107, USA.
★co-first authors.
Abstract
Keywords: Hyaluronic acid, midface, nasolabial fold, mouth, aging, face, fillers
INTRODUCTION
Treating the aging face is a popular area of medicine, as every human is subject to the physiologic process of aging. Understanding the intrinsic physiological aging process is crucial for appropriate and individualized treatment selection. The age results in thinning of the epidermis, flattening of the dermal-epidermal junction, slower wound healing, and decreased cellular proliferation[1]. Reduced synthesis of collagen due to fibroblast aging and collagen damage change the overall composition of the skin[2,3]. Extrinsic aging, most commonly attributed to sun damage but also to tobacco use and gravity, can further exacerbate the appearance of dull, discolored, and wrinkled skin. Loss of subcutaneous volume from these mechanisms is the hallmark of the aging face. In the midface, deepening of the nasolabial folds and marionette lines around the mouth are attributed to the descent of nearby fat collections[4]. Additionally, significant atrophy of the deep facial fat that provides structural volume and support to the midface occurs with aging and has a profound impact on malar projection[5-7].
A variety of treatment options for the aging face exist, and they include both surgical and nonsurgical alternatives. Since the introduction of facelift surgery in the early twentieth century, numerous developments have allowed for more satisfactory and long-lasting aesthetic results[8-10]. The modern-day rhytidectomy involves manipulation (i.e., plication, imbrication) of the superficial musculoaponeurotic system (SMAS). The deep-plane facelift has been touted as a procedure that better improves the nasolabial fold and malar fat pad[11]. This technique involves sub-SMAS dissection with the simultaneous release of midfacial ligaments, allowing for repositioning of the malar fat pad. In exchange, however, patients are subject to greater tissue trauma, longer operative time, and increased risk of damage to the facial nerve[10].Minimally invasive and noninvasive techniques such as the threadlift[12], S-Lift[13], and minimal access cranial suspension lift[14]are all attractive options, although the longevity of results is debated. No single approach to surgical intervention has been identified as ideal - patient need, patient choice, and surgeon preference tend to guide treatment selection[15,16].
Soft tissue fillers represent the frontline of nonsurgical alternatives for facial rejuvenation. There are a plethora of options for dermal fillers with variations in material, permanency, viscosity (G’), elasticity (G’),and longevity. The Food and Drug Administration (FDA) has approved different injectables for specific areas of the face and disease processes, although off-label use to achieve facial rejuvenation is common practice. In the midface, injectable fillers appeal to diminish the appearance of nasojugal and nasolabial grooves and augment the malar region. The efficacy and safety profile of injectable fillers is well-reported,although the “temporary” nature of these products often necessitates multiple procedures. Though fortunately rare, intravascular infiltration and vascular compression by filler are concerning events and may lead to devastating complications[17]. Other nonsurgical options reported in the literature include intradermal botulinum toxin injection[18,19], acoustic wave therapy[20], laser[21], and ultrasound[22].
There are a variety of injectable fillers available for the treatment of the aging midface. The objective of this review is to describe the mechanism of action, indication, technique, and evidence of efficacy and safety.Understanding the advantages and limitations of injectable fillers for midface augmentation allows providers to appropriately counsel and treat patients.
HyaIuronic acid
The first soft-tissue fillers derived from bovine collagen in 1981 were immunogenic and caused inflammatory reactions[23]. The next generation of injectable fillers included hyaluronic acid (HA), an injectable extracellular matrix component. HA is a non-sulfated glycosaminoglycan composed of polymeric disaccharides with the unique property of forming stable structures in aqueous solutions[24]. The biological functions of HA throughout the body include lubrication of joint spaces and repair of tissue injury and wound healing[25]. In addition, dermal HA is diminished during the aging process, leading to loss of skin moisture, skin atrophy, and loss of elasticity[26]. Due to its molecular consistency across species, the minimal immunogenicity and relative ease of use have allowed HA fillers to be the most commonly used soft-tissue filler today[27].
As a general guideline, injection of HA fillers is performed using 27- or 30-gauge needles along Hinderer’s lines: two intersecting lines from the ala to the tragus and lateral canthus to lateral oral commissure[28][Figure 1]. The filler is then ideally placed in the upper outer quadrant. Volume replacement targeting specific midface areas is dependent on location and requires meticulous attention to ethnic differences in bony anatomy[29]. The upper cheek, lateral cheek, anterior cheek, and medial cheek are all potential injection sites, and injectors must take precautions to avoid the zygomatic neurovascular bundle, infraorbital artery and vein, and angular artery and vein[30]. Volume replacement in the lid-cheek junction (“tear trough”) can be achieved with two or three injections along the nasojugal fold though care must be taken to avoid injury to vessels and orbital content[31]. There are two strategies to approach the submalar area: subcutaneous injection at numerous sites per side or a single medial subcutaneous injection using a fanning technique[32].Providers should take care to avoid the facial vessels and parotid duct during both techniques. Volume replacement in the preauricular area can be accomplished with three to five superficial injection sites with care to avoid the parotid gland and transverse facial artery and vein[33].
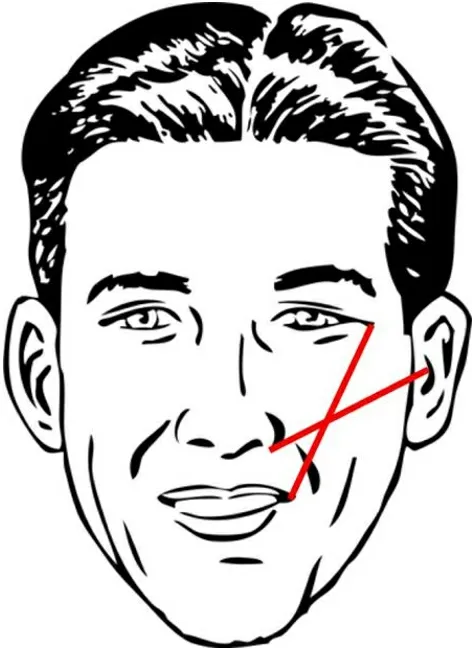
Figure 1. Hinderer’s lines (two intersecting lines: ala/tragal, lateral canthal/commissure).
The first HA-based filler approved for use by the United States FDA was Restylane (Galderma, Fort Worth,TX) in 2003[34,35]. There are now many other products that differ in concentration, elastic modulus, and degree of cross-linking, including Juvederm (Allergan Inc., Pringy, France), which has been approved for a variety of indications, including correction of facial wrinkles and folds as well as lip and cheek augmentation. Additional modifications on these products, including a new product with increased crosslinking (Teosyal resilient HA, Geneva, Switzerland), have been created to help reduce degradation due to mechanical strain[36]. Multiple randomized controlled trials have demonstrated the efficacy of HA for agerelated midface volume deficit. In a multicenter, single-blinded, controlled study, 85.6% of subjects treated with Juvederm Voluma demonstrated a significant improvement of 1 point or more at 6 months follow-up on the Mid-Face Volume Deficit Scale, a validated blinded clinician score, compared with the control group[37]. There were minimal adverse events, and more than half of the subjects reported lasting efficacy at 24 months. A follow-up study on patient satisfaction showed that nearly 93% and 79% of patients at 6 months and 2 years after treatment, respectively, noted improvement on the Global Aesthetic Improvement Scale[38]. In addition, patients reported looking 5 years younger at 6 months and 3 years younger at 2 years.Newer split-face trials are now comparing Cohesive Polydensified Matrix (CPM) (Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) with FDA-approved Vycross technology (Allergan Inc., Pringy,France), noting noninferior aesthetic results and more favorable safety and patient satisfaction profiles with CPM[39,40].
CaIcium hydroxyIapatite
Calcium hydroxylapatite (CaHa) is a unique compound with dual functionality as a replacement volumizer as well as a collagen biostimulator[41]. Due to its identical composition to human bone and teeth, CaHa is biodegradable, naturally resorbable, nontoxic, nonantigenic, and long-lasting, allowing for optimal biocompatibility and efficacy as a filler material[42]. CaHa’s biostimulatory effects work by immediately creating volume while encouraging a fibroblastic response and acting as a scaffold for newly formed collagen after natural resorption and excretion[43-45]. CaHa has a wide range of indications beyond aesthetics,including correction of orthopedic[46]and dental defects[47]and vocal fold augmentation[48]. In 2006, Radiesse(Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany) was FDA-approved for the correction of moderate to severe midface wrinkles and folds and lipoatrophy in patients with human immunodeficiency virus. Radiesse utilizes a 30:70 ratio mixture of CaHa microspheres to aqueous carrier gel and has been well studied in facial aesthetic medicine[42,49,50].In the midface, restoration of the cheekbones allows for correction of specific creases and defects while providing volume to the malar and submalar regions. By augmenting the upper maxillary region, the shadowing effect seen with midface defects is ameliorated, resulting in a younger appearance[51]. CaHa can be placed using a similar technique to HA, though a larger bore needle or cannula is recommended due to the relative viscosity[52]. To achieve optimal aesthetic results, augmentation of the entire cheek and adjacent areas should be considered, not just the area of the defect[49]. Injection depth depends on location; superior to the alar-tragal line, CaHa should be injected superior to the periosteum, while subdermal injections are ideal for targeting areas inferior to the alar-tragal line[53]. After product placement, light massaging and molding of the filler are recommended to blend the injectable and reduce tissue edema and ecchymosis,thereby helping to achieve the desired outcome[42].
There is a plethora of data supporting the long-term safety and efficacy of CaHa for facial soft-tissue augmentation. The first pivotal multi-center study comparing CaHa against human collagen in a randomized split-face trial reported significantly longer-lasting correction of nasolabial folds with less material used and fewer injections[54]. A follow-up safety study of 113 patients over 47 months showed only seven minor adverse events, including transient ecchymosis, submucosal nodules, and inflammation and edema[55]. Duration of action ranges from 12 to 18 months though long-term effectiveness of up to 30 months has been reported[56]. Despite these promising results, one systematic review reported that Radiesse was significantly more likely to result in intra-arterial complications and skin necrosis when compared with other facial fillers[57]. It is thought that the larger particle size of Radiesse increases the propensity of vascular compromise. Thus, the provider must remain vigilant to avoid vascular complications.
PoIy-L-Iactic acid
Poly-L-lactic acid (PLLA) is an absorbable, semi-permanent, biocompatible synthetic polymer that induces collagen synthesis, leading to volume restoration. After injection, PLLA particles stimulate an inflammatory response with tissue macrophages, leading to degradation into lactic acid and subsequently carbon dioxide and water[58]. This process promotes the formation of new type-I collagen as well as fibroblast activity,leading to gradual volume replacement[59]. The effect of PLLA stems from the natural host response rather than the immediate volume increase, thus leading to an extended duration of action from 12 to 24 months[60].
In the United States, Sculptra (Galderma, Fort Worth, TX) has been FDA-approved for facial lipoatrophy associated with HIV and more recently in 2009 for the correction of shallow to deep nasolabial folds and other facial wrinkles[17]. The product must be reconstituted with Sterile Water for Injection (5 mL) prior to injection to form a sterile suspension[61]. When approaching the midface, supraperiosteal injections over the zygoma, maxilla, and temples and subcutaneous injections in the submalar and preauricular areas are recommended[62]. Consensus guidelines recommend 3-5 initial sessions for optimal results, with at least four weeks between sessions. Patients should be counseled that cosmetic effects have a gradual onset and can last two years or more[62]. Due to the slowly progressive bulking effects of PLLA, it is important to not overcorrect the treatment area with too much filler[63].
Multiple retrospectives and prospective cohort studies have demonstrated the safety of injectable PLLA[64-67].For example, a pivotal randomized, single-blinded, multicenter trial in 2010 compared the efficacy and safety of PLLA with human-derived collagen for nasolabial fold deficits[68]. Subjects receiving injectable PLLA had significantly improved average Wrinkle Assessment Scale scores at all-time points up to 25 months compared with the control group. Other similar trials have reported high provider and patient satisfaction with results[69,70]. Compared to HA fillers, PLLA was found to be noninferior in the correction of moderate to severe nasolabial folds and appeared to be superior to HA in patients less than 52 years of age[71]. Main adverse effects include papules and nodules stemming from unequal distribution, inaccurate placement, and incorrect reconstitution[72]. As preparation and administration of PLLA have become increasingly standardized, however, the frequency of post-injection complications has also decreased[73].
PoIymethyI methacryIate
Polymethyl methacrylate (PMMA) is a synthetic, biologically compatible, inert, nonbiodegradable polymer originally used in dental and orthopedic procedures. Although a previous version (Artecoll, Canderm Pharma Inc, Canada) was marketed outside of the United States, newer generations of PMMA injectable fillers (ArteFill, later rebranded as Bellafill, Suneva Medical, San Diego, CA, USA) have been FDA approved for nasolabial fold augmentation since 2006[17,74]. These formulations contain PMMA microspheres, which are evenly distributed among denatured bovine collagen and lidocaine. Compared to other injectable filler materials, PMMA is a small, smooth permanent filler without electrical charge, thus preventing phagocytosis and degradation by macrophages[75]. Initial volume restoration is attributed to the collagen content in the filler though after this component is resorbed after 1 to 3 months, PMMA microspheres become permanently encapsulated with connective tissue and cells[76]. Due to the bovine collagen suspension, skin allergy testing must be conducted 2-4 weeks prior to injection, and double skin testing is often recommended to reduce the severity and incidence of allergic reactions[77]. On account of its permanent nature, the administration of PMMA requires careful attention to detail and placement. PMMA should be implanted intradermally, just superior to the dermis and subcutaneous layer[78]. The tunneling technique, or moving the needle back and forth beneath a wrinkle, allows for even distribution and layering of the product.
PMMA was initially introduced worldwide in the 1990s and became approved in 2006 after a multicenter randomized controlled trial of 251 subjects receiving Artecoll for a variety of facial wrinkles reported superior results to collagen dermal filler[78]. At 6 months follow-up, investigator and subject satisfaction ratings were higher at all injection sites, and significant augmentation was present at 12 months follow-up.After Artecoll, the next generation of PMMA was named Artefill, which boasted greater uniformity in size,surface contour, smoothness, and roundness[79]. Since then, PMMA has been repackaged as Bellafill, which offers a very favorable risk/benefit profile when administered properly. Short-term complications (tissue necrosis, infection) and long-term sequelae (granulomas, chronic inflammatory reaction) have been reported though they are fortunately rare occurrences[80]. A total of 1008 subjects receiving PMMA fillers for nasolabial folds was followed over five years, with biopsy-confirmed granulomas occurring in 1.7%[81].Almost all granulomas resolved with intralesional corticosteroid injections, and overall satisfaction rates remained high through the study period. In the event of nodules, skin changes, or contour irregularity,another study reported that surgical debulking of material could reduce the effects of these complications[82]. Long-term data also supports an excellent safety profile for this filler type. Surveillance data from 2007 to 2016 for more than half a million Bellafill syringes showed 11 total confirmed granulomas(0.002% or syringes sold)[79].
FiIIers vs. surgery
Although deep dermal fillers offer safe and excellent cosmetic results, it is important to consider which patients would likely benefit more from surgery. For those with more significant midface ptosis and facial volume loss, the more permanent and dramatic effects of rhytidectomy are more appealing. The main goals of the midface lift include effacement of the lower lid-cheek junction with volume restoration of the cheek and malar eminence[83]. Although a thorough discussion of surgical techniques is out of the scope of this review, providers must evaluate which patients would benefit from fillers, surgery, or a combination of both.During this conversation, differences in expected outcomes and recovery for the treatment options should be covered. If patients are willing to undergo general anesthesia and have significant soft tissue ptosis, most experts agree that a midface lift may be the more appropriate solution[83]. The addition of soft tissue fillers should be a separate discussion after the patient has had ample time to recover, allowing for the settling of the soft tissue.
Midface surgical options can confer longer-lasting results when compared with midface fillers. In a series of 157 patients undergoing a primary facelift at age < 50, patients reported appearing significantly younger after facelift compared with after nonsurgical intervention (8 yearsvs.4 years,P= 0.048)[84]. Thirty-two percent of patients underwent an average of 7 rounds of injectable fillers prior to surgical intervention, with an average expenditure of $7000 on nonsurgical procedures. Longevity of midface lift approaches has been reported, although most outcome measures are largely subjective, citing high patient satisfaction and quality of life scores at up to 5 years follow-up[85,86]. A recent study described a series of 143 patients undergoing an endoscopic transtemporal approach, reporting a significant, objective improvement in midfacial height that persisted for up to 15 years[87]. Despite these encouraging and lasting aesthetic results, no procedure is without its risks, which must be extensively discussed when counseling patients. The complications of midface lift surgery include asymmetry, infection, hematoma, facial and trigeminal neuropathy, and undesired scarring[88].
DISCUSSION
Management of the midface with volume augmentation can be safely and effectively achieved with the use of soft tissue injectable fillers. The selection of filler is largely dependent on patient factors, including the severity of ptosis, degree of volume loss, age, cost, preference, and surgical candidacy[88]. From the provider’s perspective, a detailed understanding of anatomy in the nasal region as well as proper technique is fundamental to minimizing the risk of complications and achieving the desired effect.
For decades, surgical rhytidectomy was established as the frontline procedure to enable patients to achieve predictable, customizable, and natural-looking results. However, the popularity of minimally invasive techniques has increased rapidly due to the introduction and wide acceptance of soft tissue fillers. Among the younger patient population that is not quite ready for a facelift, injectable fillers are an attractive, less permanent alternative to surgery. Compared to undergoing more invasive surgery, fillers offer the patient less discomfort and a shorter recovery time, making them very practical. Additionally, new and innovative advances to avoid anatomic danger zones and improve overall technique have led to safer injection practices[89-91]. Needle aspiration prior to injecting the filler has been reported to help prevent accidental intravascular injection[92]. With appropriate handling and adequate experience, injectable fillers are a safe and effective option for the aging midface.
The increasing popularity of facial fillers is well reflected in popular media and online news coverage. For example, a Google News search for articles on “facial fillers” from 2008 to 2017 reported coverage as 45%positive, 30% neutral, and 25% negative[93]. While most media coverage at national and international levels was fairly positive, there was some concern with the regulation of facial fillers, a salient issue that may incite apprehension in some patients interested in the procedure. Additionally, nearly half of the papers discussed complications associated with the procedure despite the overall rarity of vascular injury and blindness[94,95].As most cases of vision loss do not recover, and there is no consistent treatment for blindness, it is crucial for providers to understand the risks involved with the procedure and appropriately counsel patients[96].
From an economic standpoint, the short-term cost of injectable fillers is another advantage for patients seeking minimally invasive treatment. However, a study by Biskupiak and Sclafani among several facial plastic surgeries and dermatology practices in New York City estimated that the total medical cost for surgical rhytidectomy was around $10,181[97]. When comparing this value to large-volume facial soft tissues assuming 90 months of the desired effect, the medical cost for surgery was the lowest cost option among the other treatments. When considering total costs, including workdays lost, ArteFill ($11,151 for 1 treatment)and Sculptra ($14,850 for 3.75 treatments) were less costly than surgical rhytidectomy ($15,181). This discrepancy was attributed to extra fees due to anesthesia, operating room, and overhead hospital costs, as well as the lengthier recovery period for surgery. However, it is important to consider that a rhytidectomy may ultimately be more cost-effective compared to patients undergoing large-volume filler augmentation over the course of several years.
There are multiple advances in filler technology to increase longevity while improving efficacy and safety profiles. For example, a new line of dermal fillers, called resilient hyaluronic acid (RHA) (Revance Therapeutics, Nashville, TN), was designed with a cross-linking method to mimic the function of native HA better. It is thought that these products (RHA 2, 3, 4) are able to adapt to facial movements to treat dynamic wrinkles and folds, a typical indication for neuromodulators[98]. Recently FDA approved for the correction of deep dynamic wrinkles, including the nasolabial folds; RHA 2 and 3 are indicated for injection into the mid-to-deep dermis, while RHA 4 is indicated for injection into the deep dermis to superficial subcutaneous tissue[99]. One split-face, randomized controlled trial on 90 subjects with moderate to severe nasolabial folds found at least equivalent efficacy and safety profiles with comparable fillers[100]. However, immediate and long-term satisfaction for both investigators and subjects favored RHA fillers with no reports of serious adverse events. At this time of writing, other dermal fillers expected to come to the United States market include Juvederm Volite (Allergan, Dublin, Ireland), the HA-based filler meant to improve skin quality, and ProfHilo (IBSA Nordic ApS, Denmark), another HA-based filler without any chemical cross-linking agents[101,102].
CONCLUSION
As a result of volume loss and fat atrophy, the aging midface is characterized by overall drooping of soft tissues and deepening of the nasolabial folds. Although surgical rhytidectomy is a long-lasting option for these patients, recent nonsurgical avenues, including filler injections, are becoming more popular due to reduced patient discomfort and recovery times. While filler selection may depend on patient preference and individual goals, providers must remain informed on the potential complications associated with midface augmentation and provide appropriate preprocedural counseling.
DECLARATIONS
Authors’ contributions
Made substantial contributions to conception and design of the study, performed interpretation of data for the work, drafted the work, gave final approval of the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Go BC, Frost AS
Made substantial contributions to conception and design of the study, revised it critically for important intellectual content, gave final approval of the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Friedman O
AvaiIabiIity of data and materiaIs
Not applicable.
FinanciaI support and sponsorship
None.
ConfIicts of interest
All authors declared that there are no conflicts of interest.
EthicaI approvaI and consent to participate
Not applicable.
Consent for pubIication
Not applicable.
Copyright
? The Author(s) 2021.
 Plastic and Aesthetic Research2021年7期
Plastic and Aesthetic Research2021年7期
- Plastic and Aesthetic Research的其它文章
- AUTHOR INSTRUCTIONS
- Lateral abdominal wall reconstruction
- Advances in tracheal reconstruction and tissue engineering
- Immediate microvascular maxillofacial reconstruction and dental rehabilitation: protocol,case report, and literature review
- Enhanced recovery and medical management after head and neck microvascular reconstruction
- Overview of magnetic resonance lymphography for imaging lymphoedema
