OsbZIP09, a Unique OsbZIP Transcription Factor of Rice, Promotes Rather Than Suppresses Seed Germination by Attenuating Abscisic Acid Pathway
Wang Chuxin, Zhu Chengchao, Zhou Yu, Xiong Min, Wang Jindong, Bai Huang, Lu Chenya, Zhang Changquan, 2, Liu Qiaoquan, 2, Li Qianfeng, 2
Research Paper
OsbZIP09, a Unique OsbZIP Transcription Factor of Rice, Promotes Rather Than Suppresses Seed Germination by Attenuating Abscisic Acid Pathway
Wang Chuxin1, #, Zhu Chengchao1, #, Zhou Yu1, Xiong Min1, Wang Jindong1, Bai Huang1, Lu Chenya1, Zhang Changquan1, 2, Liu Qiaoquan1, 2, Li Qianfeng1, 2
(Key Laboratory of Plant Functional Genomics of the Ministry of Education / Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding / Key Laboratory of Crop Genetics and Physiology of Jiangsu Province, College of Agriculture, Yangzhou University, Yangzhou 225009, China; Co-Innovation Center for Modern Production Technology of Grain Crops of Jiangsu Province / Joint International Research Laboratory of Agriculture and Agri-Product Safety of the Ministry of Education, Yangzhou University, Yangzhou 225009, China; )
We successfully identified a novel and unique OsbZIP transcription factor, OsbZIP09, whose mutants exhibited longer seeds and less severe pre-harvest sprouting than the wild type, but shared similar germination rate as the wild type under normal germination conditions. The expression ofwas induced by abscisic acid (ABA) and declined as the germination process. As a nucleus-localized transcription factor, the conserved binding motif of OsbZIP09 was identified via DNA affinity purification sequencing technique. Further evidences indicated that OsbZIP09 directly enhanced the expression of ABA catabolism gene, thus reducing ABA accumulation. In addition, OsbZIP09 also directly bound to the promoter ofgene to inhibit its expression, thus further alleviating the suppressive effect of ABA on seed germination. These results demonstrated that OsbZIP09 likely functions as a brake of the ABA pathway to attenuate the inhibitory effect of ABA on rice seed germination via dual strategies.
OsbZIP09; pre-harvest sprouting; abscisic acid; seed germination;gene;gene; rice; transcription factor
Seed dormancy and germination are two distinct physiological processes of seed-bearing plants. Successful transition from dormancy to germination is not only a key developmental process in a plant’s life cycle but also ensures agricultural production. Seed germination is precisely controlled by various environmental cues and endogenous stimuli, especially phytohormones (Finkelstein et al, 2008; Shu et al, 2016). Nevertheless, either defect in seed germination or inappropriate release of seed dormancy will cause substantial losses in crop yield.
Abscisic acid (ABA) and gibberellins (GA) are two principal phytohormones that antagonistically regulate seed germination (Finkelstein et al, 2008; Shu et al, 2016; Nonogaki, 2019). ABA is a predominant suppressor directing seed germination, whose abundance is increased during seed maturation, thus preventing pre-harvest sprouting (PHS) (Yan and Chen, 2017). In contrast, when seed germination is activated, the accumulation of endogenous ABA decreases while that of GA increases. Typical ABA-deficient seeds have reduced dormancy but enhanced germination features (Née et al, 2017), leading to PHS that extensively reduces crop yield and grain quality. Meanwhile, plants overexpressing ABA biosynthesis genes or knock-out/down ABA catabolism genes promote endogenous ABA content and thus inhibiting seed germination (Martínez-Andújar et al, 2011; Nonogaki et al, 2014). In addition to ABA content, ABA sensitivity is also involved in regulation of seed germination. In general, ABA is perceived and transduced mainly through a core signaling pathway, including Pyrabactin Resistance1 (PYR1)/PYR1-like (PYL)/Regulatory Component of ABA Receptor (RCAR) receptors, protein phosphatases 2C (PP2Cs) and sucrose nonfermenting 1-related protein kinase 2 (SnRK2), as well as downstream transcription factors (Chen et al, 2020). When ABA is present, SnRK2s are auto- phosphorylated and subsequently activate the basic leucine zipper (bZIP) transcription factors, including ABA-responsive element (ABRE) binding proteins (AREB), ABRE-binding factors (ABF) and ABA- insensitive 5 (ABI5), thus further inducing the expression of downstream target genes and corresponding plant responses, including inhibition of seed germination (Miyakawa et al, 2013; Zhao et al, 2020). Therefore, bZIP transcription factors play a key role in mediating the ABA-responsive transcriptional network.
One of the largest and most diverse transcription factor families in plants are bZIPs, characterized by a basic DNA-binding region and an adjacent so-called leucine zipper, enabling bZIP dimerization (Dr?ge- Laser et al, 2018). bZIPs regulate a wide range of growth and developmental events, including seed maturation and germination, responses to abiotic and biotic stresses and photomorphogenesis (Alves et al, 2013; Sornaraj et al, 2016; Dr?ge-Laser and Weiste, 2018). Considering that rice is the staple food for more than half of the world’s population, and that its production is critical for ensuring global food security, the germination characteristics of rice seeds must be vigorously regulated, thus ensuring uniformity of post-harvest seed germination, and preventing PHS during the seed maturation period (Tuan et al, 2018). Although phytohormones, including ABA, are key regulators of seed dormancy and germination, they usually have pleiotropic effects on plant growth and development. Therefore, identifying specific downstream components of phytohormones and applying them in crop improvement can affect limited physiological processes associated with certain traits (Tong and Chu, 2018). As a result, cloning novel ABA-responsive OsbZIPs with specific functions in seed germination, and dissecting their molecular mechanisms, can help generate elite rice varieties with excellent germination characteristics, thus ensuring high yield and grain quality.
There are a total of 89 OsbZIP genes in rice (Nijhawan et al, 2008; Lu et al, 2009). However, most OsbZIPs in rice are still functionally uncharacterized. Among the reported bZIPs, a number of them are closely related to regulation of seed germination. Interestingly, all these OsbZIPs related to regulation of seed germination play inhibitory roles. In this study, we identified a novel and unique OsbZIP transcription factor, OsbZIP09, which promoted seed germination, contrary to other reported OsbZIPs in rice. We further demonstrated that OsbZIP09 improved rice seed germination via boosting ABA catabolism and attenuating its signaling.
RESULTS
osbzip09 mutants exhibited less PHS phenomena
In the autumn of 2018, the rice PHS phenomena were severe after several consecutive rainy days in Yangzhou City, Jiangsu Province, China. We have selected hundreds of genes with high or specific expression in rice seeds for generation of their responsive mutants via CRISPR/Cas9 technology. By examining the PHS of these mutants in the field, several lines with less severe PHS than the wild type were identified, includingmutants. To further confirm the effect ofmutation on rice PHS, a second target site was selected to generate more novelmutants for validation. The mutants generated from the original and second target sites ofwere designated asand, respectively (Fig. 1-A). We subsequently used the growth chamber to mimic the high temperature and humidity growth conditions to test the PHS phenotype of the mature rice panicles. For a better comparison, two homozygous mutants, one with 1 bp insertion () and another with 5 bp deletion () (Fig. 1-A), were selected from each mutation event. The results showed that the PHS patterns of allmutants were similar and less severe than the wild type (Fig. 1-B and -C), consistent with previous field PHS phenotype. Therefore,andwere selected as representative lines for the following analysis.
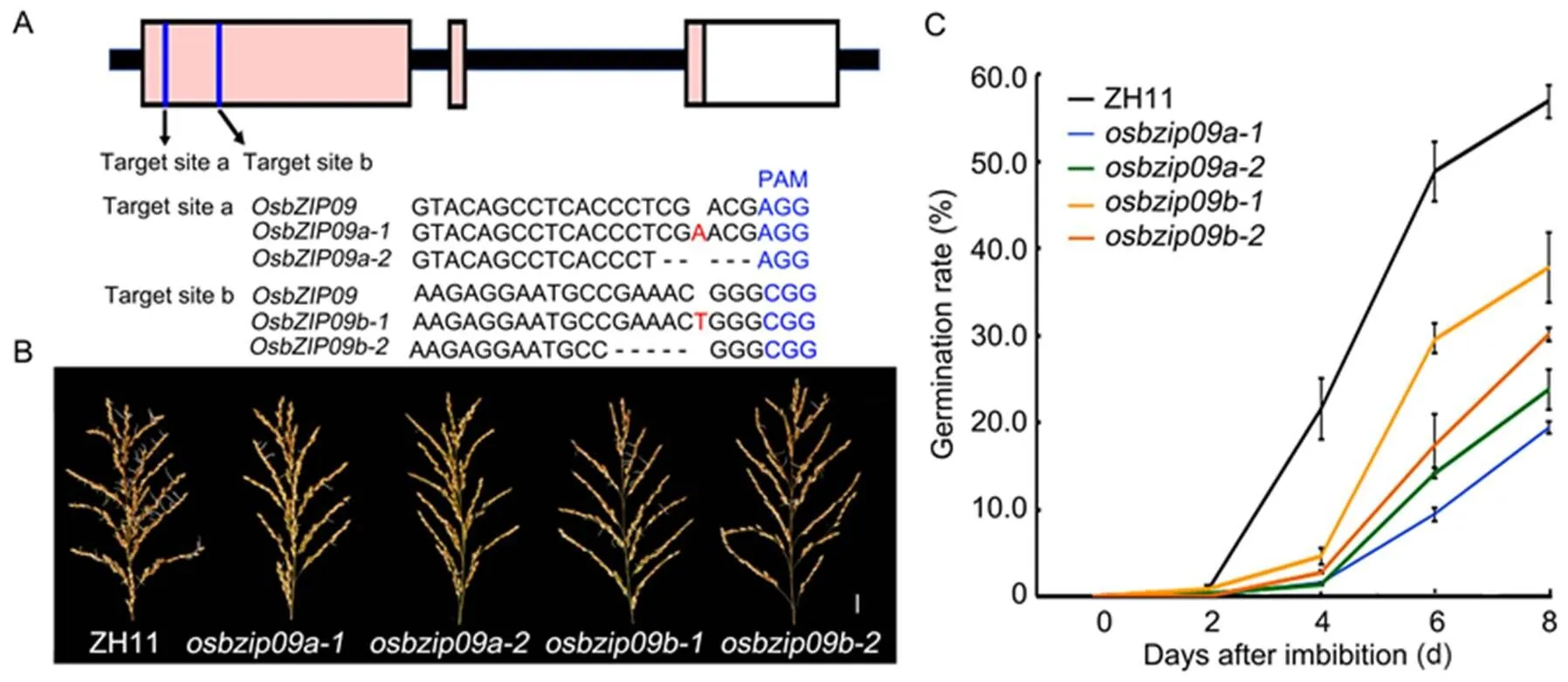
Fig. 1.Knock-out ofalleviates rice pre-harvest sprouting (PHS).
A, Schematic diagram of the two targets ingene and the mutation information of the related mutants. The protospacer adjacent motif (PAM) is highlighted in blue, insert nucleotide is highlighted in red, and deleted nucleotides are shown with minus sign ‘-’.
B,Germination phenotype of mature rice panicles after 6 d imbibition in water. Scale bar, 2 cm. ZH11, Zhonghua 11.
C, Time-course analysis of germination rates of seeds in the panicles. Data are Mean ± SD (= 30).
Agronomic traits and normal seed germination characteristics of osbzip09 mutants
In general,mutation had no observable effect on rice morphology (Fig. 2-A). The quantification data showed that there were no difference in plant height, heading date, tiller number per plant, grain number per panicle, 1000-grain weight and grain width (Fig. 2-B to -D, -F, -G and -J). Only the panicle length and grain length had certain increase, especially the grain length (Fig. 2-E, -H and -I). Slender rice, with a high length-to-width ratio, is favored by both rice breeders and consumers. Thus,mutation not only promoted PHS resistance of rice, but also improved rice grain shape. In addition, we also monitored the germination ofseeds under normal growth conditions. The result of germination assay indicated thatmutation only slightly delayed the germination process (Fig. S1).
Expression analysis and sub-cellular localization of OsbZIP09
Sinceis a novel gene, we monitored its expression pattern during rice seed development and seed germination process. The transcripts ofslightly increased as the development of seeds, and peaked around 25 d after flowering (DAF) (Fig. 3-A). During the germination period,exhibited a reversed expression pattern: the highest transcription ofwas in the dry seeds, and its expression declined gradually as seed germination progressed (Fig. 3-B). Moreover, the expression ofin response to ABA and GA, two key phytohormones antagonistically regulating seed germination, was monitored. A number of ABA and GA responsive marker genes were used to validate the hormone treatment. The expression of(),() andwas increased in response to ABA treatment (Fig. S2-A), while that of() was decreased and() was increased in response to GA treatment (Fig. S2-B), consistent with previous reports (Saika et al, 2007; Tong et al, 2014). As to, its expression was remarkably induced by ABA, but not GA (Fig. 3-C). The results were consistent with Shu et al (2016) and Née et al (2017) that ABA abundance decreased while GA content increased as seed germination process. Therefore, the declined ABA content might be the cause of decreasedexpression. Furthermore, sub- cellular localization analysis indicated that OsbZIP09 specifically localized in the cell nucleus (Fig. 3-D), coincident with its role as a transcription factor.
OsbZIP09 directly regulated expression of ABA8ox1 and LEA3
To investigate how OsbZIP09 functions as a positive regulator in regulating seed germination, DNA affinity purification sequencing (DAP-seq) was used to identify the potential direct targets of OsbZIP09. Totally, more than 1 300 binding sites were identified in the promoter region of genes. Further motif analysis identified a conserved OsbZIP09 binding sequence (C/ACACGTG/C) (Fig. 4-A), including the G-box sequence (CACGTG) and another conserved sequence (CACGTC) similar to G-box. Among these targets,() and, two genes closely related to ABA pathway, were included, with one and three G-box in the promoters, respectively (Fig. 4-Band Table S1). Moreover, thepromoter also contained a conserved CACGTC sequence (Fig. 4-B). Then the transcripts ofandduring the germination period were also examined. The result showed that their expression patterns were similar to that of, which was the highest expression at the initial stage of seed germination and then declined gradually as germination progressed (Fig. 4-C and -D).To further verify the transcriptional regulation ofandby OsbZIP09, the dual luciferase system was applied. The promoters with 1.8 and 2.0 kb lengths ofandwere amplified and cloned into the pGreenII0800- LUC vector, respectively (Fig 4-E). The data indicated that OsbZIP09 indeed can promote the expression ofbut reduce the expression of(Fig. 4-F and -G). These data suggested that OsbZIP09 can not only directly bind to the promoters of the target genes, but also differentially regulate their expression.
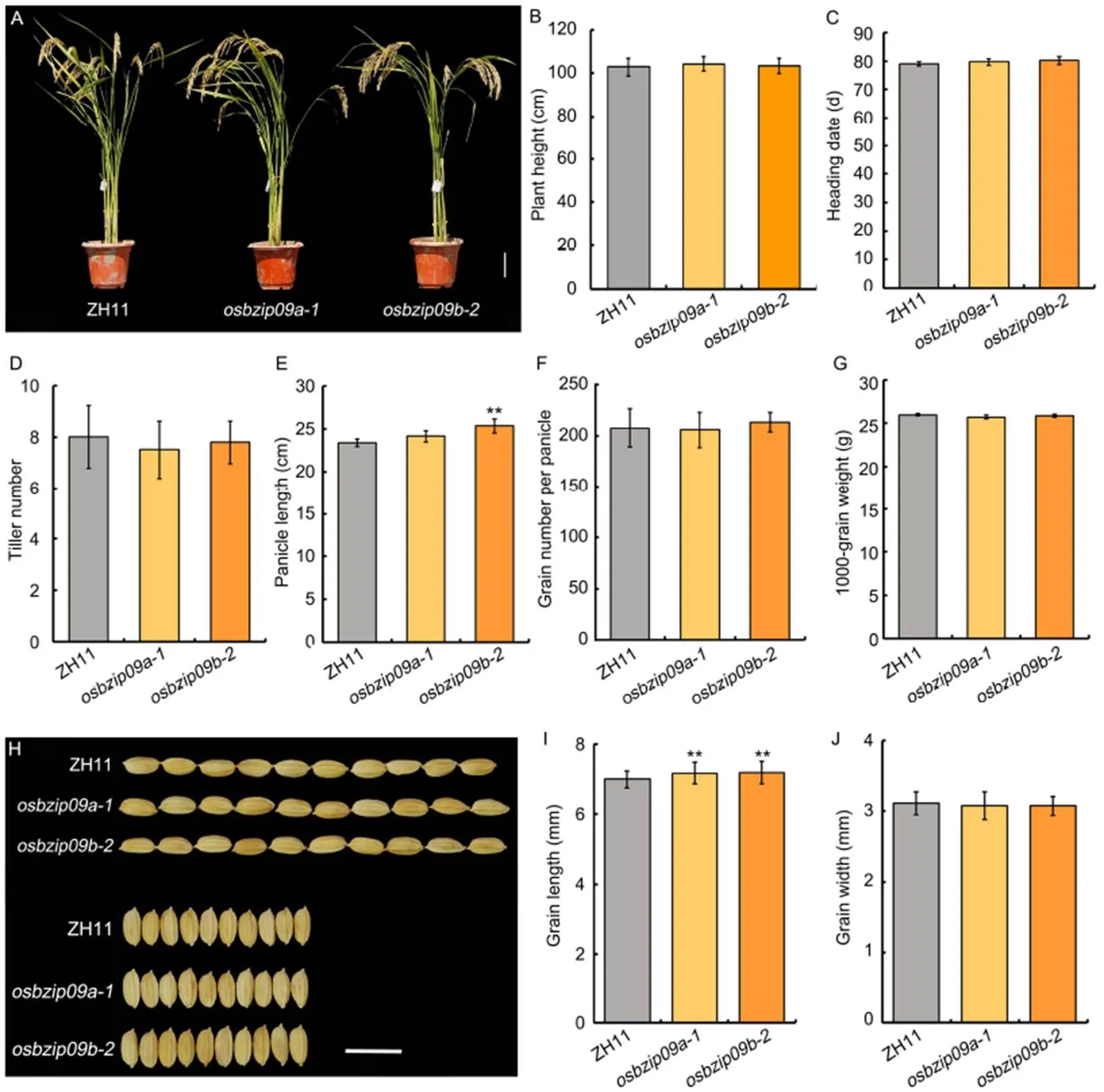
Fig. 2.Agronomic traits and normal seed germination assay ofmutants.
A, Morphology ofmutants and its wild type Zhonghua 11 (ZH11). Scale bar, 10 cm.
B?J, Plant height (B), heading date(C), tiller number per plant(D),panicle length(E), grain number per panicle(F), 1000-grain weight(G), grain shape (scale bar, 1 cm)(H), grain length (I) andgrain width(J)ofmutants and wild type.Data are Mean ± SD (= 10 in B?G, and= 30 in I and J). **,< 0.01 (-test).
OsbZIP09 enhanced ABA catabolism and weakened effect of ABA signaling

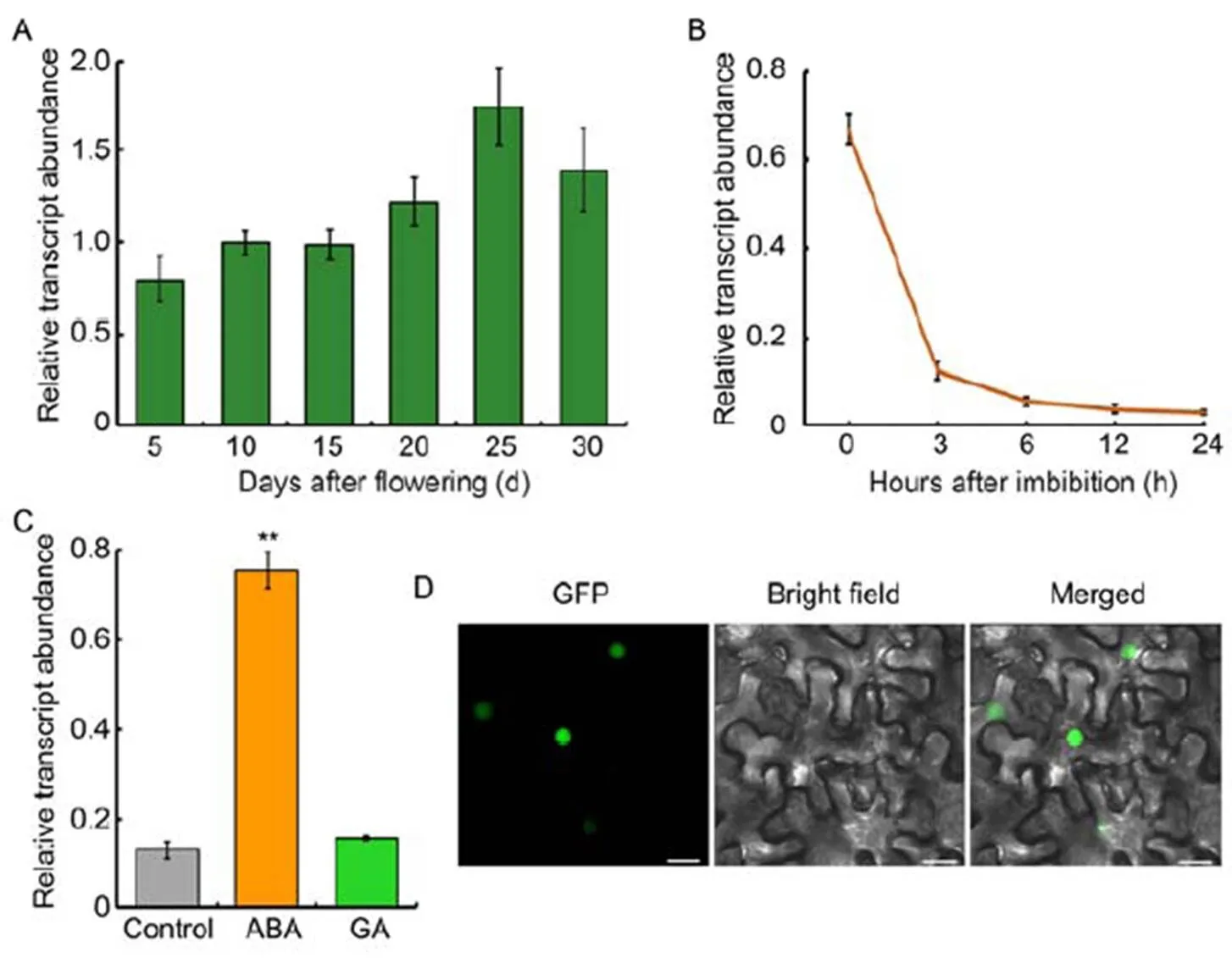
Fig. 3. Expression and sub-cellular localization analysis of OsbZIP09.
A,Expression profiling oftranscript abundance in developing seeds of rice.
B,Time-course expression analysis ofafter imbibition.
C, Expression analysis ofin responseto abscisic acid (ABA, 5 μmol/L) and gibberellins(GA, 10 μmol/L) treatments.
D, Sub-cellular localization of OsbZIP09 () in tobacco epidermal cells. Scale bars, 20 μm.
served as an internal reference gene for normalization in A?C. Data are Mean ± SD (= 3). **,< 0.01 (-test).
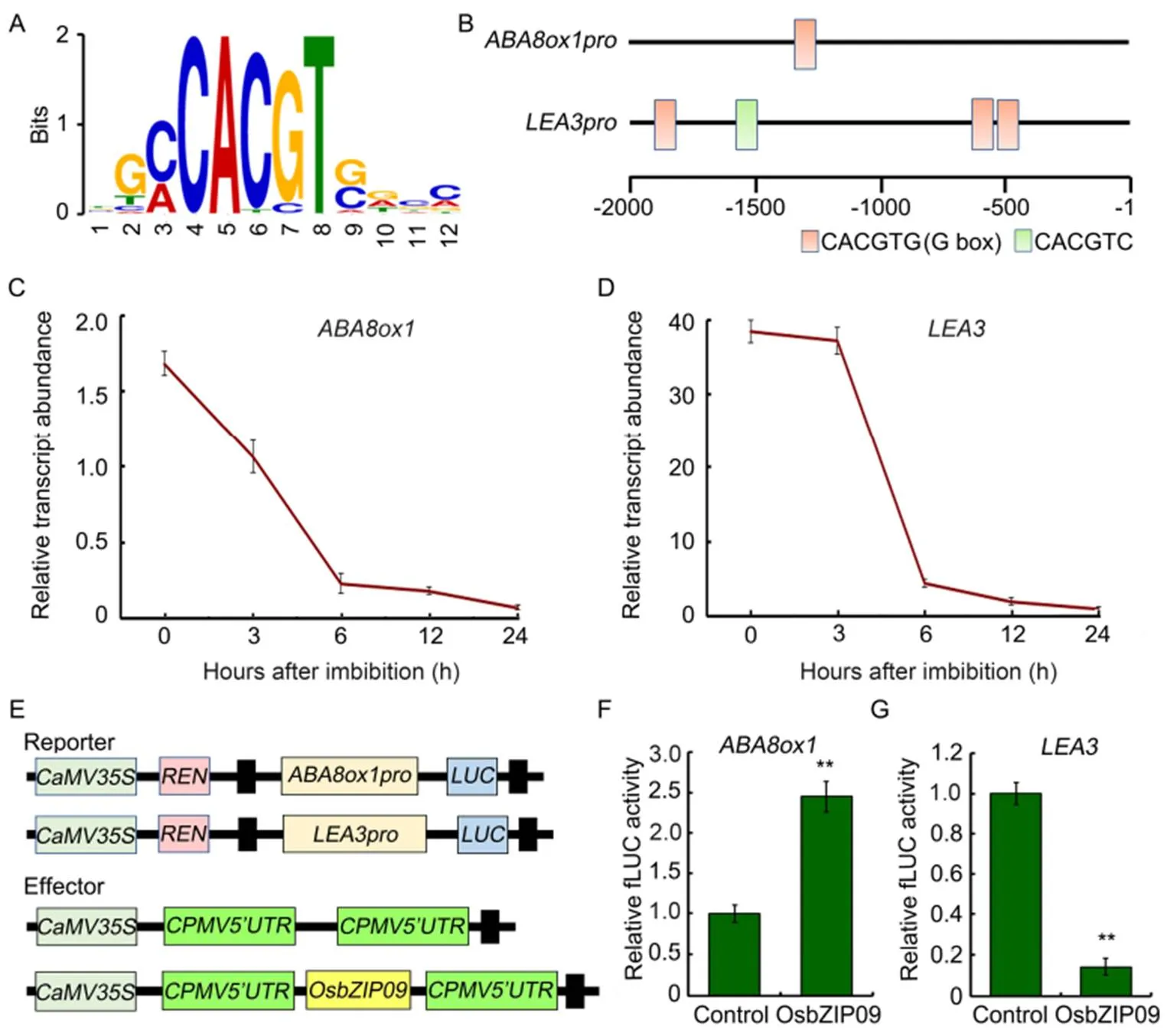
Fig. 4.Identification of OsbZIP09 binding motif and its targets involved in regulation of rice seed germination.
A, Conserved motif identified from the DNA affinity purification sequencing(DAP-seq) data.
B, Distribution of OsbZIP09 binding motifs in the 2-kb region preceding the initial codon (ATG) ofand.
C and D, Time-course expression analysis ofandafter imbibition. Data are Mean ± SD (= 3).
E, Schematic program of the reporter and effector constructs used in the dual-luciferase reporter assay.
F and G, OsbZIP09 activates the promoter activity ofbut suppresses the promoter activity of. Data areMean ± SD (= 5). ** indicates significant difference (< 0.01,-test).
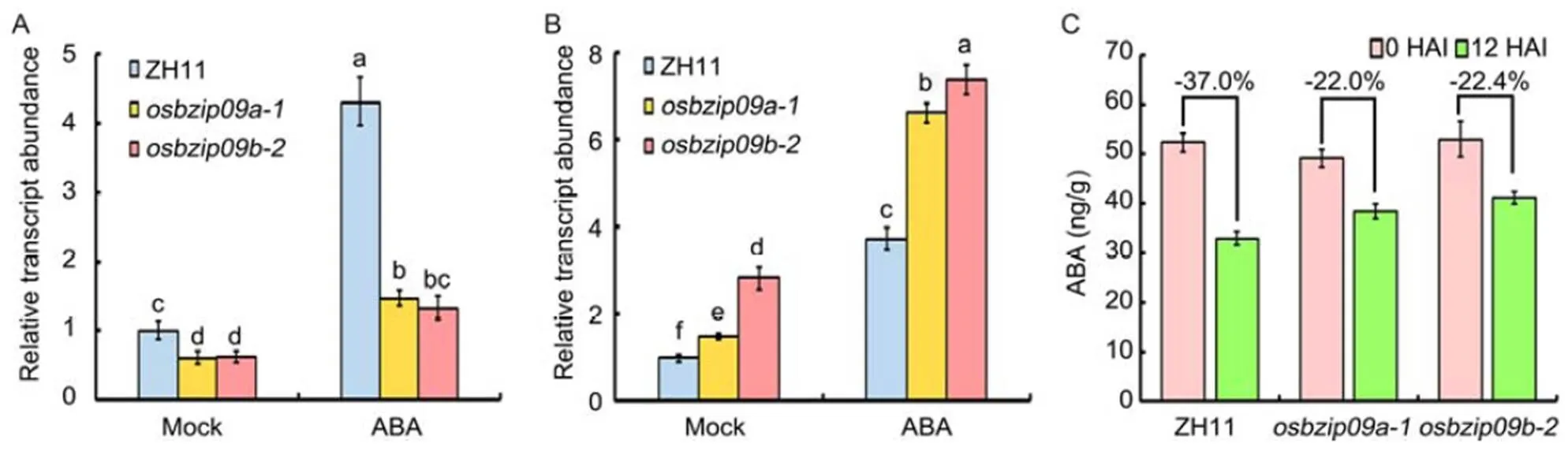
Fig. 5.Expression analysis ofandin rice seeds before and after imbibition of abscisic acid (ABA).
A and B, Expression analysis of(A) and(B) inmutants and wild type Zhonghua 11 (ZH11) with or without ABA treatment.served as an internal reference gene for normalization. Different lowercase letters indicate significant differences at< 0.05 level based on one-way analysis of variance.
C, ABA content inmutants and ZH11 before or at 12 h after imbibition (HAI).
Therefore, a simplified model was proposed to illustrate how OsbZIP09 participates in the ABA pathway to regulate rice seed germination (Fig. 6). In brief, OsbZIP09 directly enhances the expression of the ABA catabolism gene, thus decreasing ABA content and subsequently, the expression of ABA-responsive genes, including. Moreover, OsbZIP09 can also directly bind to thepromoter to inhibit its expression, thus further attenuating the suppression effect of ABA on seed germination. Meanwhile, the induction effect of ABA onexpression can become weaker as the ABA content declines.
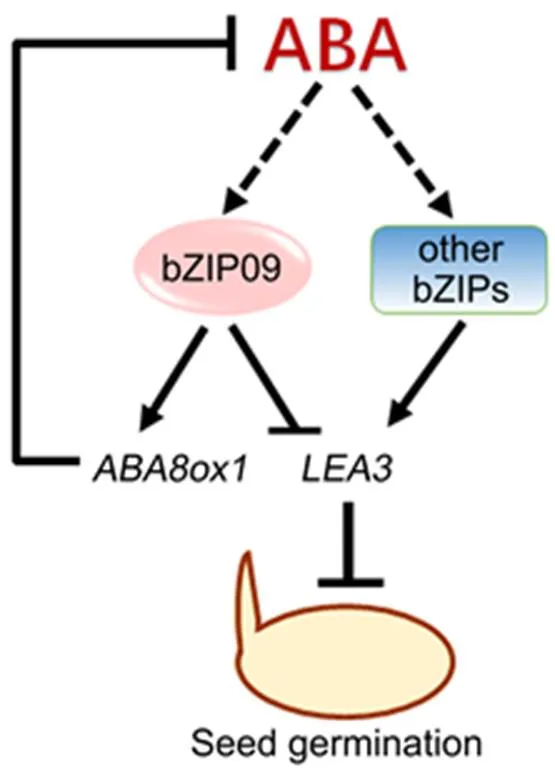
Fig. 6.Simplified model showing OsbZIP09 promotes rather than suppresses seed germination via reducing abscisic acid (ABA) accumulation and attenuating ABA-induction of downstream responsive genes in rice.
DISCUSSION
Seed germination and dormancy are quite important to both plant’s normal life cycle and crop production. A number of environmental stimuli and endogenous factors are involved in the regulation. ABA, as a major phytohormone, mainly preserves seed dormancy and suppresses seed germination via its signaling pathway. OsbZIP transcription factors are key components in ABA signaling pathway to mediate its regulation of down-stream target genes and consequently ABA- triggered plant responses, including suppressing seed germination, enhancing plants’ tolerance to stress. In this study, we identified and characterized a novel bZIP transcription factor OsbZIP09 in rice, with positive roles in regulation of seed germination but negative roles in both ABA accumulation and signaling output. This demonstrates that the OsbZIP transcription factor can also be an enhancer of seed germination in rice.
Previous reported OsbZIPs almost all play negative roles in rice germination control. For instance, over- expression ofrestores the ABA- insensitivity seed germination phenotype of, and leads to hypersensitivity to ABA (Zou et al, 2008). The/mutant is insensitive to high level of ABA at the germination and post-germination growth stages (Hossain et al, 2010). Transformation of()/into ABA-insensitivemutant can also restore its sensitivity to ABA treatment during seed germination and seedling growth (Yang et al, 2011). MOTHER OF FT AND TFL 2 (OsMFT2), a negative regulator of rice seed germination, directly interacts with three differentOsbZIPs, including OsbZIP23, OsbZIP66 and OsbZIP72, to co-regulate the expression of. Moreover, overexpression ofcan restore the PHS phenotype ofknock-out lines (Song et al, 2020). Wang Y F et al (2020) disclosed that OsbZIP72 mediates ABA-jasmonic acid (JA) crosstalk to coordinate rice seed germination. The study showed that ABA-activatedis more stable, and able to bind to()promoter to enhance its transcription and endogenous JA content, thus suppressing seed germination. In addition to ABA, DELAY OF GERMINATION 1 (DOG1) protein is another key regulator that promotes seed dormancy. OsDOG1L-3, the DOG1homologue in rice, is directly regulated by OsbZIP75 and OsbZIP78. Accordantly,overexpression induces the accumulation of OsDOG1L-3 protein and inhibits seed germination (Wang Q et al, 2020).
OsbZIP58, identified almost 20 years ago, directly interacts with OsLOL1 to promote the expression of(), a GA biosynthesis- related gene in rice (Wu et al, 2014), implying that OsbZIP58 may have the potential to promote seed germination. However, all the other OsbZIP58/Rice Seed b-Zipper 1 (RISBZ1)-related publications focus on regulation of seed storage proteins and grain filling of rice, and no germination data has been shown (Onodera et al, 2001; Yamamoto et al, 2006; Kawakatsu et al, 2009; Kawakatsu and Takaiwa, 2010). A most recent publication revealed that high temperature-triggered reduction of storage material content during rice filling is mediated by the alternative splicing of. Most importantly, the study demonstrated thatinhibits the expression of some starch hydrolyzing α-amylase genes (Xu et al, 2020), which are consideredas important positive effectors of rice seed germination. Therefore, the exact role of OsbZIP58 in modulation of seed germination is still elusive, and direct germination analysis ofrelated genetic materials is essential to clarify whether and how OsbZIP58 is involved in controlling seed germination.
In conclusion, we successfully identified a novel and unique OsbZIP transcription factor, OsbZIP09. Interestingly, OsbZIP09 acts as both transcription activator and suppressor based on its regulation onand.is a key gene involved in ABA catabolism (Saika et al, 2007)., encoding a late embryogenesis abundant protein, is sensitively induced by ABA and often used as a marker gene to indicate the effect and strength of ABA treatment (Joo et al, 2019). In fact, most transcription factors regulate the expression of downstream target genes by forming homodimer or heterodimer. Therefore, different interaction partners of the transcription factor or various flanking sequences of its binding sites determine its role as an activator or suppressor (Blauwkamp et al, 2008; MacDonald et al, 2009; Sun et al, 2010). Some important transcription factors in plants also function as both transcriptional activator and repressor, such as BZR1 and HY5 in(Sun et al, 2010; Zhang et al, 2011). In addition, the grain length of themutant was longer than the wild type, indicating the rice grain shape was improved. Most importantly, the PHS of themutants was much better than thatof the wild type, but the normal germination phenomena were similar. Thus,had the potential application in rice breeding programs. Moreover, the positive role of OsbZIP09 in regulation of seed germination is opposite with other reported OsbZIPs. For example, OsbZIP10/ABI5, a close homologue of OsbZIP09 belonging to the same sub-family, is a key transcription factor in the ABA signaling pathway, directly regulating the expression of ABA-responsive genes and suppressing seed germination. Moreover, ABI5 itself is precisely modulated by post-translational modifications (Yu et al, 2015; Yang et al, 2017; Xu et al, 2019), and also functions as an integrator to mediate crosstalk between ABA and other internal or external signals (Skubacz et al, 2016; Yadukrishnan and Datta, 2020). Therefore, it is possible that OsbZIP09 can be another generalist like OsbZIP10/ABI5, but the different point is that OsbZIP09 functions as a brake in ABA pathway. Moreover, the stability and activity of OsbZIP09 should also be regulated by other factors. Most importantly, further work will address why such striking differences exist between OsbZIP09 and other OsbZIPs in seed germination control.
METHODS
Generation of osbzip09 mutants using CRISPR/Cas9 system
Two specific target sites in the first exon of, namedand, were designed for gene editing (Fig. 1-A). The target sequences were introduced into vector pCAMBIA1300-Cas9, and the generated constructs were transformed intorice ZH11 via- mediatedtransformation. The CRISPR/Cas9 vector system and the detailed procedure are described by Wang et al (2019).
Rice growth conditions and measurement of agronomic traits
All rice plants were grown under the same climatic and management conditions during the summer in a paddy field at Yangzhou University (Yangzhou, Jiangsu Province, China). Three replicate plots were used for the experiment, and the plots were arranged in a randomized block design, with 6 rows per plot and 10 plants per row. The main agronomic traits were recorded after seed maturity. Superior rice spikelets or mature seeds from superior rice spikelets in the middle of each plot were collected for seed germination analysis.
Seed germination analysis
For germination assay mimicking PHS, matured panicles from themutants and wild type ZH11 were collected and immersed in water for 8 d, and the number of germinated seeds was counted every 2 d. Germination analysis of rice seeds under normal growth conditions was performed as described by Li et al (2020). Seeds with radicle longer than 1 mm were considered as successfully germinated (Cheng et al, 2013), and the germination rates were recorded every 12 h. Each seed germination assay included at least three independent biological replicates, and each replicate contained 30 seeds.
Transcriptional expression assay
Total RNA was isolated using an RNeasy Plant Mini Kit (Qiagen, China) or TRIzol (Invitrogen, USA). RNase-free DNase I was used to remove genomic DNA, and subsequently first-strand cDNA was synthesized using reverse transcriptase (Thermo, USA) with the oligo dT18 primer. ZH11 seeds at 0, 3, 6, 12 and 24 HAI, and seeds treated for 3 h with ABA (5 μmol/L) or GA (10 μmol/L) or the mock control, were collected for examining the expression ofvia qRT-PCR. Rice seeds ofmutants and ZH11 imbibed for 36 h were also collected and used for expression analysis ofand. Three biological replicates were included for each experiment, andserved as a reference gene for normalization.
Sub-cellular localization assay
Aconstruct was generated by in-frame fusion of full-lengthcDNA withcoding region. The fusion gene was then inserted into the pCAMBIA2300 vector driven by thepromoter, and the construct was transformed intostrain GV3101 and injected into tobacco leaves. GFP signal was then observed by a confocal microscope (Leica, Germany).
DAP-seq analysis
DAP-seq was performed by following the method described by O’Malley et al (2016). In brief, genomic DNA (gDNA) was extracted from mature seeds of rice and the gDNA DAP-seq library was prepared by attaching a short DNA sequencing adaptor on to the purified and fragmented gDNA.was fused to the HaloTag using a kit from pFN19K HaloTag T7 SP6 Flexi Vecto (Promega, USA), and expressed using a TnT SP6 High-Yield Wheat Germ Protein Expression System (L3260, Promega, USA), and was then purified using Magne HaloTag Beads (G7281, Promega, USA). The beads and-HaloTag mixture were incubated with 500 ng DNA library in 40 μL PBS buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4) with slow rotation in a cold room (4 oC)for 1.5 h. The correct DAP-seq library concentration to achieve a specific read count was calculated based on library fragment size. Negative control mock DAP-seq libraries were prepared as described above without the addition of protein to the beads. We defined target genes as those that contained DAP-seq peaks located within the transcribed regions of genes, in introns, or 5 kb upstream of the transcription start site, or 5 kb downstream of the transcription termination site. DAP-seq reads were aligned to the rice genome using Bowtie 2 (Langmead and Salzberg, 2012) with default parameters and reported only unique alignments. DAP-seq peaks were detected by MACS2 (version 2.0.10) with default parameters and the-value < 0.05 (Zhang et al, 2008). Core motifs were identified by MEME-ChIP (Machanick and Bailey, 2011).
Dual-luciferase reporter assay
-mediated transient assay was conducted with tobacco leaves by co-expressing the reporter and effector constructs. The promoters of(2.0 kb) and(1.8 kb) were each cloned into thepGreenII0800-LUC vector to generate reporter constructs. Thus, the reporter construct included the LUC reporter gene driven byorpromoter, and also thereporter within the same vector for normalization. The coding region ofwas cloned into thepGreenII62-SK vector under the control ofpromoter to serve as an effector. Then each reporter construct was cotransformed into tobacco leaves together with theeffector for transcriptional activity analysis. The signals of firefly and renilla luciferase were monitored using a Luminoskan Ascent Microplate Luminometer (Thermo, USA) with Dual Luciferase Reporter Assay Kit (Vazyme, China). The detailed method can refer to previous publication (Hellens et al, 2005). The sequences of all the primers used are listed in Table S2.
Hormone ABA and GA measurement
Mature seeds ofmutants and ZH11 before imbibition and 12 HAI were collected for measurement of endogenous ABA and GA contents by the enzyme-linked immunosorbent assay (ELISA) based method. The detailed procedure was performed as described by Yang J C et al (2001) and Yang Y M et al (2001).
ACKNOWLEDGEMENTS
This study was supported by the National Training Programs of Innovation and Entrepreneurship for Undergraduates, Science Fund for Distinguished Young Scholars of Jiangsu Province, China (Grant No. BK20200045), and the Priority Academic Program Development of Jiangsu Higher Education Institutions Program, China.We thank Professor Wang Kejian (China National Rice Research Institute) for providing the CRISPR/ Cas9 plasmids.
SUPPLEMENTAL DATA
The following materials are available in the online version of this article at http://www.sciencedirect.com/journal/rice-science; http://www.ricescience.org.
Fig. S1. Time-course germination analysis of rice seeds.
Fig. S2. Expression analysis of ABA or GA responsive marker genes.
Fig. S3. Expression analysis ofand quantification of GA3content in rice seeds before and after imbibition.
Table S1. DAP-seq data related to,and.
Table S2. Oligonucleotide primer sequences used.
Alves M S, Dadalto S P, Gon?alves A B, De Souza G B, Barros V A, Fietto L G. 2013. Plant bZIP transcription factors responsive to pathogens: A review., 14(4): 7815–7828.
Blauwkamp T A, Chang M V, Cadigan K M. 2008. Novel TCF- binding sites specify transcriptional repression by Wnt signalling., 27: 1436–1446.
Chen K, Li G J, Bressan R A, Song C P, Zhu J K, Zhao Y. 2020. Abscisic acid dynamics, signaling, and functions in plants., 62(1): 25–54.
Cheng X Y, Wu Y, Guo J P, Du B, Chen R Z, Zhu L L, He G C. 2013. A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination., 76(4): 687–698.
Dr?ge-Laser W, Snoek B L, Snel B, Weiste C. 2018. ThebZIP transcription factor family: An update., 45: 36–49.
Dr?ge-Laser W, Weiste C. 2018. The C/S 1 bZIP network: A regulatory hub orchestrating plant energy homeostasis., 23(5): 422–433.
Finkelstein R, Reeves W, Ariizumi T, Steber C. 2008. Molecular aspects of seed dormancy., 59: 387–415.
Hellens R P, Allan A C, Friel E N, Bolitho K, Grafton K, Templeton M D, Karunairetnam S, Gleave A P, Laing W A. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants., 1: 13.
Hossain M A, Cho J I, Han M, Ahn C H, Jeon J S, An G, Park P B. 2010. The ABRE-binding bZIP transcription factor OsABF2 is a positive regulator of abiotic stress and ABA signaling in rice.,167(17): 1512–1520.
Jacobsen J V, Pearce D W, Poole A T, Pharis R P, Mander L N. 2002. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley., 115(3): 428–441.
Joo J, Lee Y H, Song S I. 2019. OsbZIP42 is a positive regulator of ABA signaling and confers drought tolerance to rice., 249(5): 1521–1533.
Kawakatsu T, Takaiwa F. 2010. Differences in transcriptional regulatory mechanisms functioning for free lysine content and seed storage protein accumulation in rice grain., 51(12): 1964–1974.
Kawakatsu T, Yamamoto M P, Touno S M, Yasuda H, Takaiwa F. 2009. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice., 59(6): 908–920.
Langmead B, Salzberg S L. 2012. Fast gapped-read alignment with Bowtie 2., 9(4): 357–359.
Li Q F, Zhou Y, Xiong M, Ren X Y, Han L, Wang J D, Zhang C Q, Fan X L, Liu Q Q. 2020. Gibberellin recovers seed germination in rice with impaired brassinosteroid signalling., 293: 110435.
Lu G J, Gao C X, Zheng X N, Han B. 2009. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice., 229(3): 605–615.
MacDonald B T, Tamai K, He X. 2009. Wnt/beta-catenin signaling: Components, mechanisms, and diseases., 17: 9–26.
Machanick P, Bailey T L. 2011. MEME-ChIP: Motif analysis of large DNA datasets., 27(12): 1696–1697.
Martínez-Andújar C, Ordiz M I, Huang Z, Nonogaki M, Beachy R N, Nonogaki H. 2011. Induction of 9--epoxycarotenoid dioxygenase inseeds enhances seed dormancy.,108(41): 17225–17229.
Miyakawa T, Fujita Y, Yamaguchi-Shinozaki K, Tanokura M. 2013. Structure and function of abscisic acid receptors., 18(5): 259–266.
Née G, Xiang Y, Soppe W J. 2017. The release of dormancy, a wake-up call for seeds to germinate., 35: 8–14.
Nijhawan A, Jain M, Tyagi A K, Khurana J P. 2008. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice.,146(2): 333–350.
Nonogaki H. 2019. Seed germination and dormancy: The classic story, new puzzles, and evolution., 61(5): 541–563.
Nonogaki M, Sall K, Nambara E, Nonogaki H. 2014. Amplification of ABA biosynthesis and signaling through a positive feedback mechanism in seeds., 78(3): 527–539.
O’Malley R C, Huang S C, Song L, Lewsey M G, Bartlett A, Nery J R, Galli M, Gallavotti A, Ecker J R. 2016. Cistrome and epicistrome features shape the regulatory DNA landscape., 165(5): 1280?1292.
Onodera Y, Suzuki A, Wu C Y, Washida H, Takaiwa F. 2001. A rice functional transcriptional activator, RISBZ1, responsible for endosperm-specific expression of storage protein genes through GCN4 motif., 276(17): 14139–14152.
Saika H, Okamoto M, Miyoshi K, Kushiro T, Shinoda S, Jikumaru Y, Fujimoto M, Arikawa T, Takahashi H, Ando M, Arimura S I, Miyao A, Hirochika H, Kamiya Y, Tsutsumi N, Nambara E, Nakazono M. 2007. Ethylene promotes submergence-induced expression of, a gene that encodes ABA 8′- hydroxylase in rice., 48(2): 287–298.
Shu K, Liu X D, Xie Q, He Z H. 2016. Two faces of one seed: Hormonal regulation of dormancy and germination., 9(1): 34–45.
Skubacz A, Daszkowska-Golec A, Szarejko I. 2016. The role and regulation of ABI5 (ABA-insensitive 5) in plant development, abiotic stress responses and phytohormone crosstalk.,7: 1884.
Song S, Wang G F, Wu H, Fan X W, Liang L W, Zhao H, Li S L, Hu Y, Liu H Y, Ayaad M, Xing Y Z. 2020. OsMFT2 is involved in the regulation of ABA signaling mediated seed germination through interacting with OsbZIP23/66/72 in rice., 103(2): 532–546.
Sornaraj P, Luang S, Lopato S, Hrmova M. 2016. Basic leucine zipper (bZIP) transcription factors involved in abiotic stresses: A molecular model of a wheat bZIP factor and implications of its structure in function., 1860: 46–56.
Sun Y, Fan X Y, Cao D M, Tang W, He K, Zhu J Y, He J X, Bai M Y, Zhu S, Oh E, Patil S, Kim T W, Ji H, Wong W H, Rhee S Y, Wang Z Y. 2010. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in., 19(5): 765–777.
Tong H N, Chu C C. 2018. Functional specificities of brassinosteroid and potential utilization for crop improvement., 23(11): 1016–1028.
Tong H N, Xiao Y H, Liu D P, Gao S P, Liu L C, Yin Y H, Jin Y, Qian Q, Chu C C. 2014. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice., 26(11): 4376–4393.
Tuan P A, Kumar R, Rehal P K, Toora P K, Ayele B T. 2018. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals., 9: 668.
Wang C, Liu Q, Shen Y, Hua Y F, Wang J J, Lin J R, Wu M G, Sun T T, Cheng Z K, Mercier R, Wang K. 2019. Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes., 37(3): 283–286.
Wang Q, Lin Q B, Wu T, Duan E C, Huang Y S, Yang C Y, Mou C L, Lan J, Zhou C L, Xie K, Liu X, Guo X P, Wang J, Jiang L, Wan J M. 2020.regulates seed dormancy through the abscisic acid pathway in rice., 298: 110570.
Wang Y F, Hou Y X, Qiu J H, Wang H M, Wang S, Tang L Q, Tong X H, Zhang J. 2020. Abscisic acid promotes jasmonic acid biosynthesis via a ‘SAPK10-bZIP72-AOC’ pathway to synergistically inhibit seed germination in rice ()., 228(4): 1336–1353.
Wu J H, Zhu C F, Pang J H, Zhang X R, Yang C L, Xia G X, Tian Y C, He C Z. 2014. OsLOL1, a C2C2-type zinc finger protein, interacts with OsbZIP58 to promote seed germination through the modulation of gibberellin biosynthesis in., 80(6): 1118–1130.
Xu H, Li X F, Zhang H, Wang L C, Zhu Z G, Gao J P, Li C S, Zhu Y. 2020. High temperature inhibits the accumulation of storage materials by inducing alternative splicing ofduring filling stage in rice.,43(8): 1879–1896.
Xu X, Wan W, Jiang G, Xi Y, Huang H, Cai J, Chang Y, Duan C G, Mangrauthia S K, Peng X, Zhu J K, Zhu G. 2019. Nucleocytoplasmic trafficking of theWD40 repeat protein XIW1 regulates ABI5 stability and abscisic acid responses.,12(12): 1598–1611.
Yadukrishnan P, Datta S. 2020. Light and abscisic acid interplay in early seedling development., 229: 763–769.
Yamamoto M P, Onodera Y, Touno S M, Takaiwa F. 2006. Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes., 141(4): 1694–1707.
Yan A, Chen Z. 2017. The pivotal role of abscisic acid signaling during transition from seed maturation to germination., 36(5): 689–703.
Yang J C, Zhang J H, Wang Z Q, Zhu Q S, Wang W. 2001. Hormonal changes in the grains of rice subjected to water stress during grain filling., 127(1): 315–323.
Yang W Q, Zhang W, Wang X X. 2017. Post-translational control of ABA signalling: The roles of protein phosphorylation and ubiquitination., 15(1): 4–14.
Yang X, Yang Y N, Xue L J, Zou M J, Liu J Y, Chen F, Xue H W. 2011. Rice ABI5-like1 regulates abscisic acid and auxin responses by affecting the expression of ABRE-containing genes., 156(3): 1397–1409.
Yang Y M, Xu C N, Wang B M, Jia J Z. 2001. Effects of plant growth regulators on secondary wall thickening of cotton fibres., 35: 233–237.
Yu F F, Wu Y R, Xie Q. 2015. Precise protein post-translational modifications modulate ABI5 activity., 20(9): 569–575.
Zhang H Y, He H, Wang X C, Wang X F, Yang X Z, Li L, Deng X W. 2011. Genome-wide mapping of the-mediated gene networks in Arabidopsis that involve both transcriptional and post-transcriptional regulation., 65(3): 346–358.
Zhang Y, Liu T, Meyer C A, Eeckhoute J, Johnson D S, Bernstein B E, Nusbaum C, Myers R M, Brown M, Li W, Liu X S. 2008. Model- based analysis of ChIP-Seq (MACS)., 9(9): R137.
Zhao H Y, Nie K L, Zhou H P, Yan X J, Zhan Q D, Zheng Y, Song C P. 2020. ABI5 modulates seed germination via feedback regulation of the expression of theABA receptor genes., 228(2): 596–608.
Zou M J, Guan Y C, Ren H B, Zhang F, Chen F. 2008. A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance., 66(6): 675–683.
12 November 2020;
28 January2021
Copyright ? 2021, China National Rice Research Institute. Hosting by Elsevier B V
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/)
Peer review under responsibility of China National Rice Research Institute
http://dx.doi.org/10.1016/j.rsci.2021.05.006
Li Qianfeng (qfli@yzu.edu.cn)
(Managing Editor: Wu Yawen)
- Rice Science的其它文章
- SDF5 Encoding P450 Protein Is Required for Internode Elongation in Rice
- RGB1 Regulates Rice Panicle Architecture and Grain Filling Through Monitoring Cytokinin Level in Inflorescence Meristem and Grain Abscisic Acid Level During Filling Stage
- Application of Rice Husk Biochar for Achieving Sustainable Agriculture and Environment
- MULTI-FLORET SPIKELET 4 (MFS4) Regulates Spikelet Development and Grain Size in Rice
- A New Approach to Select Doubled Haploid Rice Lines under Salinity Stress Using Indirect Selection Index
- iTRAQ-Based Proteomics Investigation of Critical Response Proteins in Embryo and Coleoptile During Rice Anaerobic Germination

