Evaluation of Anti-inflammatory Effect of Different Polar Parts of Argyreia acuta Lour.
Wenya CHEN
Guangxi University of Chinese Medicine, Nanning 530200, China
Abstract [Objectives] To explore the anti-inflammatory effect and mechanisms of different polar parts of Argyreia acuta Lour. [Methods] The effect of different polar parts of A. acuta Lour. on the viability of mouse RAW264.7 cells was detected by the CCK-8 method. Lipopolysaccharide (LPS) was used to stimulate RAW264.7 cells to establish an in vitro inflammation model, and the NO, TNF-α and IL-6 contents were determined by Griess method and ELISA assays, respectively. [Results] Within the range of 12.5-200 μg/mL, all polar parts of A. acuta Lour. showed a proliferation effect on RAW264.7 cells, without cytotoxicity. The total extract, petroleum ether extract, ethyl acetate extract, and n-butanol extract all could inhibit the secretion of NO; and the petroleum ether extract and ethyl acetate extract could inhibit the secretion of TNF-α and IL-6. [Conclusions] The petroleum ether extract and ethyl acetate extract of A. acuta Lour. have significant anti-inflammatory activity, and the mechanism of action may be related to inhibiting the release of TNF-α and IL-6.
Key words Argyreia acuta Lour., Anti-inflammatory, RAW264.7 cell
1 Introduction
Inflammation is a defensive response of the body to stimuli. It is a very common and important basic pathological process, mainly manifested as redness, swelling, heat and pain. It is induced by biological factors, physical factors, chemical factors, necrotic tissues, allergic reactions and other factors[1]. Among them, the chemical factors that mediate the inflammatory response are also called inflammatory mediators[2], such as NO, cytoinflammatory factors TNF-α and IL-6,etc., which can be released by various cells. By binding the corresponding receptors, they affect cell growth and metabolism. These factors are in a dynamic equilibrium. Once the balance is broken, a certain degree of inflammatory reaction occurs in a cascade, and in severe cases, death may appear. With the shift of the research focus on new mechanisms of anti-inflammatory immunity in China[3], it is necessary to study the level of influence of drugs on inflammatory mediators. In recent years, natural medicines have always been a hot spot for drug research and development for high efficiency, low toxicity and rich sources[3].
A.acutaLour.is also known as Baibeiteng, Baibei Sichou, Baimian Shuiji,etc., and is usually used as medicine with dried whole plant. It is mainly distributed in Guangdong and Guangxi[4]. The main chemical components are flavonoids, resin glycosides, steroids, triterpenes, organic acids, phenols, alkaloids, coumarins,etc.Scopolamine and scopolactone, which are recognized as effective anti-inflammatory components, have been isolated[5-7].Traditional Chinese medicine believes thatA.acutaLour. has the functions of resolving phlegm, relieving cough, moisturizing the lung, stopping bleeding, and detoxifying. It is used for the treatment of acute and chronic bronchitis, tuberculosis, liver cirrhosis, nephritic edema, breast pain, traumatic hemostasis,etc[4]. In Zhuang medicine,A.acutaLour., called Godarkhab in Zhuang language, is a commonly used antidote, has effect of soothing water and air passage and removing noxious dampness, and is used to treat cough, bruises, boils, acute mastitis, morbid leucorrhea, mass formed by blood stasis and other inflammation-related diseases[8-9]. Bai Honglong[10]once usedA.acutaLour. and other traditional Chinese medicine to clinically treat senile asthmatic chronic bronchitis. Literature[11]shows thatA.acutaLour. has anti-inflammatory and hemostatic pharmacological effects[11].Studies have shown that the ethyl acetate extract and water extract ofA.acutaLour. have a significant anti-inflammatory effect on mouse ear swelling and mouse granuloma[12].At present, there have been many reports on the chemical composition and content determination ofA.acutaLour.at home and abroad. However, there are only a few reports on its hemostatic and anti-inflammatory effects. In this experiment, the toxicity and anti-inflammatory effect of different polar parts ofA.acutaLour. were studied and analyzed comprehensively at the cellular and molecular levels, using the CCK-8 method, the Griess method and the ELISA assay, respectively, in order to explore its active sites and anti-inflammatory effect.
2 Materials and methods
2.1 Samples and cellsExtracts of different polar parts (whole, petroleum ether, ethyl acetate, n-butanol, water) ofA.acutaLour. were prepared by Professor Lu Rumei’s research group from the School of Pharmacy, Guangxi University of Chinese Medicine. Mouse RAW264.7 macrophage cell line was gifted by Gao Hongwei from the Yang Shilin team of Guangxi University of Chinese Medicine.
2.2 Drugs and reagentsThe used drugs and reagents mainly included lipopolysaccharide LPS (Solarbio), DMEM medium (Jiangsu KeyGEN BioTECH Corp., Ltd.), serum FBS (Zhejiang Tianhang Biotechnology Co., Ltd.), PBS (Jiangsu KeyGEN BioTECH Corp., Ltd.), DMSO (Sigma), Griess (Sigma) and ELISA (Beijing Kangpu Tongchuang Biotechnology Development Co., Ltd.).
2.3 Main instruments and equipmentThe used instruments and equipment mainly included HR1200-IIB2 biological safety cabinet (Haier), MULTISKAN Sky full-wavelength microplate reader (Thermo Scientific), SORCALL ST 16R high-speed refrigerated centrifuge (Thermo Scientific), precision analytical balance (METTLER TOLEDO), CKX41 type inverted microscope (OLYMPUS), HVA-110 autoclave (HIRAYMA), incubator (BINDER) and Elmasonic P medium ultrasonic cleaner (Elma).
2.4 Liquid preparationAn accurate amount (20.0 mg) of the extract of each polar part (whole fraction, petroleum ether fraction, ethyl acetate fraction, n-butanol fraction, water fraction) ofA.acutaLour. was weighed. Among them, the whole fraction and water fraction were added with 1 mL of distilled water, respectively, the ethyl acetate fraction was added with 1 mL of DMSO, the n-butanol fraction was added with 0.8 mL of distilled water and 0.2 mL of DMSO, and the petroleum ether fraction was added with 0.5 mL of distilled water and 0.5 mL of DMSO. An accurate amount (1 mg) of LPS powder was poured into an EP tube, added with 1 mL of distilled water, ultrasonicated until complete dissolution, and filtered through a 0.22 μm microporous membrane to obtain the filtrate, which was then diluted with DMEM medium (culture broth) containing 10% FBS to the required concentration.
2.5 Experiments on anti-inflammatory activity of different polar parts ofA.acutaLour.
2.5.1Detection of influence of different polar parts ofA.acutaLour. on viability of RAW264.7 cells with CCK-8 method. When the RAW264.7 cells are cultured to a good condition and 80%-90% of them were adherent to the wall, they were inoculated to 96-well plates, at a density of 1×105cells/mL, with three replicates for each concentration, and then cultured in an incubator (37 ℃, 5% CO2) for 24 h. The mother liquor in Section2.4was diluted to 200, 100, 50, 25 and 12.5 μg/mL, respectively. Normal group and drug administration group were designed. After 24 h, the old culture medium was discarded. Each well of the drug administration group was added with 100 μL of drug-containing culture medium, and each well of the normal group was added with complete culture medium, followed by culture in incubator (37 ℃, 5% CO2) for 24 h. After 24 h, the old culture medium was discarded, and DMEM culture medium containing 5% CCK-8 was added to each of the wells. After culture for 30 min, theOD450value of each well was measured with a microplate reader. Cell survival rate was calculated according to formula (1):
Cell survival rate =ODDrug administration group/ODNormal group×100%
(1)
2.5.2Detection of influence of different polar parts ofA.acutaLour. on LPS-induced release of NO from RAW264.7 cells with Griess method. The inoculable cells were adjusted to 3×105cells/mL, inoculated to 96-well plates, with three replicates for each concentration, and cultured in an incubator (37 ℃, 5% CO2) for 24 h, followed by discarding of culture medium. Normal group, drug administration group and LPS group were set up. In the drug administration group, 200, 100 and 50 μg/mL were designed for each polar part. Each well of the groups, except the normal group, was added with DMEM culture medium containing 2 μg/mL LPS, placed in an incubator for 4 h to establish inflammation models, and then added with corresponding drug-containing culture medium. The wells in the normal group were added with equal volume of culture medium. After culturing in an incubator for 24 h, the supernatant was centrifuged. The OD value of each well was measured according to the instruction of the Griess kit. NO concentration was calculated according to formula (2):
NO concentration=(ODDrug administration group-ODBlank group)/(ODStandard group/ODBlank group)×Concentration of standard×Dilution multiple
(2)
2.5.3Detection of influence of different polar parts ofA.acutaLour. on LPS-induced TNF-α and IL-6 release from RAW264.7 cells with ELISA method. The inoculable cells were adjusted to 1×106cells/mL, inoculatedto 24-well plates, with three replicates for each concentration, and cultured in an incubator (37 ℃, 5% CO2) for 24 h. For the petroleum ether fraction and ethyl acetate fraction, 100, 50 and 25 μg/mL were set up for drug administration. Normal group, LPS group and drug administration group were designed. Each well of the drug administration group was added with 0.5 mL culture medium containing drug and pretreated for 1 h. Subsequently, each well of the LPS group and drug administration group was added with culture medium containing LPS to make the final concentration of LPS per well to be 2 μg/mL. The wells of the normal group were added with only culture medium, and the final volume in each well was equal. After 24-h culture in the incubator, the cell culture supernatant was collected for content determination of TNF-α and IL-6 following the instructions of the ELISA kits.
3 Results and analysis
3.1 Cell viabilityThe effect of different polar parts ofA.acutaLour. on the viability of RAW264.7 cells was detected with the CCK-8 method, and the results are shown in Table 1 and Fig.1. Compared with the normal group, different polar parts ofA.acutaLour. increased the survival rate of RAW264.7 cells to varying degrees after administered for 24 h, and they had no cytotoxicity. For the whole fraction and petroleum ether fraction, the survival rate of the cells was better within the concentration range of 50-200 μg/mL, significantly different from that in the normal group (*P<0.05,**P<0.01). The survival rate of the RAW264.7 cells was highest in 200 μg/mL of ethyl acetate extract and 50 μg/mL of water extract, suggesting that the proliferation effect of the cells was the best. Compared with the normal group, there were significant differences (*P<0.05,**P<0.01). When the concentration of n-butanol extract was 25-200 μg/mL, the cell survival effect was better, indicating a proliferation effect on the cells. Compared with the normal group, there were statistically significant differences (**P<0.01).

Table 1 Effect of different polar part of Argyreia acuta Lour. on survival rate of RAW264.7 cells n=3)
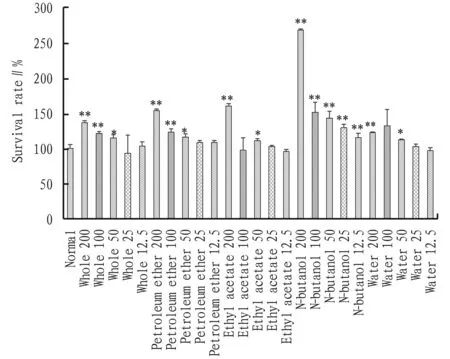
Note: Compared with normal group, *P<0.05, **P<0.01.
3.2 NO releaseThe effect of different polar parts ofA.acutaLour. on the LPS-induced NO release from RAW264.7 cells was detected by the Griess method, and the results are shown in Fig.2. Compared with the normal group, the amount of NO released by the cells in the LPS group was significantly higher(##P<0.01), indicating the success of establishing RAW264.7 cell inflammation model with LPS. Compared with the LPS group, the NO release amounts of different polar parts ofA.acutaLour. declined to varying degrees. Among them, the NO release amounts of whole fraction and petroleum ether fraction decreased significantly within the concentration range of 50-200 μg/mL in comparison to the LPS group (*P<0.05,**P<0.01), in a dose-dependent manner. At the concentration of 200 μg/mL, the n-butanol extract significantly reduced the release amount of NO (**P<0.01,vs.LPS group). At the concentration of 100 and 50 μg/mL, the NO release amount also declined, but the difference was insignificant (*P>0.05). Within the administration range, the NO release amount of water extract was basically the same as that of the LPS group (*P>0.05).
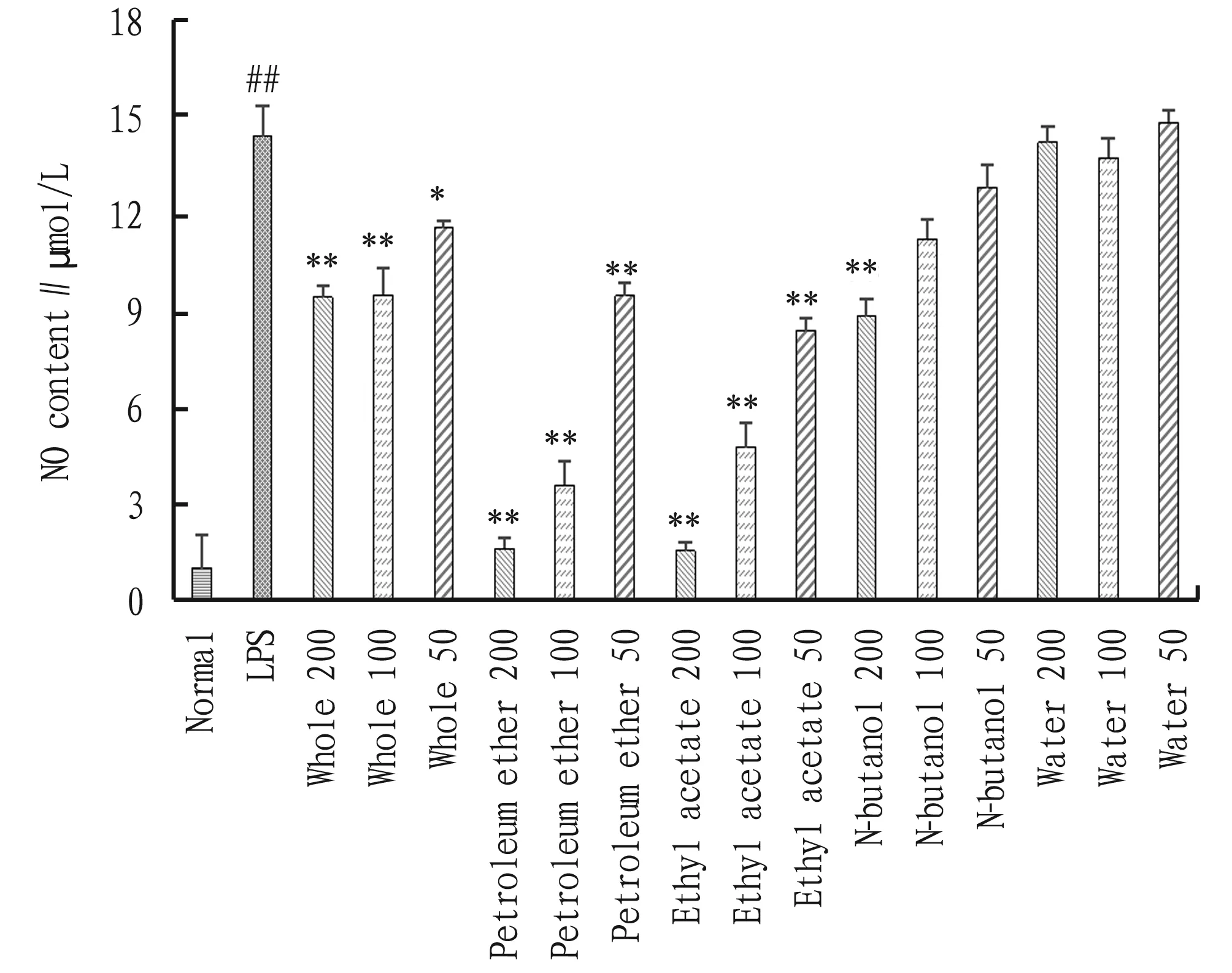
Note: Compared with normal group, ##P<0.01; compared with LPS group, *P<0.05, **P<0.01.
3.3 TNF-α and IL-6 contentsThe effect of different polar parts ofA.acutaLour. on the LPS-induced release of TNF-α and IL-6 from RAW264.7 cells was detected with ELIS assays, and the results are shown in Fig.3 and Fig.4. Compared with the normal group, the TNF-α and IL-6 release amounts from the cells in the LPS group significantly increased (#P<0.05), indicating the success of LPS-induced RAW264.7 cell inflammation model. Compared with the LPS group, the petroleum ether extract and ethyl acetate extract ofA.acutaLour. reduced the TNF-α release from the cells to varying degrees. The petroleum ether extract ofA.acutaLour. reduced the release amount of TNF-α from RAW264.7 cells significantly (*P<0.05,**P<0.01) within the concentration range of 25-100 μg/mL; and the ethyl acetate extract ofA.acutaLour. reduced the TNF-α release amount of the cells significantly (**P<0.01) at the concentration of 100 and 50 μg/mL and reduced the TNF-α release amount of the cells insignificantly (P>0.05) at the concentration of 25 μg/mL. Compared to the LPS group, the IL-6 release amount in the petroleum ether extract and ethyl acetate extract groups also declined to varying degrees. The petroleum ether extract reduced the IL-6 release amount significantly at the concentration of 100 and 50 μg/mL, and the ethyl acetate extract reduced the IL-6 release amount significantly at the concentration of 100 μg/mL (*P<0.05,**P<0.01). When the dosage of petroleum ether was 25 μg/mL and the dosage of ethyl acetate extract was 50 and 25 μg/mL, the IL-6 release amount also reduced, but the differences from the LPS group were insignificant (P>0.05).
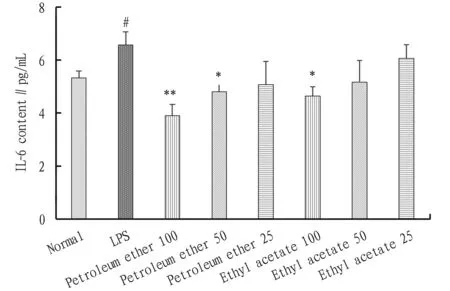
Note: Compared with normal group, #P<0.05; compared with LPS group, *P<0.05, **P<0.01.
4 Discussion
Macrophages play an important role in the inflammatory response. Under the stimulation of lipopolysaccharide (LPS), they can secrete NO, IL-6, TNF-α, IL-1β,etc., directly or indirectly mediating the inflammatory reaction process[13]. Cytotoxicity is an essential part of drug development and research. Drug can exert anti-inflammatory effect by regulating the secretion of various cytokines or inflammatory mediators. If the drug is cytotoxic, it will affect cell proliferation and its function, and the inhibiting effect on the secretion of anti-inflammatory factors or the promoting effect on the secretion of pro-inflammatory factors will mislead the analysis of experimental results. Therefore, before conducting anti-inflammatory activity study in this experiment, the cytotoxicity and toxicity of the drug were screened first. It was confirmed that the various parts ofA.acutaLour. had no cytotoxic effect on RAW264.7 cells within 24 h in the concentration range of 12.5-200 μg/mL, ensuring the reliability of the research data on the anti-inflammatory activity ofA.acutaLour and determining the concentration range of the drugs for anti-inflammatory activity experiment.
Nitric oxide (NO) is a small molecular compound that exists in the body extensively. Under normal circumstances, its content is very small. When cells are stimulated by LPS and other substances, the expression of iNOS will increase, and when a large amount of NO is induced to express, inflammation will be mediated to occur and develop[14]. The content of NO can be used as an indicator of the degree of inflammation, and it is of great significance to the amplification of inflammation. Tumor necrosis factor (TNF-α) is the most important pro-inflammatory factor secreted by macrophages, and it plays an important role in the regulation of cellular immune response and the induction of inflammation. Excess secretion of TNF-α can produce extremely strong inflammatory damage. At the same time, it can directly or indirectly induce more secretion of other inflammatory factors such as IL-6[15]. Therefore, it is a very important method to inhibit the occurrence of inflammation by effectively controlling the release of TNF-α. IL-6 is a multifunctional inflammatory factor, and it has a variety of biological functions such as immune regulation, participation in inflammation and tumorigenesis[16-17]. When the human body is in inflammation or other pathological conditions, macrophages will produce a large amount of IL-6 to promote the development of inflammation, and the release of IL-6 can reflect the severity of inflammation. Similarly, it is also an effective method to inhibit the development of inflammatory response by reducing the release of IL-6. In this experiment, both the petroleum ether extract and the ethyl acetate extract showed active inhibition on NO, IL-6, and TNF-α, indicating that they may contain anti-inflammatory components scopolamine and scopolactone. However, their monomers still need to be isolated further for detection.
5 Conclusions
Different polar parts ofA.acutaLour. are non-toxic to RAW264.7 cells in the concentration range of 12.5-200 μg/mL, and have obvious proliferation effect. Among them, the whole, petroleum ether, ethyl acetate and n-butanol extracts can inhibit the release of NO under certain conditions, and the petroleum ether and ethyl acetate extracts can inhibit the release of TNF-α and IL-6. In summary, the petroleum ether extract and ethyl acetate extract ofA.acutaLour. have a certain anti-inflammatory activity, and the anti-inflammatory mechanisms are related to the inhibition of the release of NO, TNF-α, and IL-6.
- Medicinal Plant的其它文章
- Research Progress on Pharmacological Action of 5-O-methylvisammioside
- Review of Processing Methods of Jujube
- Advances in Application of PCR Amplification in Etiologic Diagnosis
- Research Progress of Traditional Chinese Medicine in the Treatment of Acute Respiratory Distress Syndrome
- Advances in Research of Resources and Cultivation Techniques of Chinese Medicinal Material Radix Bupleuri
- Advances in Research of Pharmacological Effects of Peimine

