Investigation of in vitro antioxidant activity of dihydromyricetin and flavonoids rich extract from vine tea(Ampelopsis grossedentata)
Fu-Yan Li,Shu-Mei Chi,Hong-Bing Zhang*
1Pharmaceutical Department,Traditional Chinese Medicine Hospital of Zaozhuang,Zaozhuang 277000,China.
Abstract
Background: Vine tea from fermented Ampelopsis grossedentata leaves has been used as a herbal tea and folk medicine in the southern region of China for hundreds of years.The aim of this investigation was to analyze the total flavonoids found in vine tea, including three bioactive flavonoids, and the total phenolic contents in the aqueous methanol extracts of 10 vine tea samples.In addition, this study also aimed to examine the antioxidant activity of dihydromyricetin and vine tea's flavonoid-rich extract.Methods:The total flavonoids and total phenolic content assay of extracts from vine tea were performed by ultraviolet-visible spectroscopy and epoch microplate spectrophotometer, respectively.Three bioactive flavonoids were quantified simultaneously using high performance liquid chromatography.The antioxidant activity of dihydromyricetin and vine tea's flavonoid-rich extract was evaluated in vitro using six different methods.Results: Vine tea contained a large number of flavonoids, with dihydromyricetin as its main constituent.The flavonoid-rich extract exhibited a significant scavenging effect on superoxide anion radicals, and on 3-ethylbenzthiazoline-6-sulphonic acid and 1,1-diphenyl-2-picrylhydrazyl radicals.It also possessed definite activity in lipid peroxidation inhibition, ferric reduction, and the moderation of Fe2+ ion chelation ability.There was a significant negative correlation between dihydromyricetin content and antioxidant activity in the vine tea samples, including superoxide anion radical scavenging activity (P = ?0.754, P <0.05), lipid peroxidation inhibition activity (P = ?0.759, P <0.05),ferric-reducing antioxidant power (P = ?0.843, P <0.01), respectively.Dihydromyricetin played a dominant role in the antioxidant activities of the flavonoid-rich extract.Conclusion: Vine tea's flavonoid-rich extract could be used as a new antioxidant source to safeguard against oxidative stress.
Keywords:Vine tea,Ampelopsis grossedentata,Flavonoids,Dihydromyricetin,Phenols,Antioxidant activity
Background
Ampelopsis grossedentata(Hand.-Mazz.) W.T.Wang is a medicinal, and edible plant that grows in the mountainous areas of southern China.The plant's leaves can be fermented to create vine tea, which has been widely used as herbal tea and folk medicine in China and Southeast Asia for hundreds of years.The first detailed description of vine tea was published in traditional Chinese medicine book,Zhong Hua Ben Cao(Chinese Materia Medica; 1999).The book states that vine tea detoxicates, promotes diuresis, and reduces edema.With regard to its clinical application in traditional Chinese medicine,vine tea has been used to treat, for example, colds, sore throats, jaundice, and hepatitis [1].It has been demonstrated that vine tea contains flavonoid glycosides, flavanonol, phenols,polysaccharides and other chemical constituents [2,3].Among them, flavonoids stand out for their rich contents and significant pharmacological activity.Previous pharmacological studies have shown that vine tea possesses a wide range of biological functions,such as anti-tumor, hypoglycemic, liver protection,antioxidant, anti-bacterial, and antithrombotic activities [4-9].Dihydromyricetin (DMY), a flavanonol also known as ampelopsin,was found to be vine tea's most abundant bioactive constituent (Figure 1).Recently, it has been found to display various physiological activities, including anti-tumor,anti-inflammatory, anti-bacterial, antioxidant,anti-atherosclerosis, and hepatoprotective effects[10-15].
Reactive oxygen species (ROS), usually generated by the metabolism of oxygen from oxygen free radicals and other species (e.g., hydrogen peroxide,hydroxyl radicals and singlet oxygen) are extremely reactive non-specific molecules [16].Increasing evidence highlights that the overproduction of free radicals and ROS may result in oxidative stress,which is defined as an imbalance between oxidants and antioxidants.The overproduction of ROS can result in the onset of multiple chronic diseases (e.g., cancer,cardiovascular diseases, inflammatory diseases, and diabetes) and may also be involved in the ageing process [17, 18].In the case of oxidative stress,biological oxidative damage is provoked on relevant molecules,such as lipids,proteins,DNA,nucleic acids and carbohydrates, and ultimately leads to cell death[19].In order to reduce ROS-induced oxidative damage, it is essential to explore and research the natural substances present in medicinal and dietary plants, which can regulate and maintain proper physiological functions responsible for off-setting oxidative stress as therapeutic antioxidants.
Research focused on the relevant antioxidant mechanisms of DMY has been limited in recent years[20-22].Even less attention has been paid to systematic and in-depth research on the content and antioxidant activity of DMY and the flavonoid-rich extract derived from vine tea.There were two primary goals for our investigation.The first objective was to investigate the constituents found in the total flavonoids, three bioactive flavonoids, and total phenolic in 10 batches of vine tea.This research can provide a theoretical basis for quality control and evaluation of vine tea in the future.The second aim of this study was to evaluate the antioxidant effects of DMY and various vine tea extracts.To the best of our knowledge, this is the first report comparing in vitro antioxidant effects of DMY and vine tea's flavonoid-rich extract.The results should provide a theoretical basis for vine tea's antioxidant properties and traditional Chinese medicine use.
Material and methods
Materials
Vine tea samples were collected from Jianning County,Fujian Province of China, and purchased from Zhangjiajie City, Hunan Province of China.Detailed sample information is listed in Supplementary Table S1.All samples were authenticated by Professor Qin Minjian (Department of Resource Science of Traditional Chinese Medicines, China Pharmaceutical University,Nanjing,China).
Figure 1 Ampelopsis grossedentata (Hand.-Mazz.)W.T.Wang whole plant, the plant's leaves vine tea and its main component dihydromyricetin
Reagents
DMY was isolated and purified in our laboratory.The structure was identified with the mass spectrometry and hydrogen-nuclear magnetic resonance methods.The purity of DMY was determined to be over 98%using the high performance liquid chromatography(HPLC)-diode array detector method.Gallic acid,3-ethylbenzthiazoline-6-sulphonic acid (ABTS),1,1-diphenyl-2-picrylhydrazyl (DPPH), tween-40,β-carotene, linoleic acid, potassium persulphate,ferrozine, ascorbic acid (VC) and nitrotetrazolium blue chloride were purchased from Sigma-Aldrich Company(Shanghai,China).Folin-Ciocalteau reagent,methionine and riboflavin were obtained from Solarbio Sciences & Technology Company, Limited (Beijing,China).Acetonitrile and HPLC-grade methanol were purchased from Tiandi (USA) and Hanbon (Nanjing,China), respectively.All other reagents were of analytical grade and purchased from Nanjing Chemical Regents Company, Limited (Nanjing,China).
Sample analysis preparation and antioxidant evaluation
Sample preparation was completed per the method established in our previous study [23].Dried vine tea powder (0.5 g, 40 mesh) was sonicated with 81%methanol (21 mL) at room temperature (25℃) for 32 minutes.After ultrasonic extraction, the sample was centrifuged (2000 rpm, 15 minutes) and collected.Then, 1 mL supernatant was diluted to 25 mL for use as a stock solution for ultraviolet detection, while 2 mL supernatant was filtered through a 0.22 μm microfiltration membrane for HPLC analysis.Each sample's residual solution was dissolved to assay the antioxidant activities.
Total flavonoids and three bioactive flavonoids content assay
The total flavonoids and three bioactive flavonoids content assay of the vine tea extract was conducted using the method published in our previous study[23].We employed a ultraviolet-visible spectroscopy spectrophotometer (Model UV-2450, Japan), based on the formation of a complex flavonoid-aluminum having a maximum absorbance value at 311 nm, and also used HPLC (Agilent Technologies, USA).HPLC analysis was conducted on an Agilent Series 1260 liquid chromatography instrument equipped with an on-line degasser, a quaternary pump, a diode-array detector, a thermostatic column compartment and an auto-sampler.Analyte separation was performed on an RP-C18 column(Hedera ODS-2,4.6 mm×150 mm,5μm, Hanbon, Jiangsu) at a flow rate of 1.0 mL/min,with a sample injection volume of 10 μL, and a column temperature maintained at 30°C.The mobile phase consisted of solvents A (0.5% aqueous formic acid, v/v) and B (acetonitrile).Gradient elution was as follows: 10%?20% B (v/v) at 0?20 minutes and 20%?30% B (v/v) at 20?40 minutes.The detecting wavelength was set at 292 nm.The contents of the three analytes in each sample were estimated according to their respective calibration curves (DMY:Y = 2579.40X ?272.09, R2= 0.9999, linear range 0.3072?3.0720 mg/mL; myricitrin: Y = 966.31X +20.264, R2= 0.9999, linear range 0.1518?1.5180 mg/mL; and myricetin: Y = 1146X ?16.992, R2=0.9998,linear range 0.0150?0.1500 mg/mL).The total flavonoid contents were expressed as mg of DMY equivalents per gram (mg DMYE/g) of the sample dry weights, and estimated according to the calibration curve (total flavonoids: Y = 0.0485X + 0.0087, R2=0.9998,linear range 3.68?18.40μg/mL).
Total phenolic content assay
Two hundred microliters of the extracts were mixed with 200 μL of Folin-Ciocalteau reagent.After 5 minutes, 7 mL of 3% aqueous sodium carbonate solution was added into the mixture.The tincture was subsequently developed in the dark for 5 min at room temperature (25℃).The optical density (OD) value was measured at 765 nm using an epoch microplate spectrophotometer (BioTek Instruments, USA).Gallic acid was used as the standard, and the stock solution was serially diluted for the generation of a standard curve (Supplementary Table S2).The total phenolic content was expressed as milligrams of gallic acid equivalents per gram (mg GAE/g) of each sample's dry weight.
Antioxidant activity of extracts and dihydromyricetin in vitro
DPPH radical scavenging activity assay.DPPH radical scavenging activity was determined according to the method published by Yuan in our laboratory[24].Briefly,200 μL of a serially diluted sample solution or extract solvent (control) was mixed with 3 mL of DPPH solution (1.0 mg DPPH dissolved in 50 mL methanol).The mixture was shaken vigorously and allowed to stand at room temperature for 30 minutes.Next, the absorbance of the mixture was measured at 517 nm.VCwas used as a positive control for each sample.The DPPH radical scavenging activity was calculated by the following formula: scavenging activity (%) = [1 ?(A1?A2)/A0] × 100%, where A0and A1are the absorbance of blanks (using extract solvent instead of sample solution) and the extracts,and A2is the absorbance of the sample only (methanol instead of DPPH).Half maximal inhibitory concentration (IC50) values were generated via regression analysis of data taken from a dilution series of extraction solutions.
ABTS+ radical scavenging activity assay.The scavenging abilities of ABTS+radicals were determined using a previously published method [25].Potassium persulphate was dissolved in deionized water at 2.45 mM concentration,and ABTS was added at a concentration of 7 mM.The reaction mixture was stored at ?4°C in a refrigerator for 12 h.It was then diluted with 80% ethanol, to generate the OD value of 0.700±0.005 at 734 nm.The sample solution(0.2 mL)at various concentrations was incubated with the ABTS+solution (3 mL) at room temperature for 30 minutes, and the absorbance at 734 nm was immediately recorded.VCwas used as the positive control.The ABTS+radical scavenging activity was calculated by the following formula: scavenging activity (%) = (1 ?A1/A0) × 100%, where A0and A1are the absorbance of blanks (the extract solvent lacking sample solution)and the extracts, respectively.The IC50values were generated by regression analysis of data from a dilution series of extraction solutions.
Superoxide radical scavenging activity assay.The superoxide radical scavenging activity was measured using the method published by Yuan[24].The reaction was carried out in a mixture containing the sample solution (0.2 mL, a serially diluted sample solution),phosphate buffer (1.5 mL, 50 mmol/L, pH 7.4),methionine (0.3 mL, 130 mmol/L), nitrotetrazolium blue chloride (0.3 mL, 750 μmol/L), disodium ethylenediamine tetraacetic acid (EDTA-Na2) solution(0.3 mL, 20 μmol/L), riboflavin (0.3 mL, 20 μmol/L),and purified water (0.5 mL) by incubating the mixture under a daylight lamp for 30 min at room temperature(25℃).VCwas used as the positive control.The epoch microplate spectrophotometer was used to measure the mixture at 560 nm.The superoxide radical scavenging activity was calculated by the following formula:scavenging activity (%) = (1 ?A1/A0) × 100%, where A0and A1are the absorbance of blanks (the extract solvent lacking sample solution) and the extracts,respectively.The IC50values were generated by regression analysis of data from a dilution series of extraction solutions.
Ferric-reducing antioxidant power (FRAP) assay.The reduction potential of each sample was determined according to the method with a slight modification [26].The reaction mixture with 0.5 mL sodium phosphate buffer (0.2 M, pH 6.6), 0.5 mL potassium ferricyanide (1%, w/v) and 0.5 mL of different concentrations of the sample solution was incubated at 50°C for 20 minutes.After the addition of 0.5 mL trichloroacetic acid (10%, w/v), the mixture was centrifuged at 4000 rpm for 10 minutes.The upper layer(0.5 mL)was then mixed with 0.1 mL of distilled water,and 0.1 mL of fresh ferric chloride (0.1%, w/v).The absorbance was measured at 700 nm.VCwas used as the positive control.The reducing power was calculated by the following formula: reducing power(OD) = (A1?A0) × 100%, where A1is the absorbance of the sample, and A0is the absorbance of control wherein FeCl3solution has been replaced by water.The concentration for 50% of maximal effect (EC50)value represented the effective concentration at which the absorbance was 0.5 for reducing power.
Lipid peroxidation inhibition activity assay.The antioxidant activity of each extract was evaluated using a β-carotene-linoleic acid model system,according to the method published by Yuan [24].The β-carotene (3 mL, 0.01 mg/mL dissolved in chloroform) was added to a round-bottom flask containing linoleic acid (40 mg) and Tween-40 (400 mg).After removing the chloroform under a nitrogen flush for 5 minutes, purified water (100 mL) was added with vigorous stirring to form an emulsion.Initial absorbance of the emulsion (A1) was measured at 470 nm.The emulsion (3 mL) was added to each tube containing 0.2 mL extract solution and kept in a water bath at 50°C for 1 h.Absorbance (A0) was measured at 470 nm.VCwas used as reference for comparison.The lipid peroxidation inhibition activity was calculated as: scavenging activity (%) = (1 ?A1/A0) × 100%, where A1is the absorbance of the extracts at the initial and A0is the absorbance of the reaction.The IC50values were generated using regression analysis data from a dilution series of extraction solutions.
Fe2+ ion chelating ability assay.The Fe2+chelating activity was investigated based on the method with slight modifications [27].The reaction mixture was treated with 0.4 mL of the extract solution, 0.05 mL of ferrous chloride solution (2 mM), and 2 mL of methanol.The reaction was initiated by adding the reaction mixture into 0.1 ml ferrozine solution(5 mM),followed by vigorous shaking.The resulting mixture was incubated at room temperature (25℃) for 10 minutes, then absorbance was determined at 562 nm against a blank.EDTA-Na2solution was used as a positive control.The chelating ability was calculated using the following formula: chelating rate (%) = (1 ?A1/A0)×100%,where A0and A1are the absorbance of blanks (using extract solvent instead of sample solution) and the extracts, and IC50values were generated by regression analysis data from a dilution series of extraction solutions.
Statistical analysis
All analyses were performe d in triplicate.Results were expressed as mean ± standard deviation.Statistical analyses of the data were performed using the correlation analysis and student'st-test (SPSS Statistics 19.0, IBM Corporation, USA).The difference was considered significant atP<0.05.
Results
Total flavonoids, three bioactive flavonoids, and total phenolic contents
The chromatograms of aqueous methanol extracts ofAmpelopsis grossedentata(Hand.-Mazz.) W.T.Wang and standard controls of dihydromyricetin, myricitrin and myricetin are shown in Figure 2.From the summarized results (Table 1), we concluded that total flavonoid contents in vine tea samples was close to or more than 500 mg/g on a dry weight basis, except for sample T-10, which confirms that vine tea contains a large number of flavonoids.It was also clear that DMY content in the 10 vine tea samples distinctly exceeded 200 mg/g on a dry weight basis,which led us to conclude that DMY is one of vine tea's leading bioactive flavonoids.As for as the quantitative determination of myricitrin, its values ranged from 6.40±0.12 to 8.26± 0.08 mg/g on a dry weight basis,except for sample T-2.The determination of myricetin content ranged from 2.39±0.02 to 3.75±0.11,except for sample T-8.As shown in Table 1, it can be concluded that the total phenol contents of the other nine vine tea samples significantly exceeded 300 mg/g on a dry weight basis, with a range between 324.67 ±3.03 and 371.46±0.96,except for sample T-3.
Antioxidant activity of dihydromyricetin and flavonoid-rich extract
Scavenging activity on DPPH radicals.As a stable free radical compound, DPPH has been widely accepted as a tool for estimating the free radical scavenging capacity of antioxidants [28].The DPPH scavenging activities of the methanol extracts from 10 vine tea samples at different concentrations (12.5?200μg/mL) are presented in Figure 3-A and Supplementary Table S3.The flavonoid-rich extract was effective as a scavenger and exhibited dose-dependent DPPH radical scavenging activity.As shown in Supplementary Table S3, the IC50values of each sample displayed no significant differences within the range of 40.84 ± 0.38 to 48.53 ± 0.76μg/mL.As shown in Figure 3-B, it demonstrates that DMY displayed more DPPH radical scavenging activity than VC, with IC50values of 16.22 ± 0.03 and 19.14 ± 0.13 μg/mL, respectively.This confirms that DMY had fairly strong scavenging activities.The suggests that DMY content and vine tea scavenging activities on DPPH were negatively correlated (P=?0.565,P=0.089)(Table 2).
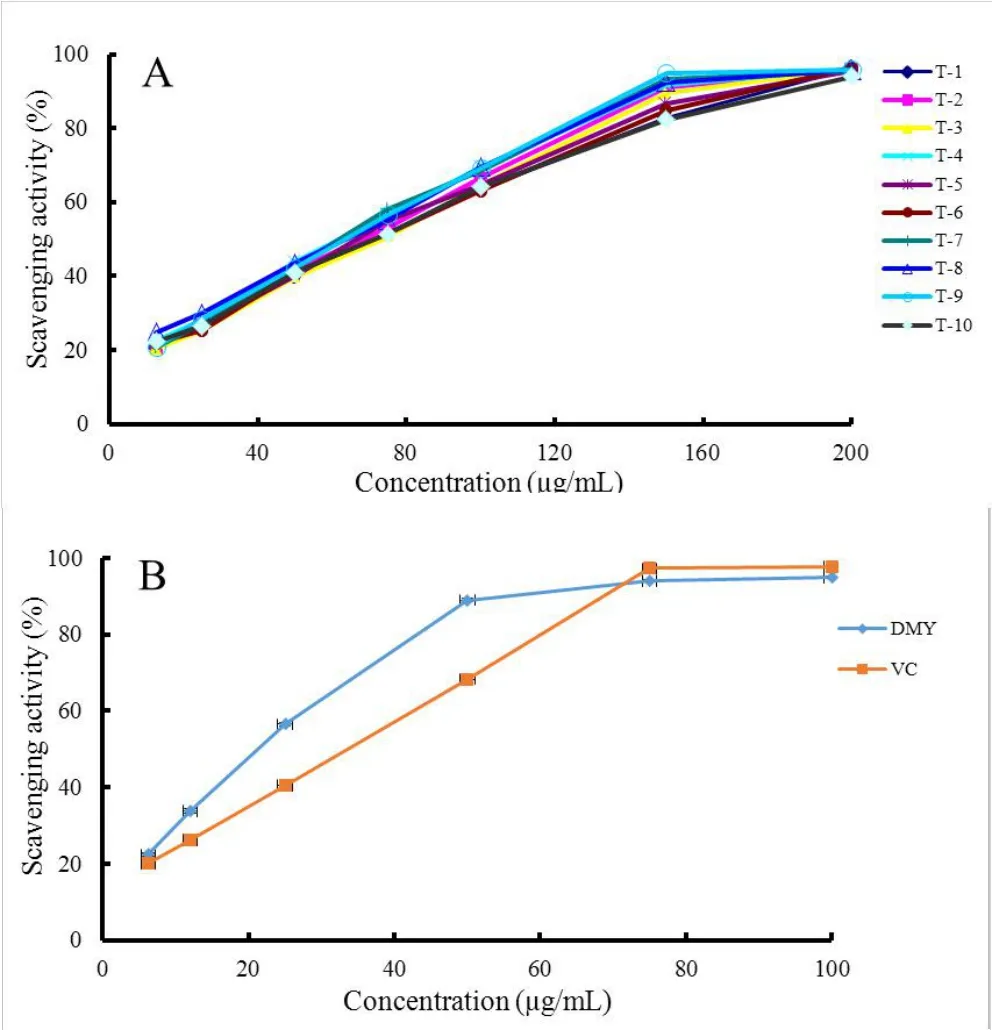
Figure 3 DPPH radical scavenging activity of flavonoid-rich extract from ten vine tea samples(A)and compare of scavenging activity between DMY and VC (B).DMY, dihydromyricetin; VC, ascorbic acid.
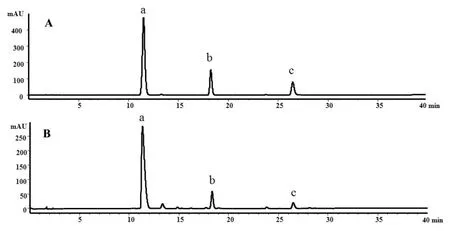
Figure 2 Typical chromatograms of standard controls (A) and aqueous methanol extracts of vine tea(Ampelopsis grossedentata)(B).a:dihydromyricetin,b:myricitrin and c:myricetin.
Scavenging activity on ABTS+radical.The model of scavenging ABTS+radical is a widely accepted method to evaluate the free radical scavenging activities of antioxidants[28].The results indicate that,in the range of 10?50 μg/mL, the scavenging abilities of the flavonoid-rich extracts were dose-dependent(Figure 4-A).All vine tea samples showed excellent ABTS+radical scavenging activity (83.85%?94.65%)at a concentration of 50 μg/mL in the reaction mixtures (Supplementary Table S4).The scavenging activities of DMY and VCobviously increased with increased concentrations (Figure 4-B).The IC50values of DMY and VC were 3.96 ± 0.17 and 24.31 ± 0.14μg/mL, respectively (P <0.05).It was obvious that DMY displayed distinctly greater scavenging activities than VC.Meanwhile, the IC50values were within the range of 22.52 ± 0.08 and 28.50 ± 0.24 μg/mL, which was almost similar to VC.As shown in Table 2, there was a negative correlation between DMY content and vine tea scavenging activity on ABTS+radical (P =?0.450,P=0.192).
Scavenging activity on superoxide radicals.
Superoxide radicals, originated either from metabolic processes or oxygen activation, is considered to be the primary ROS.As a harmful compound to cells, the formation of superoxide radicals can induce oxidative damage in lipids,proteins and DNA[29].

Figure 4 ABTS+ radical scavenging activity of flavonoid-rich extract from ten vine tea samples(A)and compare of scavenging activity between DMY and VC (B).DMY, dihydromyricetin; VC, ascorbic acid.
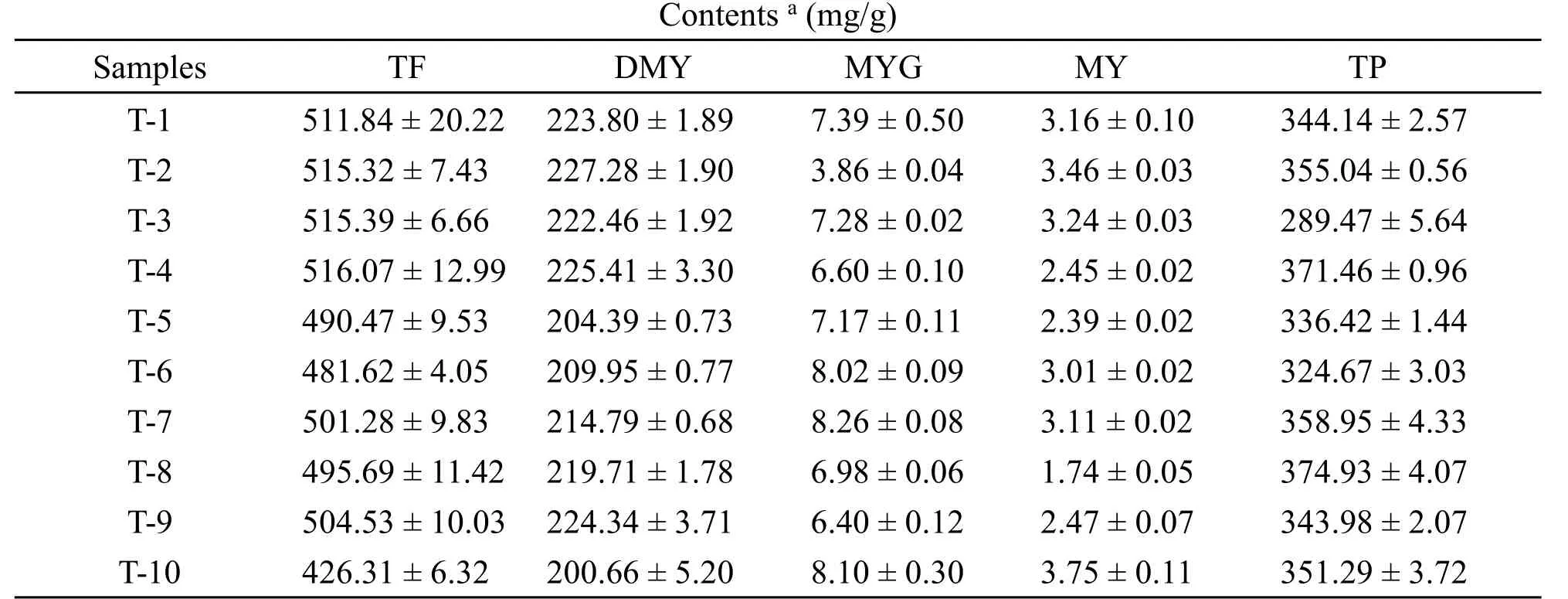
Table 1 Contents of the total flavonoids, dihydromyricetin, myricitrin, myricetin and the total phenolic in vine tea from different origins

Table 2 The results of correlation analysis between DMY content and antioxidant capacities of the flavonoid-rich extracts from vine tea samples
As shown in Figure 5-A,vine tea scavenging activities were correlated with increasing concentrations, and exhibited excellent scavenging effects on superoxide radicals within the range of 90.92%?94.35% at a concentration of 100μg/mL(Supplementary Table S5).In terms of IC50values, the sample T-4 possessed the highest scavenging activity with an IC50value of 15.88±0.22μg/mL(Supplementary Table S5).As described in Figure 5-B, the scavenging activities of DMY and VCalso increased with increasing concentrations.Above all, the scavenging activities of the flavonoid-rich extracts and DMY were much higher than VC, and the IC50values for DMY and VCwere 13.25±0.16 and 117.58±1.46μg/mL,respectively(P<0.05).The correlation analysis results demonstrate that DMY content and vine tea sample scavenging activities on superoxide radicals had a significant negative correlation(P=?0.754,P<0.05)(Table 2).

Figure 5 Superoxide radical scavenging activity of the flavonoid-rich extract from ten vine tea samples(A) and compare of scavenging activity between DMY and VC (B).DMY, dihydromyricetin; VC,ascorbic acid.
FRAP assay.The reducing power of a compound may serve as a significant indicator of its potential antioxidant activity.The FRAP assay is a simple and direct method to assess “antioxidant power” [26].The antioxidants can transform Fe3+into Fe2+, so that the reducing power could be monitored by measuring the Prussian blue formation at 700 nm.As shown in Figure 6-A and 6-B, a dose-response relationship was found in the reducing ability of the flavonoid-rich extracts, DMY, and VC.The reducing powers of vine tea samples were lower than DMY and VC, as presented in Supplementary Table S6.The reducing ability of DMY (EC50= 80.72 ± 0.52 μg/mL) was a little higher than that of VC(EC50= 99.60 ± 0.87μg/mL).As can be seen in Table 2, there was a noticeably negative correlation between DMY content and the vine tea's reducing power (P= ?0.843,P<0.01).
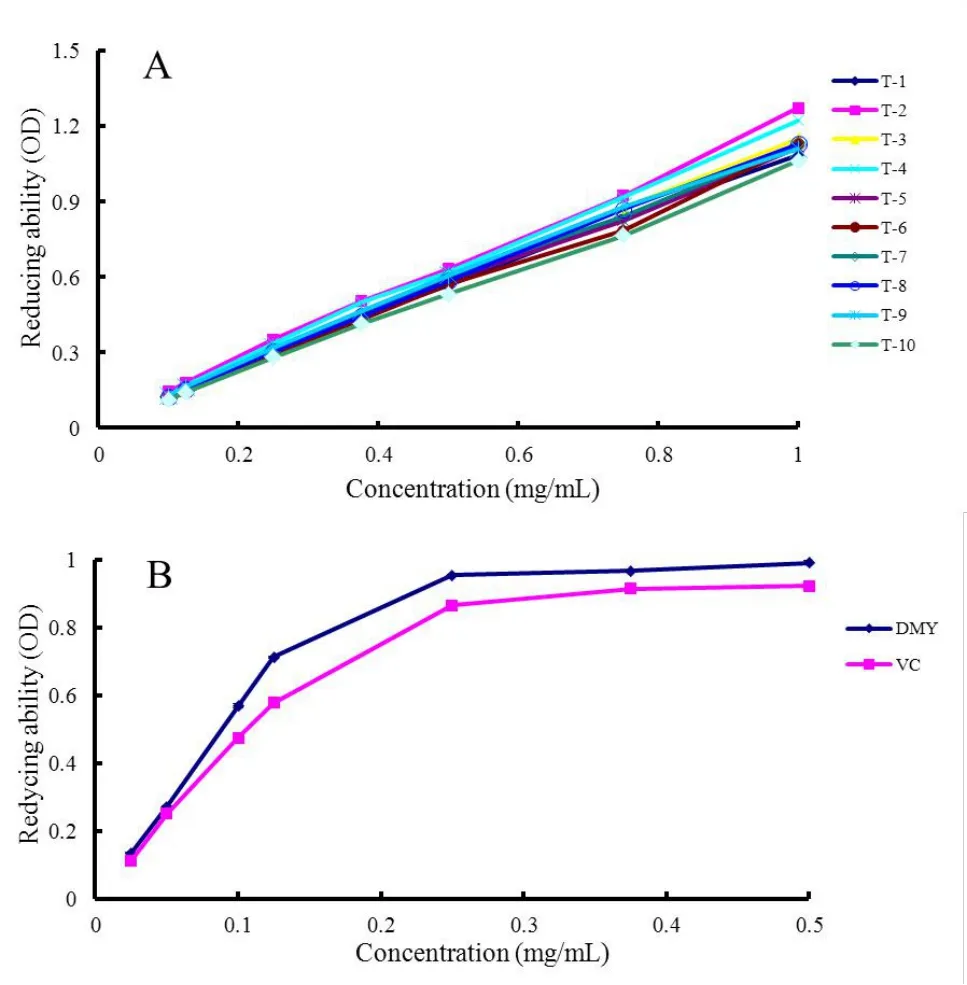
Figure 6 Fe3+ ion reducing power of flavonoid-rich extract from ten vine tea samples (A) and compare of scavenging activity between DMY and VC (B).DMY,dihydromyricetin;VC,ascorbic acid.
Lipid peroxidation inhibition activity.Lipid peroxides are an important class of ROS and have a significant impact on cell functions.They are believed to be a primary factor in neurodegenerative diseases,such as Alzheimer's disease [30].According to Figure 7-A and 7-B, the inhibition effects of the flavonoid-rich extracts and DMY increased with increasing concentrations.As shown in Supplementary Table S7, all vine tea samples exhibited definite lipid peroxidation inhibition effects and revealed no obvious differences within the range of 192.07 ± 7.53 to 211.14 ± 6.11 μg/mL, other than sample T-10.The inhibition activity of DMY was much higher than the vine tea samples, and VC(P<0.05).The IC50values for DMY were 20.18 ± 1.23 μg/mL (Supplementary Table S7).As can be seen in Table 2, there was a significant negative correlation between DMY content and the inhibition activity of vine tea samples (P=?0.759,P<0.05).DMY exhibited promotive effects on lipid peroxidation observed for VCat a lower concentration.Only the much higher concentration of VCdemonstrated some inhibition effects.
Chelation ability with Fe2+ion.The ability to chelate metal ions is supposed to be an antioxidant mechanism,since it reduces the concentration of the catalyzing transition metal in lipid peroxidase [31].Figure 8-A and Supplementary Table S8 show the obtained ferrous chelating abilities of the flavonoid-rich extract from vine tea.The results suggest that extracts combined with the Fe2+ion in a dose-dependent manner.As depicted in Figure 8-B, the values indicate that EDTA-Na2has an extremely strong chelating capacity, with the chelation rate reaching 91.46 ±0.23% at a concentration of 0.5 mM.DMY at a much higher concentration possessed a very weak metal ion chelating ability.As shown in Table 2, there was no significantly positive correlation between DMY content and the chelation ability of vine tea samples(P=0.076,P=0.836).
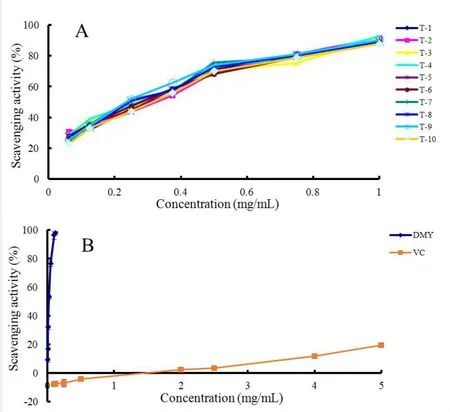
Figure 7 Inhibition effects on lipid peroxidation of flavonoid-rich extract from ten vine tea samples(A)and compare of scavenging activity between DMY and VC (B).DMY, dihydromyricetin; VC, ascorbic acid.

Figure 8 Fe2+ ion chelating ability of the flavonoid-rich extract from ten vine tea samples(A)and EDTA (B).DMY, dihydromyricetin; EDTA,ethylenediamine tetraacetic acid.
Discussion
This study found that vine tea contained a large number of flavonoids.DMY was confirmed to be the main bioactive constituent in the flavonoid-rich extracts taken from vine tea samples.It is difficult to compare our results with previously reported experimental data[32,33].These differences might be caused by different factors, such as plant gene types,habitats or harvesting times,the mode of conservation,processing methods, or extraction methods.Overall,these results could provide a theoretical basis for the quality control and evaluation of vine tea.
Since there is currently no standard method to evaluate the antioxidant abilities of natural products,we used six different experimental methods to comprehensively evaluate the antioxidant activity of vine tea.This investigation revealed that vine tea with higher DMY content exhibited stronger radical scavenging activities.DMY played a key role in scavenging superoxide anion radicals, ABTS+, and DPPH radicals in the flavonoid-rich extracts from vine tea, which is in agreement with Wang's previously reported results[34].
Due to the fact that we used different antioxidant activity evaluation methods with different mechanisms,the same active component or monomer composition may demonstrate various antioxidant activities.In this investigation, we found that higher DMY content in vine tea demonstrated stronger inhibition of lipid peroxidation and ferric-reducing antioxidant powers.Compared to the analysis results of other methods, the flavonoid-rich extract possessed moderate metal ion chelating abilities,which may be attributed to its types or structure[35].
Conclusion
The results in this study revealed that vine tea contains a variety of flavonoids,with DMY as the most leading bioactive constituent among those flavonoids.The flavonoid-rich extracts from our vine tea samples demonstrated remarkable antioxidant activities on superoxide anion radicals, ABTS+and DPPH radicals,and also strongly inhibited lipid peroxidation,ferric-reducing power, and moderate metal ion chelating ability.These findings imply that vine tea extracts could reduce oxidative stress.DMY plays a dominant role in vine tea's antioxidant activities,which could be used as a potential resource of natural antioxidants in functional foods and health products.Further investigations of the in vivo antioxidant activities of DMY and vine tea's flavonoid-rich extract are in progress in our laboratory.
 Traditional Medicine Research2021年1期
Traditional Medicine Research2021年1期
- Traditional Medicine Research的其它文章
- The dynamic changes and mechanisms of Rehmanniae radix processing based on Maillard reaction
- Advances in anti-inflammatory and immunoregulatory mechanisms of sinomenine
- Hua-Zhuo-Kai-Yu decoction inhibits apoptosis in nonalcoholic fatty liver disease
- Qiming granule in treating type 2 diabetic kidney disease patients:study protocol for a randomized controlled trial
- Bibliometric analysis of acupuncture research through the Web of Science database from 1990 to 2019
- Jian-Gan-Xiao-Zhi decoction ameliorates high-fat high-carbohydrate diet-induced non-alcoholic fatty liver disease and insulin resistance by regulating the AMPK/JNK pathway
