Anti-inflammatory and anti-apoptotic effects of N-acetylcysteine in diabetic rat corneal epithelium
Sae-Byeok Hwang, Jin Hyoung Park,3, Ji-Yun Park, Soon-Suk Kang, Ho Seok Chung,Hun Lee2,, Jae Yong Kim2,, Hungwon Tchah2,
1Research Institute of Miso Eye Clinic, Gyeonggi-do 13640,Republic of Korea
2Biomedical Research Center, Asan Institute for Life Science,Asan Medical Center, Seoul 05505, Republic of Korea
3Miso Eye Clinic, Gyeonggi-do 13640, Republic of Korea
4Department of Ophthalmology, University of Ulsan College of Medicine, Seoul 05505, Republic of Korea
5Research Institute for Biomacromolecules, University of Ulsan College of Medicine, Seoul 05505, Republic of Korea
6Department of Ophthalmology, Dankook University Hospital,Dankook University College of Medicine, Cheonan 31116,Republic of Korea
Abstract
● KEYWORDS: N-acetylcysteine; apoptosis; inflammation;diabetes; corneal epithelium; rat
INTRODUCTION
Diabetes mellitus (DM) is associated with a number of potential complications, including diabetic keratopathy[1], which may be characterized by delayed corneal wound healing, epithelial defects, fragility, recurrent erosion,corneal edema, and non-healing ulcers[2]. Although the mechanism by which diabetic keratopathy develops is not fully understood, the principal clinical impact is delayed wound healing, which has been the subject of most of the studies conducted to date. The molecular defects that underpin delayed wound healing principally concern inflammatory and apoptotic pathways. Corneal wound healing is delayed as a result of glucose-induced reactive oxygen species (ROS) generation[3-5],followed by the induction of apoptosis by the sequential effects of advanced glycation end-products (AGEs), oxidative stress,and nuclear factor-kappa B (NF-κB) activation[5-7]. Therefore,the inhibition of apoptotic signaling pathways is likely to be required by a potential therapy for diabetic keratopathy.
Several therapies have been developed that accelerate epithelial wound healing and protect cells against apoptosis[8-10], but we hypothesized that the antioxidant N-acetylcysteine(NAC) could represent an effective treatment for diabetic keratopathy. NAC has been reported to have beneficial effects against diabetic cardiomyopathy, hepatic oxidative stress,and painful diabetic neuropathy[11-14]. It has also been reported that NAC reduces oxidative stress in the corneas of rats with keratoconjunctivitis[15], and inhibits inflammation and apoptosis in human conjunctival epithelial cells that are maintained in a highglucose environment[11]. In addition, a previous study of cultured porcine cornea showed that NAC improves corneal epithelial wound healing through an inhibition of intracellular ROS production[3,16]and a reduction in glucose-induced apoptosis[17].However, although these studies measured markers of wound healing and assessed the Akt and extracellular signal-related kinase signaling cascades, they did not assess inflammatory and apoptotic signaling in diabetic corneas.
Therefore, in the present study, we aimed to investigate the effects of NAC in streptozotocin (STZ)-induced diabetic rat cornea and in a human corneal epithelial cell line (HCEC)maintained in a high-glucose environment. We measured the expression of the receptor for advanced glycation end-products(RAGE) and inflammatory cytokines, and evaluated apoptotic signaling by assessing the activation of the cleaved caspase-3(CCAP-3)/poly-ADP ribose polymerase (PARP) cascades.
MATERIALS AND METHODS
Ethical Approval The study was approved by the Institutional Animal Care and Use Committee of the Asan Institute for Life Sciences at the Asan Medical Center. Animals were treated in compliance with the Institute of Laboratory Animal Resources Guide.
Corneal Epithelial Cell Culture and Treatments HCECs that had been immortalized by transfection with plasmid pRSV-T contains SV-40 (simian virus 40) early region genes and the Rous Sarcoma virus long terminal repeat were obtained from ATCC (American Type Culture Collection;Manassas, VA, USA) and cultured in keratinocyte serumfree medium (Thermo Fisher Scientific, Waltham, MA, USA)supplemented with bovine pituitary extract (Thermo Fisher Scientific, USA), human recombinant epidermal growth factor(Thermo Fisher Scientific, USA), 500 ng/mL hydrocortisone, and 0.005 mg/mL insulin. The plates were coated with a mixture of 0.01 mg/mL fibronectin, 0.03 mg/mL bovine collagen type I, and 0.01 mg/mL bovine serum albumin (BSA), dissolved in culture medium, and incubated in a 37℃ incubator overnight before use. The HCECs were incubated at 37℃ in a 5% CO2atmosphere, trypsinized using 0.05% trypsin-EDTA, and passaged every 3-4d. Cell cultures were allocated to four groups: 0, 5, 50 mmol/L glucose, and 50 mmol/L glucose with NAC (3 mmol/L) groups. The cells were incubated in medium containing these concentrations of glucose and NAC for 24h,after which measurements were made.
Rat Maintenance and Treatments We induced diabetes in 6-week-old male Sprague-Dawley rats weighing 150-160 g.They were maintained at 25℃±1℃ with a relative humidity of 40%±5% under a 12h light-dark cycle (lights on 08:00 to 20:00). The rats were divided into three groups: a control group(n=3), a DM group (n=3), and a DM with NAC group (n=3).After 1wk of acclimation, the control group was administered citrate buffer, and the DM and DM+NAC groups were intraperitoneally administered 65 mg/kg STZ. Starting 1d later,the blood glucose levels were measured using a glucometer.Rats with >17 mmol/L (300 mg/dL) in heparinized tail vein blood were used for the experiment. Then, 10 μL of 200 μg/mL NAC was topically applied to corneas of the DM+NAC group and phosphate buffer saline (PBS) was topically administered to corneas of the DM group three times daily for 4wk. At the end of this period, the rats were terminally anesthetized, and their eyeballs were isolated, fixed in 10% phosphate-buffered formalin for 24h, and then embedded in paraffin.
Immunofluorescence To characterize the expression of RAGE, the eyeballs were cut into 4 μm thick sections, which were baked at 60℃ for 30min. After deparaffinization, the slides were heated in 0.01 mol/L sodium citrate buffer (pH 6.0)at 90℃-100℃ for 30min for antigen retrieval. The tissues were then blocked with 0.1% BSA and 5% donkey serum (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA)at room temperature (RT) for 30min. After washing three times for 10min each, the slides were incubated with an anti-RAGE antibody (Abcam, Cambridge, UK) overnight at 4℃.After three further 10min washes, they were incubated with Alexa Fluor 488 AffiniPure Donkey Anti-Rabbit IgG (H+L)secondary antibody (Jackson ImmunoResearch Laboratories,Inc.) at RT for 30min, washed four times for 10min each, and stained with 4’-6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) for 5min. The slides were photographed using confocal microscopy (Carl Zeiss,Inc., Jena, Germany) at a high magnification (400×). The fluorescence intensity of the images was analyzed using Image J software (version 1.62f; available by ftp at http://rsb.info.nih.gov/nih-image; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry Some tissue sections were stained using hematoxylin and eosin. In addition, interleukin-1β(IL-1β) and CCAP-3 expression was characterized following immunohistochemical staining using the Dako REAL EnVision Detection System (Dako, Carpinteria, CA, USA) and DAPI (Vector Laboratories). The slides were photographed using microscopy (Carl Zeiss, Inc., Jena, Germany).
Enzyme-linked Immunosorbent Assay We measured the concentration of tumor necrosis factor (TNF)-α in the culture medium using an eBioscience Human TNF alpha ELISA Ready-SET-Go kit (Thermo Fisher Scientific, USA), according to the manufacturer’s protocol. The medium was centrifuged at 13 000× g for 10min at 4℃ and the supernatant was collected for this purpose.
Western Blot Analysis To quantify the levels of PARP and cleaved PARP, HCECs were collected and lysed using PROPREP Protein Extraction Solution (iNtRON Biotechnology,iNtODEWORLD, Inc., Gyeonggi-do, Korea). The cell lysates were centrifuged at 13 000× g for 15min at 4℃ and the supernatants were collected. The protein concentrations of the supernatants were determined using the Bradford method. Aliquots of supernatant containing 30 μg were mixed with equal volumes of Laemmli sample buffer, boiled, and resolved by 12% SDS-PAGE. Proteins were then transferred to Amersham nitrocellulose western blotting membranes (GE Healthcare, Chicago, IL, USA). The membranes were blocked for 1h using a 0.2% casein-based blocking reagent containing 0.1% Tween-20 and then incubated with primary antibodies against PARP or cleaved PARP (Cell Signaling Technology, Inc.,Danvers, MA, USA) overnight at 4℃. The membranes were then washed three times for 10min each with 0.1% Tween-20/Tris-buffered saline and then incubated with a horseradish peroxidase-conjugated anti-IgG secondary antibody (1:10 000 dilution). Specific protein bands were visualized using Western ECL Substrate (Bio-Rad Inc., Hercules, CA, USA). All images were scanned and densitometry analyses were performed using Image J software (NIH, MD).
TUNEL Assay To identify apoptotic cells, we used the TdT-mediated dUTP nick-end labeling (TUNEL) technique,which labels the cut ends of DNA fragments in the nuclei of apoptotic cells. Tissue sections were deparaffinized, labeled with fluorescein-dUTP (in situcell death detection kit; Roche Diagnostics, Seoul, South Korea), and stained with DAPI(Vector Laboratories, Burlingame, CA, USA. The slides were evaluated and photographed using confocal microscopy (Carl Zeiss, Inc., Jena, Germany) at a high magnification (400×).
Annexin V and Propidium Iodide Staining Cells that had been treated with or without HG and NAC were trypsinized with 0.05% trypsin-EDTA, collected, and centrifuged. The cell pellets were washed using ice-cold PBS, resuspended in binding buffer containing 0.01 mol/L HEPES/NaOH (pH 7.4),0.14 mol/L NaCl, and 2.5 mmol/L CaCl2, and incubated with fluorescein isothiocyanate (FITC)-labeled annexin V (BD Biosciences, Inc., San Jose, CA, USA) and propidium iodide(PI; BD Biosciences, Inc.) at RT in the dark for 15min. Positive cells were then counted using a FacsCanto II Flow Cytometer(BD Biosciences, Inc.).
Statistical Analysis All quantitative measurements were performed at least in triplicate and the data are expressed as means±standard deviation (SD). The data were analyzed using the Mann-WhitneyUtest or Student’st-test, and statistical significance was accepted whenP<0.05.
RESULTS
Expression of RAGE and Pro-inflammatory Cytokines We first compared the expression of RAGE in the corneas of control and diabetic rats that had or had not been treated with NAC. RAGE expression was low in the control group(1.00±0.34-fold), whereas it was very high in the STZ-induced diabetic corneal epithelium (2.46±0.13-fold compared to control group). However, NAC treatment reduced the level of expression (1.83±0.11-fold compared to control group,P<0.05; Figure 1).
We next measured the expression of the pro-inflammatory cytokine IL-1β. Diabetic corneal epithelium showed more intense cytoplasmic staining for IL-1β than control epithelium,but the administration of NAC reduced the fluorescence intensity (Figure 2A). We next quantified the TNF-α concentration in the media surrounding HCECs exposed to 5 or 50 mmol/L glucose for 24h and found that it was higher in the 50 mmol/L glucose cells (310±2.00 pg/mL) than in 5 mmol/L glucose cells (121±6.93 pg/mL). However, this effect of glucose of 50 mmol/L was ameliorated by NAC cotreatment (153.67±2.31 pg/mL; Figure 2B).
Extrinsic Apoptotic Signaling in Rat Cornea and HCECs The amount of CCAP-3 in apoptotic cells was assessed by immunohistochemical staining of paraffin-embedded corneas.Whereas no staining for CCAP-3 was visible in the control group, there was heavy cytoplasmic and perinuclear staining in basal and wing cells in the DM group, as shown in Figure 3A.However, NAC-treated corneal epithelium showed levels of CCAP-3 that were more similar to those of the control group.The levels of the pro-apoptotic factor, cleaved PARP (89 kDa),were also quantified in HCECs exposed to glucose stress for 24h, with or without NAC treatment, by Western blotting.The quantity of cleaved PARP was higher in the 50 mmol/L glucose group (7.43±0.56-fold) than in the 0 mmol/L glucose group (1.99±0.07-fold), but this effect of 50 mmol/L glucose was ameliorated by treatment with NAC (5.55±0.31-fold;Figure 3B).
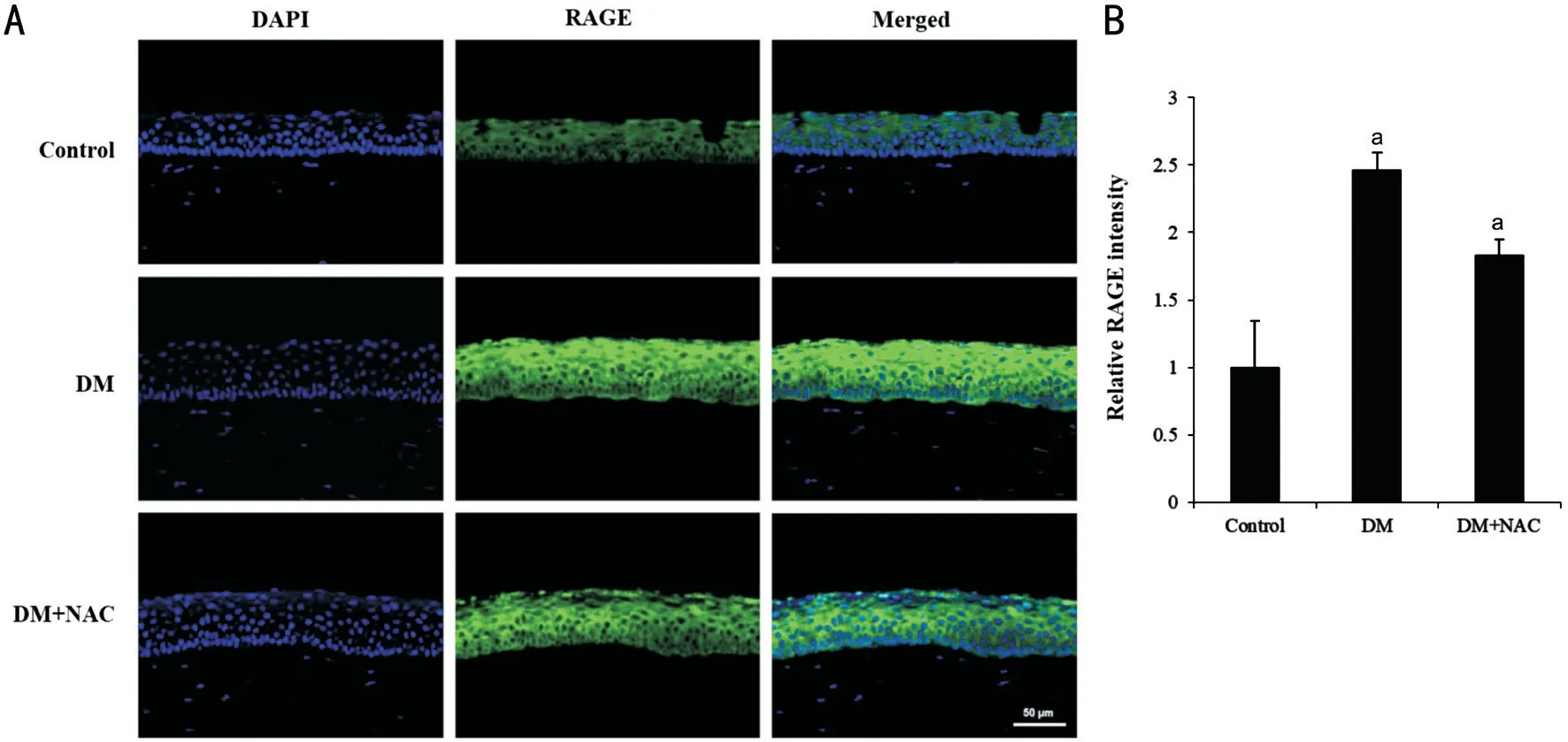
Figure 1 NAC reduces the expression of RAGE, an AGE receptor that is associated with pro-inflammatory gene activation, in the corneal epithelium of diabetic rats RAGE expression was detected by immunofluorescence in the STZ diabetic rats of DM+NAC group were topically applied with 10 μL of 200 μg/mL NAC for 4wk. A: Immunofluorescent staining was performed using an anti-RAGE antibody (green).The nuclei were counterstained using DAPI (blue). Representative data of one of three mice are shown. White scale bar: 50 μm. B: Relative RAGE expression was semi-quantified. The error bars represent the standard deviation of separate sample. aP<0.05; DM: Diabetes mellitus;NAC: N-acetylcysteine; DAPI: 4’-6-diamidino-2-phenylindole; RAGE: Receptor for advanced glycation end-products.
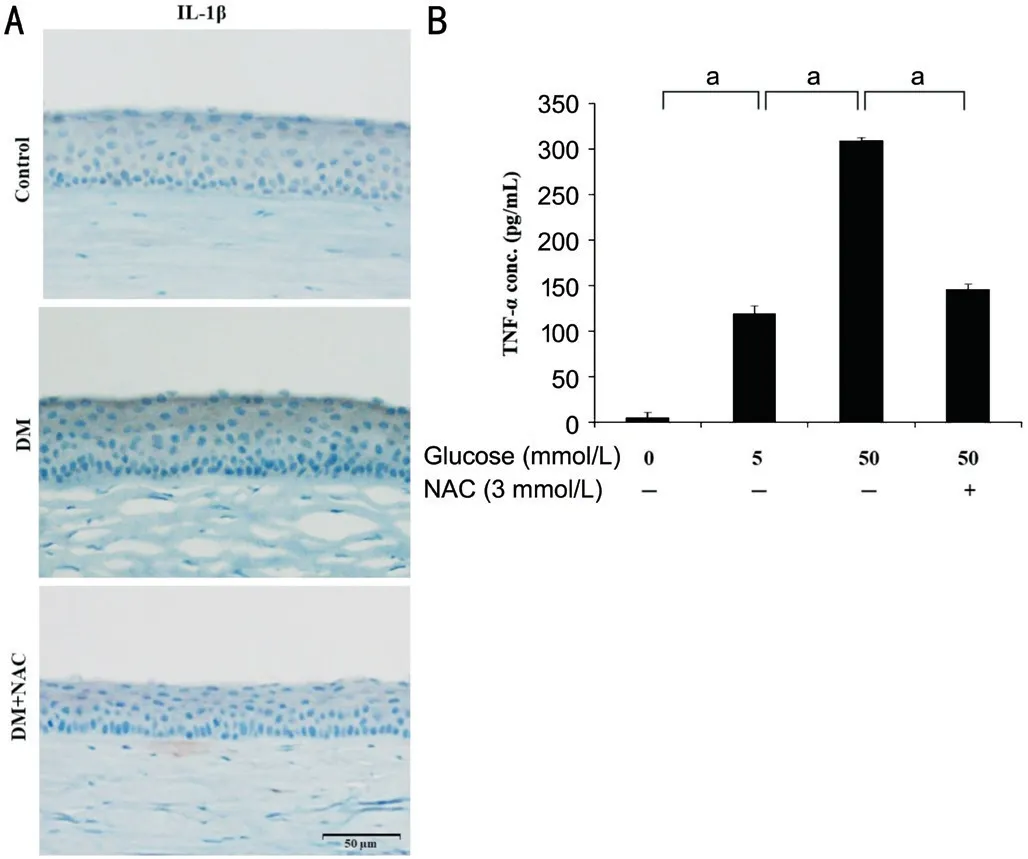
Figure 2 NAC inhibits inflammatory cytokine expression in the corneal epithelium of diabetic rats and in high glucose treated HCECs Immunohistochemistry analysis for IL-1β was performed on diabetic rats that were topically administrated with NAC (10 μL of 200 μg/mL). And cells were treated with glucose (0, 5, 50 mmol/L) or glucose with NAC (3 mmol/L) for 24h. A: Representative images of the immunohistochemical staining for IL-1β in the corneal epithelium of diabetic rats that were or were not treated with NAC (n=3). Black scale bar: 50 μm. B: The concentration of TNF-α in the media surrounding HCECs that were exposed to high-glucose concentrations and treated with or without NAC was quantified using an ELISA.Representative data from three independent experiments are shown.The error bars represent the standard deviation of three independent experiments. aP<0.05; DM: Diabetes mellitus; NAC: N-acetylcysteine;IL-1β: Interleukin-1β; TNF-α: Tumor necrosis factor-α.
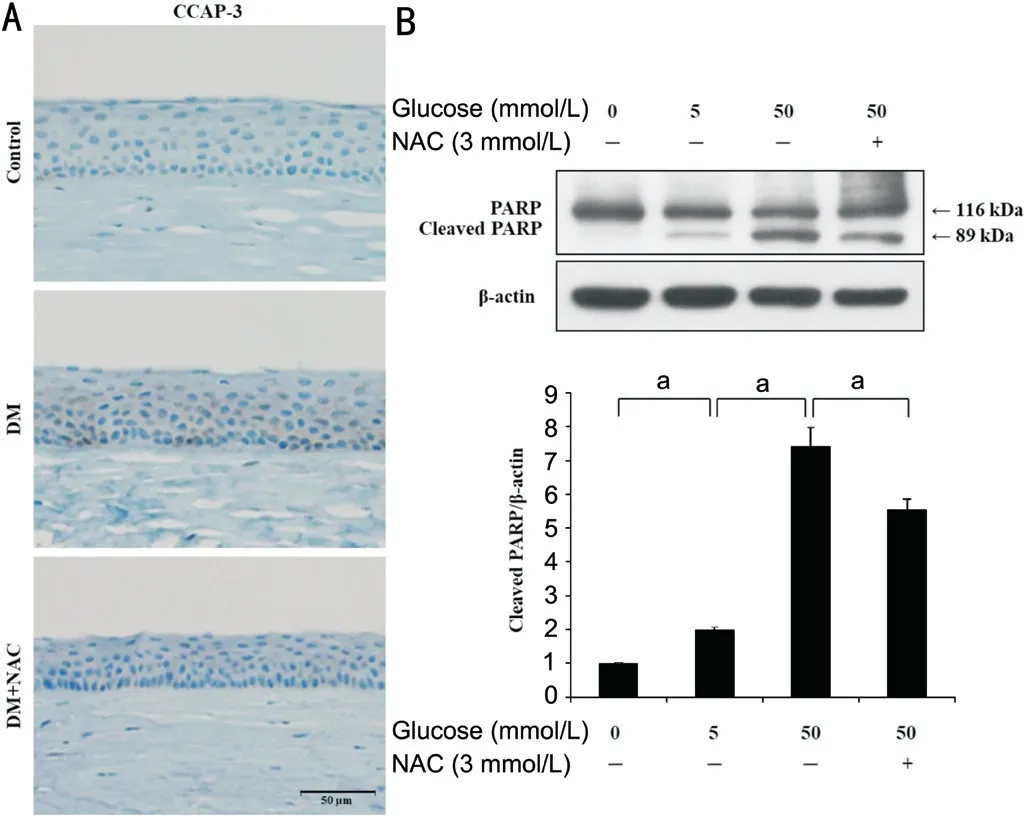
Figure 3 NAC reduces the levels of extrinsic apoptotic factors in diabetic corneal epithelium and HCECs In the corneal epithelium of the STZ induced-diabetic rats that is topically applied with or without 10 μL of 200 μg/mL NAC for 4wk,immunohistochemistry analysis for CCAP-3 was performed. A:Representative immunohistochemistry for CCAP-3 in the corneal epithelium of diabetic rats that were or were not treated with NAC.Representative data of one of three mice are shown. Black scale bar:50 μm. B: Cleaved PARP levels were measured by Western blotting in HCECs subjected to glucose (0, 5, 50 mmol/L) stress±co-treated with NAC (3 mmol/L) for 24h. β-actin was used as a loading control.Representative data for three independent experiments and the quantitative densitometry results are shown. aP<0.05; DM: Diabetes mellitus; NAC: N-acetylcysteine; CCAP-3: cleaved caspase-3; PARP:Poly-ADP ribose polymerase.
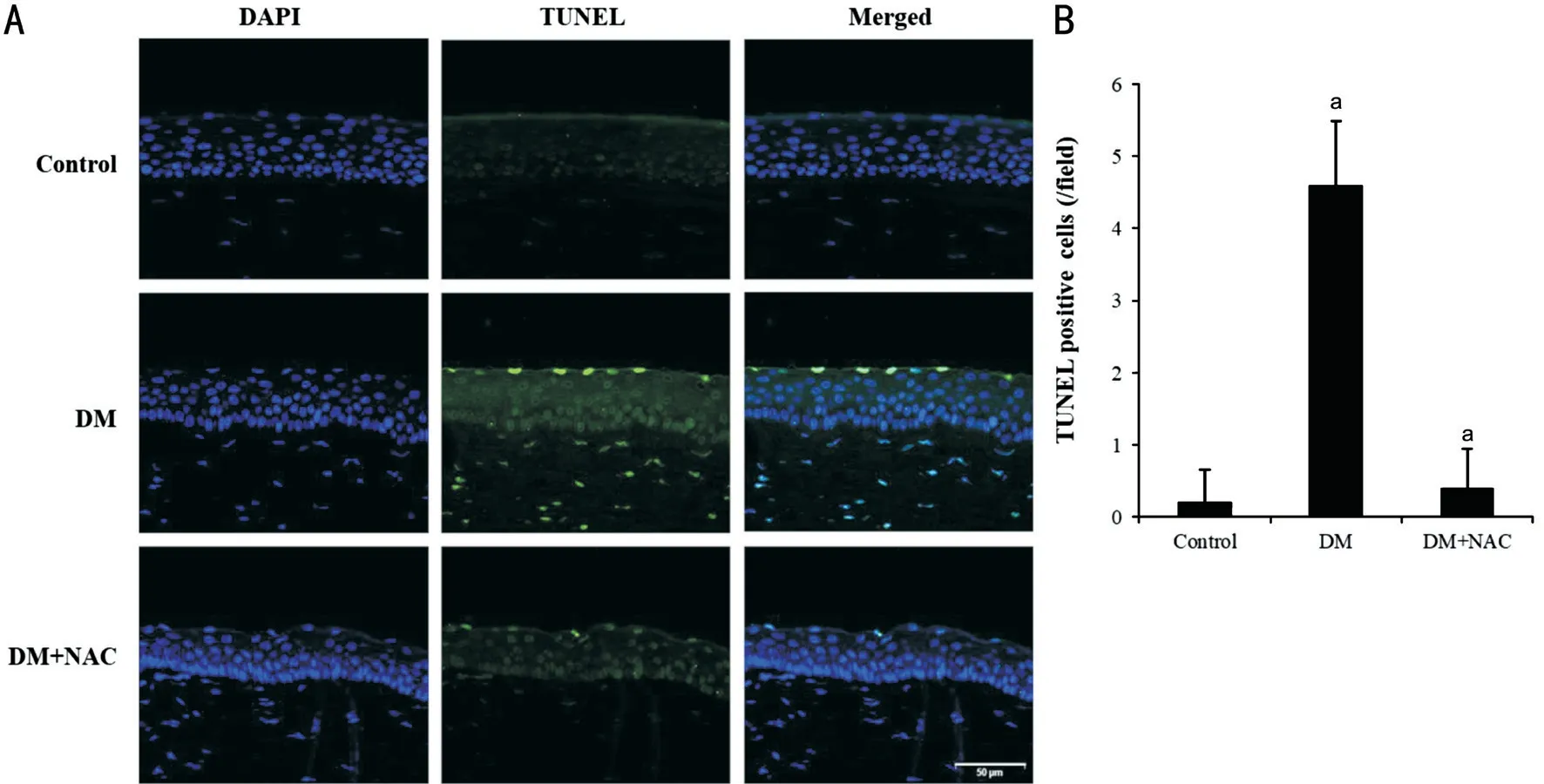
Figure 4 NAC reduces apoptosis in diabetic corneal epithelium Few TUNEL-positive nuclei were detected in corneal epithelial cells of STZ induced-diabetic rats of DM+NAC group were topically applied with 10 μL of 200 μg/mL NAC for 4wk. A: TUNEL assay (green) of corneal epithelium from diabetic rats that were or were not treated with NAC. The nuclei were counterstained using DAPI (blue). Representative images of one of three mice are shown. White scale bar: 50 μm. B: The number of TUNEL-positive cells in epithelium was counted. The field is 0.03 mm2.aP<0.05. DAPI: 4’-6-diamidino-2-phenylindole; DM: Diabetes mellitus; NAC: N-acetylcysteine.
Apoptosis in Rat Corneal Epithelium and HCECs Apoptotic cells were identified in corneal epithelium using the TUNEL assay. Few TUNEL-positive nuclei were detected in the control group (Figure 4), but they were numerous in the corneal epithelium of diabetic rats. Moreover, apoptosis was also apparent in stromal keratocytes. By contrast, very few TUNEL-positive nuclei were present in corneas from the DM+AC group (Figure 4).In the HCECs subjected to glucose stress for 24h, apoptotic cells were identified using flow cytometry. As shown in Figure 5, 17.35%±1.5% of the cells with 5 mmol/L glucose were FITC-positive, whereas 25.85%±1.1% of the cells with 50 mmol/L glucose were positive. However, NAC treatment reduced this to 19.33%±2.15%, which implies that the apoptosis induced by 50 mmol/L glucose is ameliorated by treatment with NAC. Similarly, there were fewer PI-positive cells among those treated with NAC than among those incubated in medium with 50 mmol/L glucose alone. A comparison of the percentage of PI- and FITC-double positive cells showed that apoptotic cells were at the lowest in control group (11.2%)and at the highest in the 50 mmol/L glucose group (21.1%)(Figure 5A).
DISCUSSION
In the present study, we found high levels of RAGE, IL-1β,and CCAP-3 in the corneal epithelium of STZ-induced diabetic rats. However, topical administration of NAC ameliorated the effects of diabetes on these pro-inflammatory and pro-apoptotic factors. Similarly, in HCECs incubated in a high-glucose environment, the expression of the pro-inflammatory cytokine TNF-α and cleaved PARP were upregulated, as was apoptosis.However, co-treatment with NAC reduced the levels of TNF-α and cleaved PARP. Thus, we have identified beneficial effects of NAC in diabetic keratopathy bothin vitroandin vivo.
Hyperglycemia causes the development of biomechanical abnormalities to occur, such as irreversible collagen crosslinking, which is mediated by the accumulation of AGEs in various locations[2,18-20], including vascular epithelial cells[18],corneal nerve fibers, aqueous humor, and cornea[20]. AGEs have been detected in the corneal epithelial cells of diabetic monkeys[21]and human diabetic patients[22]. The interaction of excess AGEs with their receptors activates intracellular signaling pathways that induce the formation of ROS and the release of several cytokines, which is mediated through NFκB activation[1,2,7,23]. Moreover, the accumulation of AGEs in corneal endothelial cells induces apoptosis as a result of oxidative stress[24]. The deposit had also relative with defects in cell migration leads to delayed wound healing[3,6,17,20,24].Consistent with this, in the present study there was higher RAGE expression in the corneal epithelium of diabetic rats than in that of control rats.
The AGE-RAGE system accelerates the development of oxidative stress. ROS are generated by the oxidized NADPH subunits p22phox[6]or p47phox[3]in corneal epithelium.There are numerous lines of evidence that hyperglycemia is associated with greater ROS production[5,25-27]. NAC is an antioxidant that has been shown in many previous studies to reduce free radical concentrations[11,13-15,17], including in highglucose-treated primary rabbit corneal epithelial cells[17]and primary human conjunctival epithelial cells[11]. It has also been shown to reduce the total oxidant status and oxidative stress index of rats in which keratoconjunctivitis is induced by cytosine arabinoside treatment[15]. In addition treatment with either an anti-RAGE antibody or an inhibitor of NADPH oxidase prevents the increase in ROS production[3,6]. These previous findings suggested that NAC would reduce the ROS production in diabetes, and indeed, in the present study, NAC treatment ameliorated the diabetes-associated increase in RAGE expression in rat corneal epithelium.
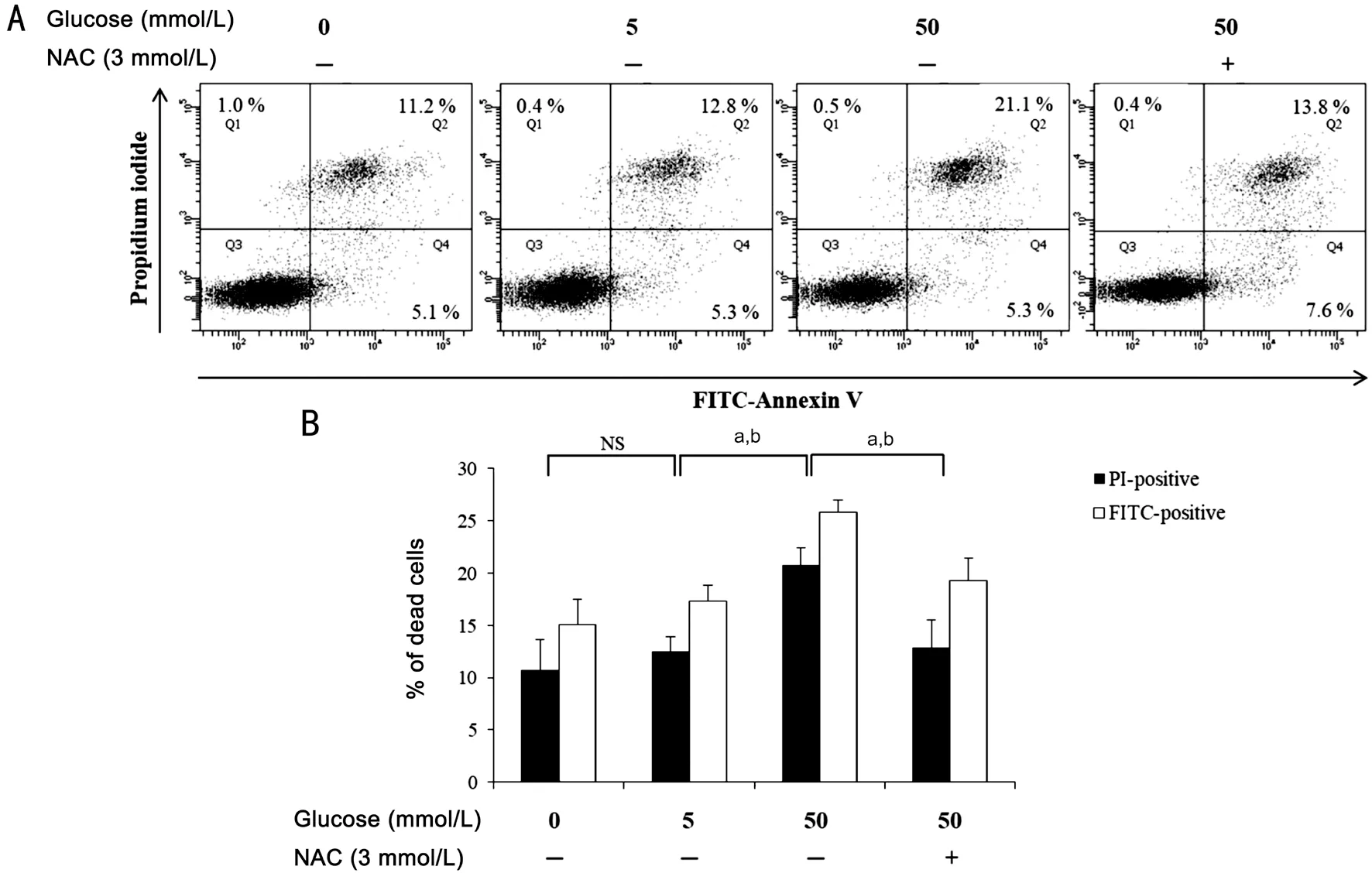
Figure 5 NAC reduces apoptosis in HCECs exposed to high-glucose concentrations and treated with or without NAC Apoptosis analysis involved culturing HCECs in the presence of glucose media (0, 5, 50 mmol/L) with or without NAC (3 mmol/L). A: The level of apoptosis was determined using flow cytometry. B: The percentage of FITC-labeled cells was calculated as the sum of the values in quadrants Q2 and Q4.The percentage of PI-labeled cells was calculated as the sum of the values in quadrants Q1 and Q2. Representative data for three independent experiments are shown. aP<0.05 for PI-positivity; bP<0.05 for FITC-positivity; NS: Not significant; NAC: N-acetylcysteine.
High-glucose concentrations are associated with increases in the production of several cytokines in bothin vitroandin vivomodels. For example, in the High-glucose-treated corneal cells,the expression of IL-1β, TNF-α, IL-10, and IL-4 was high[25,27],and patients with diabetes have been shown to have high levels of TNF-α, IL-6, and IL-8[28]. We found high expression of IL-1β in the corneal epithelium of diabetic rats and that of TNF-α in HCECs. However, NAC has anti-inflammatory effects in diabetic human conjunctival epithelial cells[11]. In the present study, the expression of IL-1β was high in diabetic rat cornea and that of TNF-α was high in HCECs, but NAC treatment ameliorated both of these defects.
It is well known that the inflammation associated with hyperglycemia can result in cellular apoptosis[25,27,29]. Consistent with this, the levels of CCAP-3 and Bax are upregulated[25]and that of blc-2 is downregulated in corneal epithelium exposed to high-glucose[29]. In the present study, we found that the concentration of CCAP-3 was high and TUNEL assay showed that apoptotic cells were more frequent in the corneal epithelium of diabetic rats. In addition, the concentration of cleaved PARP, a marker of apoptosis, was high in highglucose-treated HCECs. However, NAC treatment reduced the concentration of CCAP-3 and the number of apoptotic cells. Previous studies have shown that apoptosis results from the formation of ROS, and the consequent activation of the c-Jun N-terminal kinase/p38 mitogen-activated protein kinase and endoplasmic reticulum stress signaling pathways[6,17].But PARP is a component of the extrinsic apoptotic pathway,whereas the endoplasmic reticulum stress pathways are components of the intrinsic apoptotic pathway; therefore, high glucose may induce apoptosis through both the intrinsic and extrinsic pathways. However, further study is required to test this hypothesis.
The fact that treatment with NAC mitigated the pro-inflammatory and pro-apoptotic effects of hyperglycemia suggests that it might be of therapeutic use. However, it has been shown that high concentrations of NAC induce dry eye disease by inhibiting mucin-16 in an animal model[30]. Therefore,further studies are required to identify the most appropriate concentration of NAC for clinical topical ocular use.
Systemic glucose levels increased after intraperitoneal injection of STZ[7,31]. But we need to identify glucose levels after treatment of topical NAC. Although, corneal NAC presumably is not getting into circulation and improving insulin sensitivity,we think that further studies are necessary to assuring benefits of systemic versus local NAC delivery.
Rats that are administered STZ develop pathology similar to that of type 1 diabetes[28], which develops as a result of a pancreatic insulin deficiency. By contrast, type 2 diabetes is characterized by insulin resistance, and its prevalence is much higher than that of type 1 diabetes[32]. Therefore, the effects of NAC in type 2 diabetes should also be investigated using animal models of type 2 diabetes, such as diet-induced obese ordb/dbmice[28].
It is known that hyperglycemia is associated with inflammation and that the mechanisms involve RAGE signaling and the release of pro-inflammatory cytokines, such as TNF-α, which activate the extrinsic apoptotic pathway and thus caspase-3/PARP signaling. However, in the present study, even though the design of experiment needs some improvement, we have shown that NAC inhibits the activation of these proinflammatory and pro-apoptotic pathways in bothin vitroandin vivomodels of exposure to high glucose. Therefore,NAC may be useful for the management of conditions such as diabetic keratopathy.
ACKNOWLEDGEMENTS
Authors’ contributions: Conception and design: Park JH and Tchah H; analysis and interpretation: Hwang SB, Kang SS, and Park JH; writing of the manuscript: Hwang SB, Park JH, and Chung HS; data collection: Hwang SB and Park JY; literature searching: Hwang SB, Park JH, Kim JY, and Kang SS; critical revision: Kang SS, Park JH, Kim JY, Lee H, and Tchah H; final approval of the manuscript: Tchah H.
Foundation:Supported by a Student Research Grant (2019)from the University of Ulsan College of Medicine, Seoul,Republic of Korea.
Conflicts of Interest:Hwang SB, None; Park JH, None;Park JY, None; Kang SS, None; Chung HS, None; Lee H,
None; Kim JY, None; Tchah H, None.
 International Journal of Ophthalmology2021年12期
International Journal of Ophthalmology2021年12期
- International Journal of Ophthalmology的其它文章
- Upregulation of ASPP2 expression alleviates the development of proliferative vitreoretinopathy in a rat model
- Mesenchymal stem cell-derived exosomes inhibit the VEGF-A expression in human retinal vascular endothelial cells induced by high glucose
- Protective effects of umbilical cord mesenchymal stem cell exosomes in a diabetic rat model through live retinal imaging
- New technique for removal of perfluorocarbon liquid related sticky silicone oil and literature review
- Quantitative analysis of retinal vasculature in normal eyes using ultra-widefield fluorescein angiography
- Evaluation of the long-term effect of foldable capsular vitreous bodies in severe ocular rupture
