Ef fects of weeding and fertilization on soil biology and biochemical processes and tree growth in a mixed stand of Dalbergia odorifera and Santalum album
Peng Zhang · Xiaofei Li · Shiyu Xue · Zhiyi Cui ·Daping Xu · Zengjiang Yang
Abstract In southern China, the eucalyptus plantation industry has been severely restricted by government policy over concerns on negative environmental impacts. In its place, large-scale plantations of high-value tropical tree species such as nitrogen-f ixing Dalbergia odorifera and hemiparasite Santalum album have been widely cultivated including in mixed-species plantations. However, despite their poor growth, little information is available on suitable silvicultural practices of these plantations. Therefore, we subjected an 8-year-old mixed stand of D. odorifera and S.album to weeding, fertilization, weeding + fertilization, or no (CK) treatments and measured soil microbial biomass,respiration, nutrients, nitrogen mineralization and leaching and tree growth and litter production. Weeding and fertilization decreased microbial biomass but increased soil respiration, inhibited mineralization, had not ef fect on leaching of soil nitrogen, and improved the nutrient status of plantation soil. All practices improved the growth of D. odorifera. In the mixed plantation, fertilization increased litter production and nutrient content, but weeding and weeding + fertilization decreased growth of S. album and litter production in mixed plantation because weeding decreased the number of S. album haustoria in underground plant roots. In conclusion, fertilization is recommended; however, weedingrelated practices are inappropriate for D. odorifera and S.album mixed plantations. These conclusions have important implications for managing other parasite or mixed-species plantations.
Keywords Plantation practices · Microbial biomass · Soil respiration · Mineralization · Leaching · Hemiparasite
Introduction
Management practices are applied to improve the production and quality of plantation forests by increasing soil nutrient availability and regulating competition among plants(Erb et al. 2018; Sida et al. 2018). Weeding and fertilization are the most common of these practices around the world for tree species such asPopulusspp.(Pokharel and Chang 2016),Eucalyptusspp.(Carrero et al. 2018),Pinus banksiana(Pokharel et al. 2017),Cunninghamia lanceola(Wang et al. 2008) andPhyllostachys edulis(Li et al. 2016; Song et al. 2020).
Biological and biochemical processes in the soil such as microbial activity and nutrient transformation contribute to maintaining soil ecological functions. Weeding changes energy and nutrient inputs into the soil by decreasing vegetation cover in plantations (Rey et al. 2011; Zhang et al. 2018).Thus, weed af fects not only soil physicochemical properties,such as temperature, water content (?zkan and G?kbulak 2017), pH (Li et al. 2014), and nutrient availability (Rey et al. 2011), but also the quantity and activity of roots and microbes in the soil (Fierer et al. 2012; Allison et al. 2013).
Fertilization is commonly used to increase nutrient content and availability in the soils and thus improve productivity of plantations (Fox 2000). It also can af fect soil biological and biochemical properties processes such as soil microbial biomass, nutrient cycling and respiration rate(Lee and Shibu 2003). Numerous studies have explored the ef fects of fertilizer application, especially nitrogen (N), on soil biological and biochemical processes, but results have been inconsistent; fertilizer application has signif icantly increased soil microbial biomass (Li et al. 2010; Song et al.2020), microbial community diversity (Ramirez et al. 2010)and soil respiration rate (Bowden et al. 2004) and also had negative or neutral ef fects (Samuelson et al. 2009; Sun et al.2011; Wang et al. 2017). The inconsistencies can be due to dif ferences in tree species, plantation age, site conditions and fertilizer content and dosage (Peng et al. 2008). In addition, little is known about the ef fects of combined weeding and fertilization on plantation ecosystems. A better understanding of these ef fects will help better manage plantation ecosystem functions.
In southern China, concerns about negative environmental impacts from eucalyptus plantation industry have led to severe governmental policy restrictions. Instead, high-value tropical tree species such asDalbergia odoriferaandSantalum albumhave been widely grown in large-scale mixedspecies plantations. Both species are renowned for their valuable heartwood and widely used as religious, cosmetic,furniture and medicinal materials (Dhanya et al. 2010; Cui et al. 2019).Santalumspecies are hemiparasites that take up water and nutrients from host plants through haustoria in the roots (Lu et al. 2014). In addition,D. odoriferais a good host forS. albumbecause it is strong nitrogen-f ixer (Lu et al.2017). However, little information is available on suitable cultivation practices for these plantations (Cui et al. 2017).Furthermore, herbicides must be replaced by manual weeding to avoid harmingS. albumbecause of its parasitic characteristics, and intensive farming and fertilization must be implemented in place of extensive traditional management practices. Weeding promotes growth of trees by minimizing neighboring competition, or it may inhibit the growth ofS.albumby decreasing the number ofS. albumhaustoria in plant roots. However, little is known about the mechanism by which weeding and fertilization regulate ecosystem functions in mixed stands ofD. odoriferaandS. album. A better mechanistic understanding of these management practices will help manage forest plantations more ef fectively.
A weeding and fertilization experiment was conducted in a mixed-species plantation ofD. odoriferaandS. albumto study changes in soil microbial biomass, respiration, nutrients, tree growth and litter production. We examined (a)whether weeding decreases and fertilization increase soil microbial biomass and soil respiration, (b) whether weeding and fertilization improve soil nutrients and N transformation,and (c) whether a combined treatment of weeding and fertilization promotes growth of trees over the single and the control treatments.
Materials and methods
Site description
At the experimental plantation in Foshan City, Guangdong,China (22°47′N, 112°32′E), mean annual precipitation is 1681 mm and mean air temperature is 23.4 °C. The dry season from October to March received 18% of the annual precipitation during the study period (Fig. 1).
The mixed-species plantation ofD. odoriferaandS.albumin the study was established in 2009 with a species ratio of 1:1, alternately planted with a spacing 2.5 m × 2.5 m.At the beginning of treatments in April 2017, the survival rate was ~ 94% (750 trees/ha) forD. odoriferaand ~ 88%(700 trees ha ?1 ) forS. album.At 8 years of age,D. odoriferahad a mean DBH of 7.63 ± 1.46 cm and mean height of 6.29 ± 0.87 m andS. albumhad a mean DBH of 7.04 ± 1.51 cm a mean height of 5.53 ± 0.86 m.The soil in the mixed plantation is classif ied as haplic acrisols according to the FAO soil classif ication. Initial data for soil status are shown in Table 1.
Experimental design and sampling
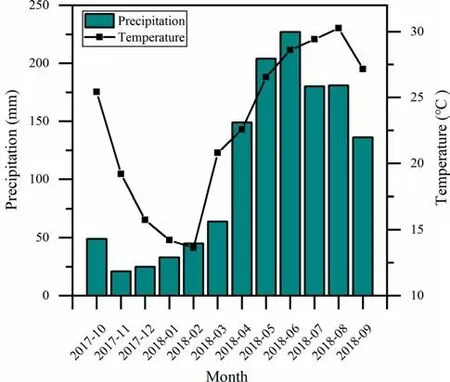
Fig. 1 Mean monthly temperature and precipitation in the experimental area in Foshan City, Guangdong, China from October 2017 to September 2018. Source: Meteorological Bureau
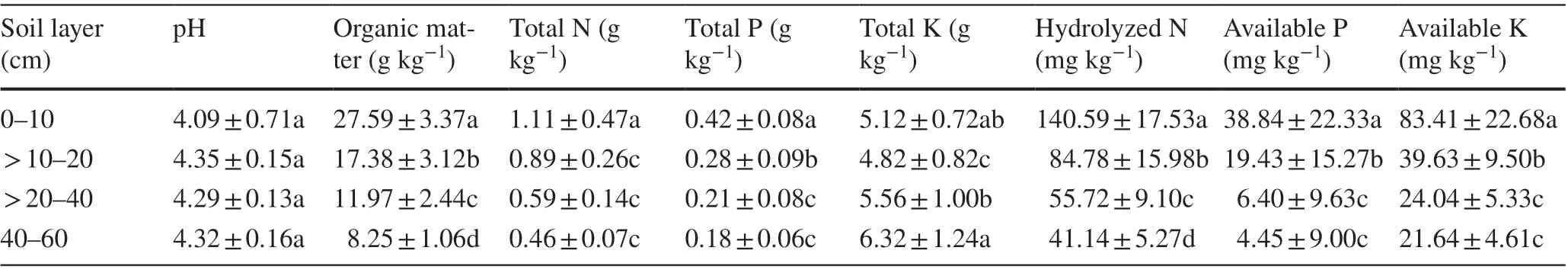
Table 1 Initial soil nutrient status in dif ferent layers in experimental plots in 2017
The experiment was laid out in a randomized complete block design with four treatments (control [CK], weeding [W],fertilization [F], and weeding + fertilization [W + F]) and four replicates. Each of the 16 replicate treatment plots was 400 m 2 . For the weeding treatment, ground vegetation was removed manually with a spade and spread evenly on the ground. For the fertilizer treatment, about 0.5 kg of Norwegian compound fertilizer (N15P15K15, Yara International,Oslo, Norway) was applied to each hole, dug at the center point between two trees. All treatments were carried out twice a year (in April and August). Soil samples were collected from all treatment plots one time per season (summer:June, autumn: September, winter: December, spring: March)in 2017 and 2018. Soil samples were collected at 0–10 cm depth in each plot using a f ive-point sampling method and then stored on dry ice and transported to a laboratory for analysis.
Soil microbial carbon and nitrogen analysis
The chloroform fumigation extraction method (Brookes et al. 1985) was used to analyze soil microbial biomass carbon (MBC) and nitrogen (MBN). A fresh soil sample of 20 g was fumigated with alcohol-free trichloromethane for 24 h, then extracted in 0.5 M K2SO4(1:2.5 w/v). C and N contents were obtained using a TOC analyzer (multi N/C 3100, Analytik Jena, Germany).
Soil respiration measurement
Three polyvinyl chloride (PVC) collars (20 cm diameter and 10 cm height) were inserted 7 cm into the soil on the diagonal of each plot. Soil respiration rates were measured monthly from October 2017 to September 2018, using an LI-8100 automatic soil CO2f lux system (LI-COR, Lincoln,NE, USA). Measurements of soil respiration in all PVC collars were completed between 09:00 and 11:00 h on a sunny day. At the same time, soil temperature (T) and soil moisture (W) at 10-cm depth were measured by a TRIME-PICO TDR probe (IMKO, Ettlingen, Germany). The calculation to determine annual soil CO2f luxes was described by Inoue and Koizumi ( 2012).
Soil nutrient, nitrogen mineralization and leaching determination
Soil samples were homogenized and f ine roots, stones and other materials (> 2 mm) were discarded. Soil pH was measured using a glass electrode pH meter. Ammonium and nitrate nitrogen concentrations were measured using ion-selective electrodes (Greenberg et al. 1985). Available phosphorus was extracted using ammonium hydrochloride and determined using the molybdenum antimony colorimetric method (Olsen and Sommers 1982). Available potassium was determined using atomic absorption spectroscopy (Bahr et al. 2018).
Net N mineralization and leaching rates were measured in situ using a sequential coring technique (Adams and Attiwill 1986; Raison et al. 1987). Five sampling points were selected randomly along the diagonal line of each plot. At each sampling point, three PVC collars (4.6 cm in diameter and 15 cm in height) were hammered into the soil to a depth of 10 cm, and one of the three collars with soil(S0) was collected and taken to a laboratory for the various measurements of the soil. The other two collars were left in situ for 30 days. Another collar had an open top to allow rain to pass through (S1), and the other collar had a covered top and perforations in the upper 5 cm of the sidewall (S2) for ventilation. Net N mineralization was def ined as the increase in ammonium plus nitrate N between (S0)and (S2), and net leaching was calculated between S0and S1. Initial soil samples were collected in June (summer),September (autumn), December (winter) and March (spring)in the following year. Each soil sample was oven-dried, and the moisture content was measured by weighing method. A fresh soil sample of 10 g was mixed with 50 mL of 2 M KCl,shaken for 1 h, and f iltered through f ilter paper. NH4-N and NO2-N + NO2-N concentrations were then measured using automated colorimetry.
Tree growth and haustorial number
Height and DBH of all trees in the 16 treatment plots were measured using a height meter and caliper, respectively,at the start of the experiment and 1 year later. Four 20 cm × 20 cm subplots were selected randomly in each plot within the vertical projection of the crown ofS. album. The roots of all underground plant parts within each subplot were dug out, and the haustoria were counted.
Litter collection and analysis
Litter was collected monthly from October to September during 2017–2018. Three 1 m × 1 m litter traps 50 cm tall were placed across the diagonal of each plot. Litter from the three traps was mixed into one sample, oven-dried and weighed using an analytical scale (0.0001 g) (Souza et al.2019). Total N, P and K of the litter were measured using the Kjeldahl method (Vanlauwe et al. 1996), phosphovanadomolybdate method of Hanson ( 1950) and f lame photometry(Herrera et al. 2008), respectively.
Statistical analyses
A one-way ANOVA and least signif icant dif ference tests were used to determine the statistical signif icance of differences at the 0.05 level in mean soil microbial biomass,soil respiration, soil nutrient, nitrogen mineralization and leaching, litter production and growth increment in height and in DBH (Increment in height or DBH = Height or DBH 1 year after treatment ? Height or DBH before treatment)in response to the weeding or fertilization treatments for each sampling date. All data were tested for homogeneity of variance and normality of residuals before conducting the ANOVA. Data analyses were performed using SPSS 22.0(SPSS Inc., Chicago, IL, USA).
Results
Soil microbial biomass
Microbial biomass showed strongly seasonal variations with the highest in the summer and the lowest in the winter(Fig. 2). MBC and MBN contents dif fered depending on the treatment. In the spring after 1 year of treatment, MBC with weeding, fertilization and weeding + fertilization treatments decreased signif icantly by 34.1%, 27.8%, and 11.3%,respectively, compared with the control treatment (Fig. 2 a).MBN in the weeding and fertilization treatments decreased signif icantly by 37.7% and 47.8% compared with the control(Fig. 2 b). In general, all the treatments signif icantly reduced the soil microbial biomass compared to the controls.
Soil respiration
Soil respiration rate f luctuated from month to month during the experiment; the unimodal curve for each treatment showed a maximum from May to November and minimum from December to April (Fig. 3 a). CO2f lux values peaked in October and ranged from 7.28 to 10.48 μmol m ?2 s ?1 . The CO2f lux ranged from 2.29 to 2.79 μmol m ?2 s ?1 and was lowest in February. During each month, the weeding + fertilization samples generally had the maximum soil respiration rate.
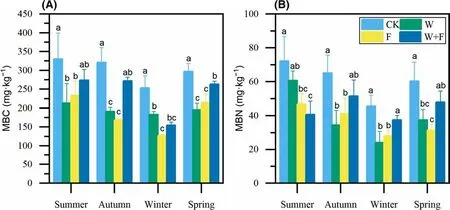
Fig. 2 Mean (± SD) soil microbial carbon ( a, MBC) and nitrogen( b, MBN) in each season after dif ferent cultivation practices. Dif ferent letters above the histobars denote signif icant ( p < 0.05) dif ferences among treatments in the same season as determined by a one-way ANOVA and least signif icance dif ference test; blue lines represent standard deviations ( n = 4). Control (CK: no weeding or fertilization),weeding (W), fertilization (F), and weeding + fertilization (W + F)
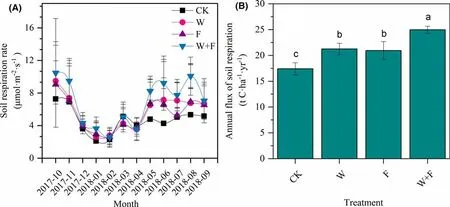
Fig. 3 Monthly dynamics of soil respiration rate ( a) and annual f lux of soil respiration ( b) under dif ferent treatments. Dif ferent letters denote signif icant ( p < 0.05) dif ferences among treatments in a oneway ANOVA and least signif icant dif ference test; bars represents standard deviations ( n = 12). Control (CK, no weeding or fertilizer),weeding (W), fertilization (F)
Compared with the CK, the weeding, fertilization and weeding + fertilization treatments increased the annual f lux in soil respiration by 22.2%, 17.1% and 45.5%, respectively(Fig. 3 b). Thus, compared to CK, all the cultivation practices in this study signif icantly increased the soil respiration.
Soil nutrient, nitrogen mineralization and leaching
Soil pH (4.59–5.03) varied little among treatments throughout the year (Fig. 4 a). The ammonium nitrogen content in the soil peaked in autumn for the three treatments and the CK (Fig. 4 b). Compared with CK, ammonium nitrogen contents in the weeding and weeding + fertilization treatments increased signif icantly by 65.3 and 75.1%, respectively.Unlike ammonium nitrogen, nitrate nitrogen was highest in the spring (Fig. 4 c). Nitrate nitrogen contents for all cultivation treatments were signif icantly greater than for the CK.Over the year, the nitrate nitrogen content for the CK was minimal compared to the three cultivation practices. Available phosphorus was highest in summer (Fig. 4 d), and was signif icantly increased by weeding + fertilization over the four seasons. Available potassium for the three treatments was signif icantly greater than for the CK and was highest with weeding + fertilization (Fig. 4 e). These results indicated that all the cultivation in this study signif icantly improved the nutrient levels of the plantation soil compared to the CK.
Nitrogen nitrification was higher in the spring and autumn, and nitrification rate was lowest in winter(Table 2). In addition, the nitrogen nitrif ication rate was lowest in the weeding treatment (1.39 mg kg ?1 month ?1 ).The CK group had the highest net nitrif ication rate in each season. The ammonium rate was highest in the fertilization treatment in the winter and lowest in the weeding treatment in the autumn. The net nitrogen ammonium rate had signif icant seasonal variation. Soil ammonia rates were lower in the autumn and spring. Ammonium nitrogen accumulated in the summer and winter and was highest in the winter (Table 2). Rates of nitrogen mineralization were higher in the spring and autumn. The leaching rate of soil nitrogen dif fered signif icantly among the seasons,and the mean leaching rate was highest in the autumn, followed by summer, spring and winter (Table 3). Brief ly, the variation patterns in soil mineralization and nitrif ication rates were consistent with variations in the temperature throughout the year.
Compared with CK, the weeding + fertilization, fertilization, and weeding treatments signif icantly reduced annual mineralization by 7.5%, 20.3% and 22.9%, respectively (Fig. 5 a). The annual nitrogen leaching in the weeding treatment was signif icantly lower than in the other treatments; the fertilization and weeding + fertilization treatments did not dif fer signif icantly from the CK(Fig. 5 b). For all four treatments, the annual mineralization was greater than the annual nitrogen leaching. In brief, all cultivation practices signif icantly inhibited mineralization but did not increase leaching of soil nitrogen.
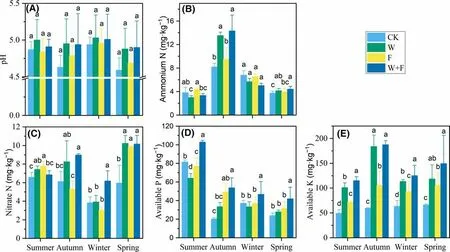
Fig. 4 Soil pH ( a), ammonium nitrogen ( b), nitrate nitrogen ( c),available phosphorus ( d), available potassium ( e) in dif ferent seasons after dif ferent treatments. Dif ferent letters denote signif icant( P < 0.05) dif ferences among treatments in the same season as determined by a one-way ANOVA; error bars represent standard deviations ( n = 4). Control (CK, no weeding or fertilizer), weeding (W),fertilization (F)
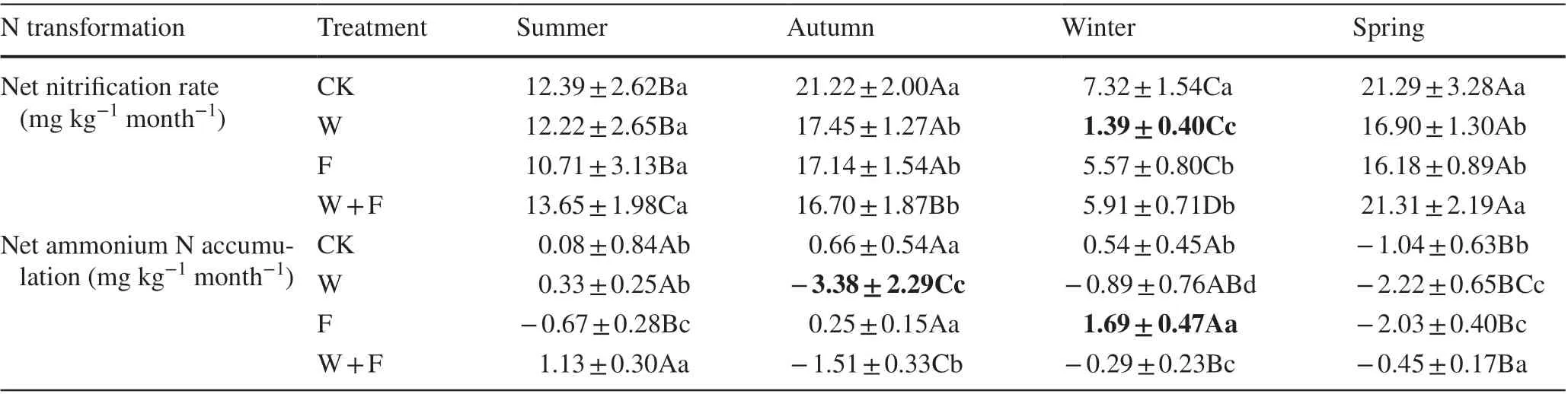
Table 2 Ef fects of dif ferent cultivation practices and seasons on nitrogen nitrif ication and ammonium
Tree growth
No statistically signif icant dif ferences were found among the four treatments with regard to mean height and DBH for either species before treatments started (Table 4).
In the evaluation of increment change in height and DBH (Fig. 6), the greatest increase forD. odorifera(Fig. 6 a) was found in the weeding + fertilization treatment(49.50 cm), followed by fertilization (42.40 cm), weeding(33.65 cm) and CK (27.90 cm). All cultivation treatments signif icantly increased the height increment compared to the CK. The DBH increment varied between 5.55 and 9.11 mm (Fig. 6 c) and was signif icantly higher for the weeding + fertilization and fertilization treatments than in the CK; weeding did not signif icantly af fect the DBH increment. Compared to the CK, all cultivation treatments increased the growth ofD. odorifera.

Table 3 Ef fects of dif ferent tending treatments and seasons on nitrogen mineralization and leaching

Fig. 5 Annual mineralization( a) and annual nitrogen leaching( b) under dif ferent treatments.Dif ferent letters denote signif icant ( P < 0.05) dif ferences among treatments in a one-way ANOVA and the bar represents standard deviation ( n = 4).Control (CK), weeding (W),fertilization (F)
Table 4 Initial mean height and diameter at breast height of individuals of Dalbergia odorifera and Santalum album when treatments started
UnlikeD. odorifera,S. albumattained the greatest height increment in the fertilization treatment (Fig. 6 b). Compared with the CK, the weeding and weeding + fertilization treatments led to signif icantly lower changes in height increment (30.03 and 23.6%, respectively). However, fertilization increased the height increment by 23.2%. The DBH increment with weeding was signif icantly lower than that in the CK, whereas fertilization and weeding + fertilization treatments did not dif fer signif icantly from the CK (Fig. 6 d).Compared to the CK, fertilization signif icantly increased and weeding signif icantly reduced the growth ofS. album.
Haustorium number
The number ofS. albumhaustoria in plant roots after the various treatments is shown in Fig. 7. Signif icantly more haustoria formed in the fertilization treatment than in the other treatments, up to 2.94, 9.65, and 181.83 times as much as CK, weeding + fertilization, and only weeding, respectively. However, the haustorial number was much lower in the weeding and the weeding + fertilization treatments compared to CK. Thus, fertilization increased haustorial production, but weeding reduced haustorial production.
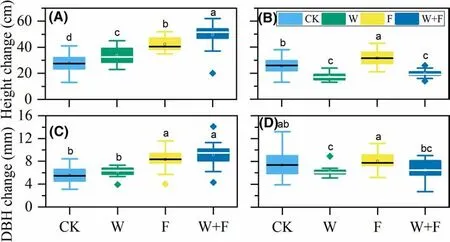
Fig. 6 Height increment for Dalbergia odorifera ( a) and Santalum album ( b) and DBH variation for D. odorifera ( c)and S. album ( d) under dif ferent treatments. Dif ferent letters denote signif icant ( P < 0.05)dif ferences among treatments in a one-way ANOVA and least signif icant dif ference test; bars represent standard deviations( n = ~ 120). Control (CK, no weeding or fertilizer), weeding(W), fertilization (F)
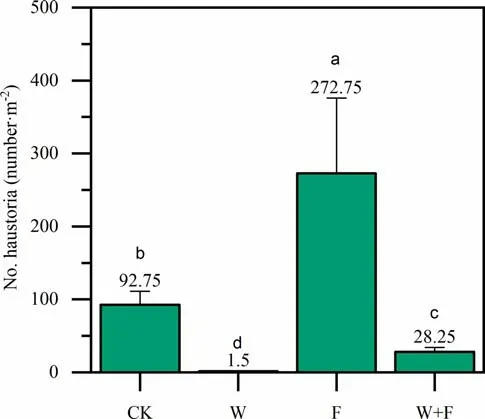
Fig. 7 Number of Santalum album haustorium per square meter of soil in roots of Dalbergia odorifera under dif ferent treatments. Dif ferent letters denote a signif icant ( p < 0.05) dif ference among treatments in a one-way ANOVA and least signif icant dif ference test; bars represent standard deviations ( n = 16). Control (CK), weeding (W), fertilization (F)
Litter production
Total litter biomass dif fered signif icantly among treatments,ranging from 6.71 to 10.38 t ha ?1 a ?1 (Fig. 8 A). Compared with levels in the CK, total litter biomass increased by 26.9%with fertilization, but decreased by 6.6% with weeding + fertilization and 18.0% with weeding.
Annual total nutrient content of litter varied among treatments (Fig. 8 b). On average, the annual litter nutrient content was in the order nitrogen (142.10?246.45 kg ha ?1 a ?1 ) > potassium (65.62–127.61 kg ha ?1 a ?1 ) > phosphorus(8.38–14.99 kg ha ?1 a ?1 ). The highest content of total litter nutrient was in the fertilization treatment(389.04 kg ha ?1 a ?1 ), whereas values in the fertilization and weeding + fertilization treatments did not dif fer signif icantly compared to the CK (Fig. 8 b). Thus, compared to the CK,fertilization signif icantly increased the amount of litter and nutrient return, whereas weeding reduced the litter production of the mixed plantation.
Discussion
Ef fects of weeding and fertilization on soil biology process
In this mixed-species plantation, cultivation practices decreased microbial biomass, but increased soil respiration,indicating that both weeding and fertilization signif icantly af fected soil biological processes. Soil microbial biomass has an important role in nutrient cycling and is therefore essential for plant growth, which is very sensitive to environmental factors (Fliessbach et al. 1994). Compared with CK, weeding and fertilization decreased soil microbial biomass in line with previous f indings (Stewart et al. 2018;Sun et al. 2018). The ef fects of fertilization on soil communities depend heavily on the contents of soil nutrients(Allison and Martiny 2008). In N-limited soil, N addition will directly increase the microbial populations and activity(Hobbie and Vitousek 2000; Compton et al. 2004). On the contrary, N addition will decrease soil microbial biomass in the soils of N saturation and even N inhibition (Guo et al.2017). Additionally, weeding reduced microbial biomass as a result of lower input of organic matter into the soil (Wardle et al. 1999).
Soil respiration rates in the dif ferent cultivation practices measured in this study were greater than in the CK,and weeding + fertilization generally yielded the highest rate. Total soil respiration consists of autotrophic and heterotrophic components (Wang et al. 2017). Autotrophic respiration is mainly from plant roots, and heterotrophic respirations is primarily from decomposition of organic matter by soil microbes (Zhao et al. 2018). Weeding and fertilization af fect soil respiration through stimulating plant growth and altering microbial biomass and activity (Olsson et al. 2005; Allison and Martiny 2008). After our weeding treatments, the plant residues were spread evenly on the surface of the plantation soil, which allowed more organic matter to be returned to the soil and degraded. Because fertilization accelerates nutrient availability and improves root growth, the rate of root respiration increased in this study. These results are in line with those reported by Zhu et al. ( 2016), Nguyen and Marschner ( 2017) and Spohn and Schleuss ( 2019). Therefore, cultivation practices decreased soil microbial biomass, but increased soil respiration. They also promoted root growth and microbial activity and degradation ability, ultimately increasing root and microbial respiration.
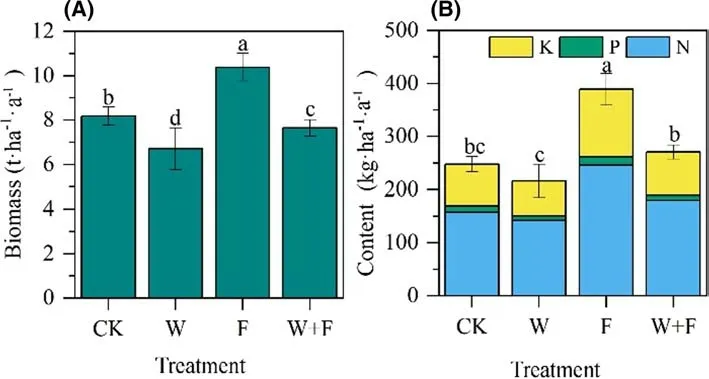
Fig. 8 Annual litter production ( a) and nutrient content( b) under dif ferent treatments.Dif ferent letters denote signif icant ( P < 0.05) dif ferences among treatments in a one-way ANOVA and least signif icant dif ference test; bars represent standard deviation ( n = 4). N:nitrogen; P: phosphorous; K:potassium. Control (CK), weeding (W), fertilization (F), and weeding + fertilization (W + F)
Ef fects of weeding and fertilization on soil biochemical processes
In our study, all cultivation practices basically improved plantation nutrients. Soil nitrogen mineralization was inhibited, and leaching was not increased by the practices. Weeding and fertilization can improve sustainable and ef ficient use of nutrient of plantation land (Zhou et al. 2018). On the one hand, fertilization directly adds nutrients to the plantation soil (Bom et al. 2019), and plant residues left behind after weeding accelerate nutrient return to the soil (Tanaka et al. 2012). Consistent with the reduction in microbial populations by the cultivation practices in this study, the process of mineralization of soil nitrogen was inhibited, and leaching was not increased. Zhang et al. ( 2017) concluded that carbon/energy resources decrease with loss of plant diversity,thus leading to reduced soil microbial diversity. Therefore,in this study, weeding could inhibit soil nitrogen processes by reducing the microbial population. Furthermore, weeding + fertilization inhibited soil nitrogen processes less than weeding did because the combined practice added more nutrients required for microbial process.
The variations in soil mineralization and nitrif ication rates were consistent with the temperature variations throughout the year. Soil temperature is positively correlated with total nitrogen mineralization (Li et al. 2020). When the soil is full of water in the wet season, net nitrogen mineralization decreased. Therefore, soil mineralization rates were higher in the spring and autumn.Overall, cultivation practices inhibited mineralization but did not increase nitrogen leaching from the soil, and the nutrient status in the plantation soil improved. The cultivation practices were thus benef icial for preserving soil fertility and accumulating nitrogen.
Ef fects of weeding and fertilization on tree growth of D.odorifera and S. album
ForD. odorifera, all cultivation practices promoted higher growth compared to CK. Generally, weeding and fertilization can increase growth rates by controlling competing vegetation and increasing inputs to raise the availability of nutrients (Fox et al. 2007; Campoe et al. 2014). In many of these stands, fertilization will increase plant growth by increasing leaf area. ForS. album, unlike forD. odorifera, growth was signif icantly increased by fertilization and decreased by weeding. These results are consistent with fertilization increasing and weeding decreasing the number ofS. albumhaustoria in plant roots in this study. Although weeding removed nutrient-competing vegetation, it also reduced the nutrient sources in the host. Weeding promoted the growth ofD. odorifera, while reducing the growth ofS.album, perhaps due to the decrease in the number of haustoria that resulted in the weeding + fertilization and weeding treatments. Thus, the combined treatment of weeding and fertilization did not promote the expected higher growth compared to either of the single treatments or the lack of treatments (CK).
Inputs from litter, a major carbon and nutrient source,represent important components in the biogeochemistry in forest ecosystems (Attiwill et al. 1978). Changes in plant growth can also lead to the change in litter; the faster a plant grows, the more litter it produces (Belovsky and Slade 2000). In our study, fertilization signif icantly increased the amount of litter and nutrient return. The growth of trees increased rapidly with increased soil nutrients after fertilization as did the herbaceous shrubs; thus,S. albumobtained more nutrients from these herbaceous hosts for its growth(Xu et al. 2011). Conversely, the weeding treatment signif icantly reduced the growth ofS. album, resulting in decreased litter production.
In summary, the ef fects of the cultivation practices on the growth and litter production ofD. odoriferaandS. albumwere inconsistent. Fertilization signif icantly increased the growth of trees and the amount of litter and nutrient return,improving production in the mixed plantation ofD. odoriferaandS. album. However, weeding-related practices decreased the growth of trees and litter production in the mixed plantation by reducing the number ofS. albumhaustorium in roots. Considering the short duration of the study,we need to continue monitoring tree growth for a few more years to determine whether these relationships continue over time.
Conclusion
In this mixed plantation, the ef fects of weeding and fertilization on soil biological processes are ref lected in the reduction of microbial biomass and the increase of soil respiration. Weeding and fertilization inhibited mineralization but did not increase leaching of soil nitrogen, and nutrient status of the soil improved. Thus, these cultivation practices will help preserve soil fertility and the aid nitrogen accumulation.Inconsistent with non-parasitic plantations, the combined treatment of weeding and fertilization did not promote better growth compared to the single and control treatments. Cultivation practices improved the growth ofD. odorifera, but weeding and weeding + fertilization decreased the growth ofS. albumand litter production in the mixed plantation because weeding decreased the number ofS. albumhaustoria in roots. In conclusion, fertilization is recommended,but weeding practices are inappropriate forD. odoriferaandS. albummixed plantations. These f indings hold important implications for management practices for other parasite or mixed plantations.
Author contributions PZ wrote the manuscript. XFL and SYX collected data. ZJY and DPX developed the study design. ZYC provided expert knowledge used in the writing and revision of the manuscript.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
 Journal of Forestry Research2021年6期
Journal of Forestry Research2021年6期
- Journal of Forestry Research的其它文章
- Genome-wide identif ication and cold stress-induced expression analysis of the CBF gene family in Liriodendron chinense
- Characterization and expression analysis of genes encoding Taxol biosynthetic enzymes in Taxus spp.
- Critical ef fects on the photosynthetic ef ficiency and stem sap f low of poplar in the Yellow River Delta in response to soil water
- Floristic composition and structure of the Kibate Forest along environmental gradients in Wonchi, Southwestern Ethiopia
- Inf luence of soil microorganisms and physicochemical properties on plant diversity in an arid desert of Western China
- Metabolic diversity and seasonal variation of soil microbial communities in natural forested wetlands
