Study on Synergistic Effect and Mechanism of Sunscreen Bemt and Mbbt
Yang Yunyun
JALA Group Co.,China
Abstract The synergistic effect and mechanism of two chemical sunscreens,BEMT and MBBT,have been studied.UV absorption spectrum study suggested that UV absorption peaks of BEMT and MRRT shifted when the polarities of solvents varied.In ethanol,a typical semi-polarity solvent,UV absorption capacity of BEMT was reduced compared to its capacity in a non-polarity solvent of isooctane.While the addition of MBBT can offset the impact of solvent polarity.In terms of emulsions with BEMT as the only sunscreen agent,in vitro test indicated that SPF increased as the solvent polarity rose,and the addition of MBBT can offset this impact.Sunscreen efficient can be significantly enhanced via combining water phased and oil phased sunscreen agents.
Key words Chemical sunscreen;BEMT;MBBT;Synergistic effect
As the prevalent awareness of importance of UV protection in recent years,there is an increasing demand of sunscreen products globally.According to statics,over 70% of marketed sunscreen products feature a SPF value higher than 30.Consumers,especially those from Asia,have specific requirements of SPF.Besides,they also pursue sunscreen products which are fresh and non-greasy.However,sunscreen products with high SPF usually contain various sunscreen agents at high dosages,because it is quite difficult for one sunscreen agent to deliver a broad-spectrum protection.Moreover,limited kinds of sunscreen agents and varied regulatory restrictions in different countries pose challenges to formulators.Synergistic effect can boost sun-blocking efficacy,thus achieve higher SPF at lower dosages of sunscreen agents.This means that sunscreen systems with synergistic effect can probably meet consumer needs and at the same time,comply with regulations.That’s why more attention has been paid on the studies of synergistic effect[1,2],and much scientific research have been conducted in this area[3,4].
Every sunscreen agent has its distinctive UV absorption range.BEMT and MBBT are two novel chemical sunscreen agents with broad spectrum.BEMT is an oil soluble sunscreen agent,while the most popular commercialized type of MBBT is a non-aqueous-nonoleaginous particle dispersion with the particle diameter of less than 200 nm (active content around 50%).BEMT and MBBT have always been included in the same system,but their synergistic effect hasn’t been studied yet.This research has studied the synergistic effect of BEMT and MBBT with UV-visible spectrophotometer and in vitro SPF analyzer,and mechanism of this effect has also been explored.
1 Experiment
1.1 Instrument and reagent
Spectrophotometer UV WIN 5 UV (Beijing Purkinje General Instrument Co.,Ltd.);Ultrasonic cleaner (Kun Shan Ultrasonic Instruments Co.,Ltd);Analytical balance (Sartorius Scientific Instruments(Beiing) Co.,Ltd.);Ultraviolet transmittance analyzer UV-2000s (Labsphere);PMMA plate (Beijing JInghongfan Trading Co.,Ltd.).
Deionized water (lab);Anhydrous alcohol (AR,Sinopharm Chemical Reagent Co.,Ltd.);Isooctane(AR,Sinopharm Chemical Reagent Co.,Ltd.);Steareth-21 (CG,BASF);Bis-ethylhexyloxyphenol methoxyphenyl triazine (CG,BASF);Bisethylhexyloxyphenol methoxyphenyl triazine (and)Acrylates/C12-22 alkyl methacrylate copolymer(CG,BASF);Water/Methylene bis-benzotriazolyl tetramethylbutylphenol/decyl glucoside/Propylene glycol/Xanthan gum (CG,BASF);Caprylic/Capric triglyceride (CG,BASF);Dicaprylyl carbonate (CG,BASF);Disodium EDTA (CG,Nouryon Chemicals International B.V.);Glycerin (CG,SOCC);C14-22 alcohols/C12-20 alkyl glucoside/Water/Glucoside(CG,Seppic);C12-15 alkyl benzoate (CG,Evonik);Phenoxyethanol (CG,Dow).
1.2 Sample preparation
Samples have been prepared with formulas listed in Table 1.Preparation process was as follows:Phase A and phase B were heated to 80~85 ℃ respectively.Both phases were stirred until fully dissolved.Added phase B into Phase A for emulsification at 75~80 ℃.Homogenized the emulsion for 5min and cooled it down to 40~45 ℃.Added phase C and phase D.Continued stirring and cooling down the emulsion to below 38 ℃.Discharged the sample for testing.
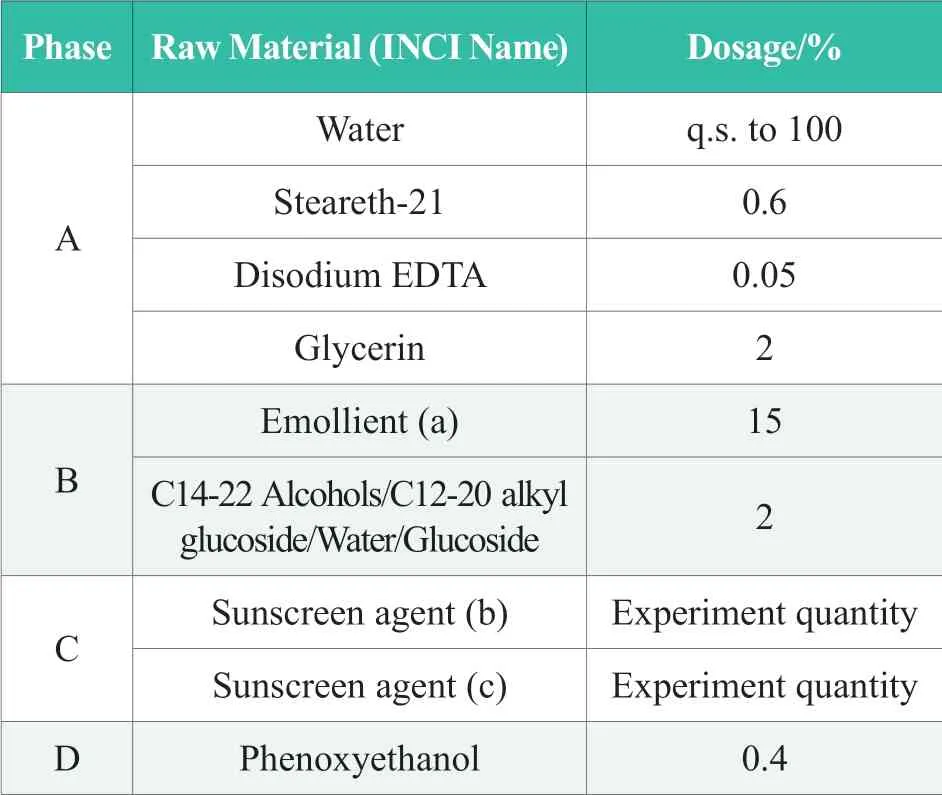
Table 1.Formulation for test samples
1.3 Instrumental analysis
1.3.1 UV-visible spectrophotometric analysis
The sample solution was prepared by dissolving 0.0600 g sample formulated as Tab.1 with ethanol or isooctane in a 50 mL volumetric flask.The volumetric flask was then put in the ultrasonic cleaner for 30 min.Diluted the solution with the solvent (ethanol or isooctane) as solution 1.1 mL Solution 2 was prepared by diluting solution 1 with the solvent (ethanol or isooctane)till its concentration becomes one tenth/one twentieth of the original value in a volumetric flask.Added solution 2 in 1 cm quartz cell as a test sample.The absorbanceconcentrations calibration curves of the blank sample and the absorbance-concentrations calibration curves of test samples were scanned at 250~450 nm.
1.3.2 In vitro SPF test
(0.027 6±0.000 3) g Sample prepared as Table 1 was transferred and spotted on PMMA plate (5 cm×5 cm) with a pipette.The sample was spread evenly by the forefinger wearing a disposable finger-cot within specific play time.The PMMA plate was then kept away from light and dried for 30 min.Then tested the SPF value of each sample by UV-2000s.Each test sample was applied on three plates,and each plate was tested in 5 different areas.
2 Results and discussions
2.1 The identification of Test Method
Currently the most popular SPF tests include in vivo and in vitro methods.Although in vivo test is known as the ‘golden standard’ because of its reliability,it is quite time-consuming and expensive.Therefore,it is not suitable for fundamental research.In vitro test generates data by instruments,such as spectrophotometer and SPF analyzer whose mechanism is based on ultraviolet transmittance theory.Besides,simulation calculators,such as DSM SUNSCREEN OPTIMIZER?,are also frequently recommended for SPF estimation.In vitro tests are widely used to evaluate the UV absorption capability of sunscreen agents.Featuring simple operation,the test data of in vitro tests are still relevant,particularly for chemical sunscreen agents,though these data are sometime not consistent with the data of in vivo tests.So they are of vital importance for raw material study and formula design of sunscreen products[5,6].
Three o/w chemical sunscreen products with in vivo SPF data were tested by spectrophotometer and ultraviolet transmittance analyzer to verify the feasibility of in vitro tests in this study.Samples to be tested by spectrophotometer were diluted with ethanol till its concentration becomes one twentieth of the original value.Then test samples were scanned at 290~320nm (UVB).The maximum UV absorbance Aλmax,invro SPF value tested by ultraviolet transmittance analyzer UV-2000s and simulated SPF value calculated by DSM SUNSCREEN OPTIMIZER? of each sample were listed in Table 2.
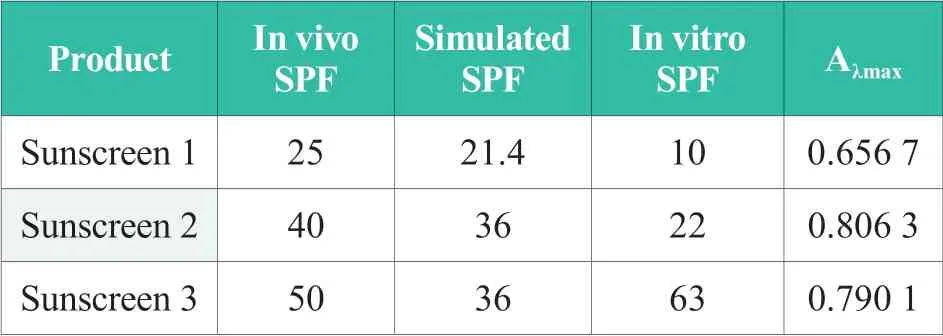
Table 2.SPF value in vivo,simulated,in vitro and Aλmax of three sunscreens
In comparison,sunscreen products with distinct in vivo SPF values shows nearly the same simulated SPF values and Aλmax(sunscreen 2 and 3).In order to evaluate the linkage among in vivo SPF values,simulated SPF values and in vivo SPF values,linear analysis of these data and Aλmaxwere conducted as Figure 1.As shown in Fig.1,there was a significant liner correlation between Aλmaxand simulated SPF values withR2>99%.While the liner correlation between Aλmaxand in vivo SPF values was weak withR2≈50%.Given that the simulation calculator generated SPF values on the basis of the addition of sun block capability of individual sunscreen agent,the close simulated SPF values of sunscreen 2 and 3 indicated that the sun block efficiencies of them are almost the same,so Aλmaxof them were also quite consistent.The analysis of SPF value difference between sunscreen 2 and 3 was as follows.The sun block capability of sunscreen 2 mainly came from sunscreen agents themselves.While in sunscreen 3,both sunscreen agents and other boosters,including triacontanyl PVP,ammonium styrene/acrylates copolymer,etc.,contributed to its sun block capability[7,8].
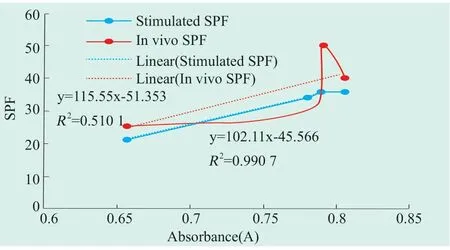
Figure 1.Correlation between SPF value in vivo and Aλmax
This feasibility analysis indicated that although spectrophotometric test could evaluate UV absorption capability of a sunscreen agent,it couldn’t evaluate the synergistic effect of other boosters,such as a film former.
The correlation analysis of in vitro SPF value and in vivo SPF value is illustrated in Figure 2.TheR2of 0.833 shown that there was some extent of correlation between them.
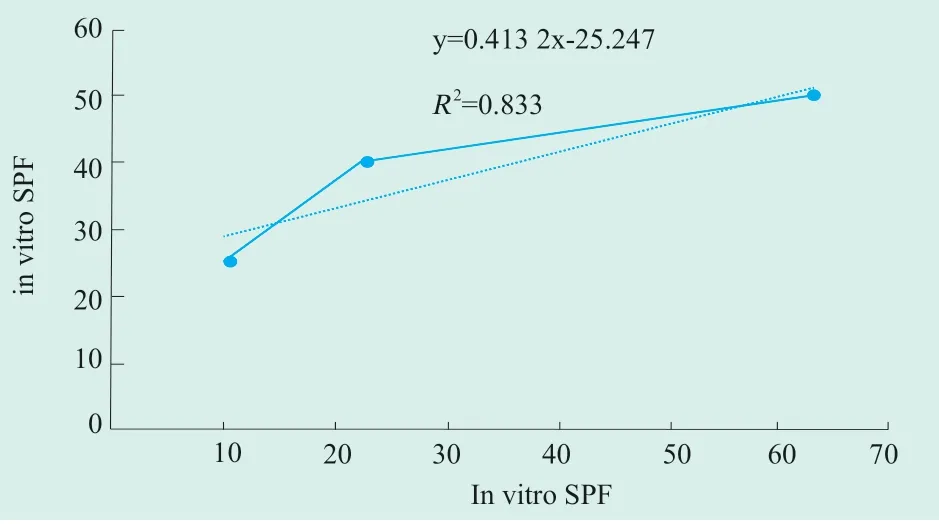
Figure 2.Correlation between SPF value in vivo and in vitro of UV-2000s
These experiments suggested that Aλmaxindicated UV absorption capability of a sunscreen agent,and in vitro SPF value shown some extent of correlation with in vivo result,although sometimes they are not completely consistent.In our research work,sun block capabilities of BEMT,MBBT and their combos were studied,so UV-visible spectrophotometric analysis and in vitro test were applied.
2.2 In vitro test of synergistic effect of sunscreen agents
According toSafety and Technical Standards for Cosmetics(2015 Edition),the upper limit of BEMT and MBBT is 10% respectively[9].Fifteen formulation options were designed for test samples (Table 1) to study the synergistic effect of BEMT and MBBT (Table 3).All test samples were prepared as the process listed in 1.2,and uv-visible spectrophotometric analysis and in vitro test were conducted on all samples.
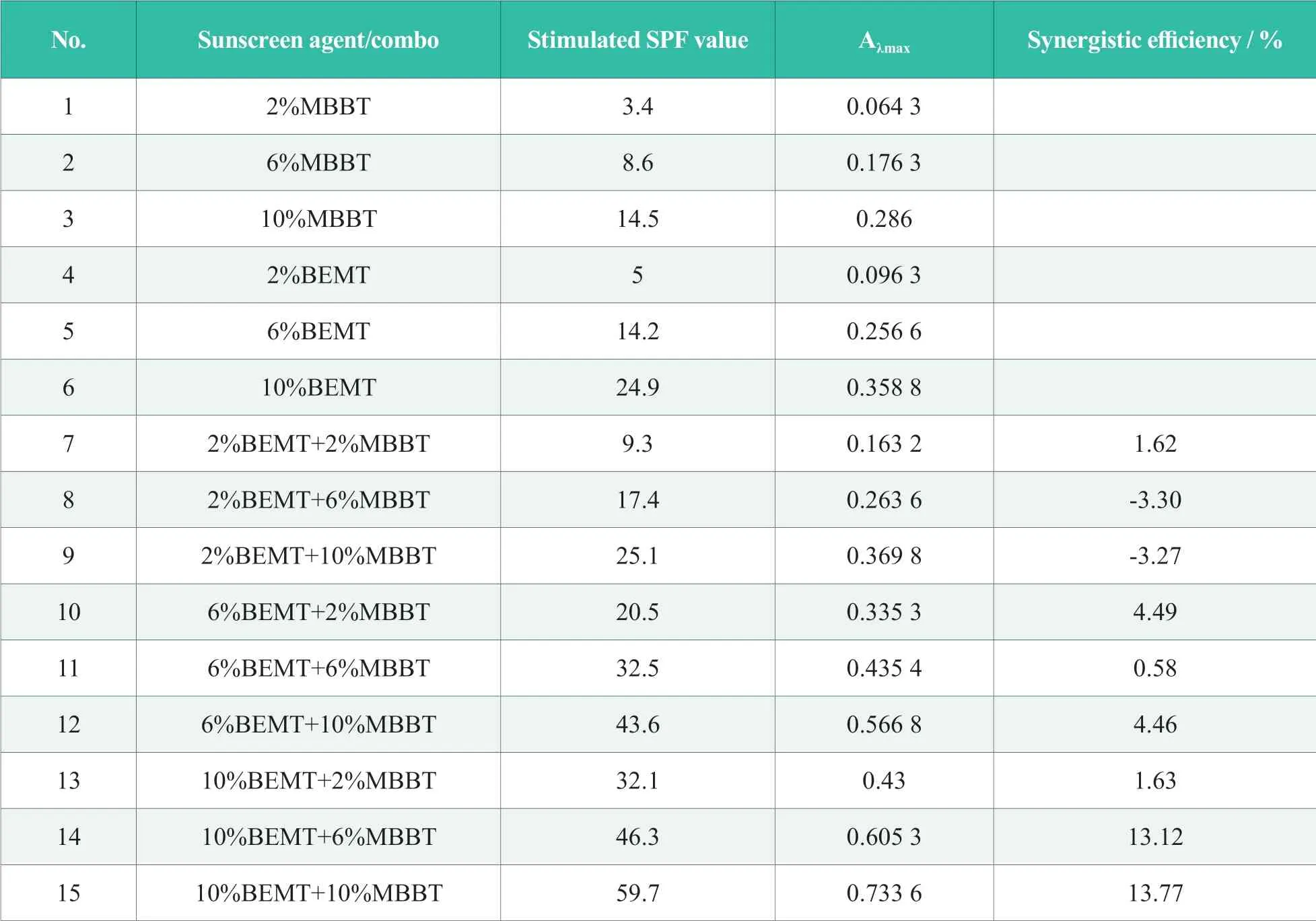
Table 3.UV results for 15 sunscreen regimens and synergistic efficiency (ethanol)

2.2.1 UV-Visible spectrophotometric analysis
Louise etc.[10]has proven that solvent polarity has an impact on Aλmaxand ? of sunscreen agent by UV-visible spectrophotometric analysis.The maximum absorption wavelength of highly polar sunscreen agent shown blue shift in polar solvent,while the maximum absorption wavelength of lowto-moderate polarity sunscreen agent shown red shift in polar solvent.The shift of the maximum absorption wavelength might have an impact on sun block capability.
Ethanol as a moderate-polarity solvent and isooctane as a low polarity solvent were selected in this study.Diluted the samples of BEMT and MBBT by solvent (ethanol or isooctane) till its concentration becomes one twentieth of the original value to make the test samples.Then,test samples were scanned at 250~450 nm and spectrums were shown in Figure 3.These spectrums indicated that BEMT had two absorption peaks in each solvent,with its Aλmaxof 342 nm and 355 nm in ethanol and isooctane respectively.Aλmaxof 311 nm and 305 nm in ethanol and isooctane correspondingly featured another absorption.Similarly,BEMT also had two absorption peaks,with its Aλmaxof 346 nm and 351 nm in ethanol and isooctane respectively.And another absorption peak had its Aλmaxof 304 nm both in ethanol and isooctane.Aλmaxinformation suggested that in UVB range(290~320 nm),as the polarity of solvent increased,Aλmaxof BEMT made a red shift;while in UVA range(320~400 nm),as the polarity of solvent increased,Aλmaxof BEMT and MBBT made a blue shift.
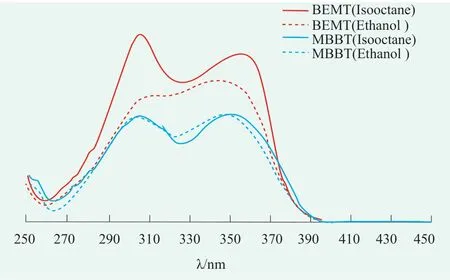
Figure 3.UV-absorption spectra of MBBT and BEMT in ethanol and isooctane
BEMT,one of the novel broad-spectrum sunscreen agents as a derivative of hydroxyphenyl triazine,exhibits a strong absorption capability of UVB.Because of the absorption capability of UVA contributed by its hydroxyl group,BEMT has two absorption peaks.The triphenyl-trizine part of BEMT which contributes to its UVB absorption has a low polarity,so the polarity of its excited state is higher that its of ground state.That’s why its excited state is more stable in ethanol which is a moderate polarity solvent and makes a red shift.The Coplanar orientation of hydroxyphenyl group and triazine ring in BEMT molecule contributes to its UVA absorption.The strength of coplanar orientation depends on the hydrogen bond between nitrogen atom in triazine ring and hydroxyphenyl group.The polarity of solvent might exert an impact on the strength of this hydrogen bond by twisting hydroxyphenyl group and triazine ring.Higher polarity of solvent means more energy needed to return to the original coplanar orientation and blue shift has been witnessed[11,12].
As a sunscreen featuring a structure of o-hydroxybenzotriazole,the UVA absorption of MBBT comes from its benzotriazol group,and its ortho hydroxyl structure contributes to the UVB absorption,showing two absorption peaks in UV range[13].Similar to BEMT,polarity of solvent has an impact on the strength of hydrogen bond between benzotriazol group and ortho hydroxyl group.So as the polarity of solvent increases,blue shift has been witnessed.
UV-visible spectrophotometric analysis of 15 samples listed in Table 3 were conducted as follows.Diluted the samples with ethanol till its concentration becomes one twentieth of the original value to make the test sample.Then,test samples were scanned at 250~450 nm and Aλmaxin UVB range was recorded and listed in Tab.3.Data analysis shown that the liner correlation between Aλmax and stimulated SPF value wasY=0.011 5x+0.069 withR2of 0.985,indicating a good correlation.The synergistic effect between BEMT and MBBT was assessed by synergistic efficiency(Synergistic efficiency (%)=[Aλmax(BEMT+MBBT)-(Aλmax(BEMT)+ Aλmax(MBBT))]/ (Aλmax(BEMT) +Aλmax(MBB))×100%).The figures of synergistic efficiency suggested that there were little synergistic effect between these two sunscreen agents when their dosages were low.When dosages were increased to 10%BEMT+6%MBBT and 10%BEMT+10%MBBT,synergistic effects were quite significant with synergistic efficiency over 10%.As in UV-visible spectrophotometric analysis,test dosages of sunscreen agents were diluted to one thousandth of the original value,so it might be difficult to observe synergistic effect at such low level.When dosages of sunscreen agents were increased,remarkable synergistic effects were observed,indicating that the higher the dosages of sunscreen agents might result to more significant synergistic effect.
2.2.2 In vitro test by UV-2000s
The low concentration of sunscreen agents in test samples caused by the dilution process of UV-visible spectrophotometric analysis imposed difficulties on the observation of synergistic effect.So some combos were selected and SPF value of these systems were tested by UV-2000s in order to comprehensively evaluate the synergistic effect between BEMT and MBBT,as shown in Table 4.The synergistic efficiency data suggested that there was a synergistic effect between BEMT and MBBT,and the higher the dosages of sunscreen agents might result to more significant synergistic effect.
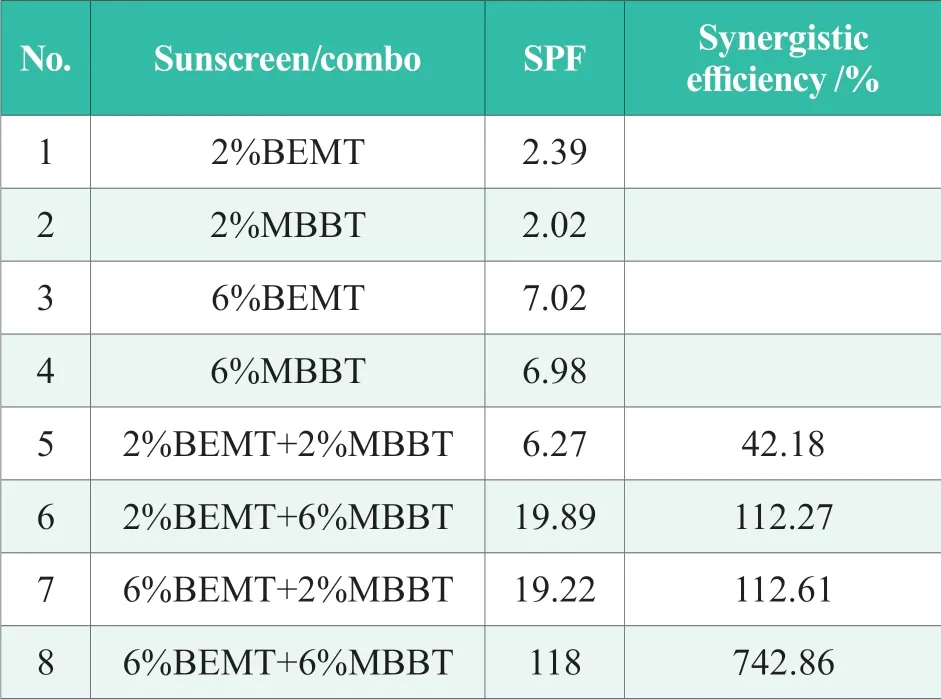
Table 4.SPF value in vitro and synergistic efficiency of BEMT and MBBT
To summarize,test results of both UV-visible spectrophotometric analysis and in vitro test by UV-2000s indicated that there was a synergistic effect between BEMT and MBBT,and the higher the dosages of sunscreen agents might result to more significant synergistic effect.
2.3 Mechanism study of synergistic effect of BEMT and MBBT
Synergistic effect of sunscreen agents mainly comes from following approaches:compatibilities;suitable solvent to better dispersion of crystalline sunscreens,critical absorption wavelength increasing and enhancement of the ratio of UVA/UVB;the application of granular sunscreen agents;the application of both water soluble and oil soluble sunscreen agents;improvement of UVA protection[14,15].
BEMT and MBBT are quite stable,so the interaction between them are relatively low.Besides,both of them show a strong UVA protection,so there is little space to improve UVA protection of this combo.Based on these analyses,our study has focused on solvent effect,scattering effect of granular sunscreen agents and synergistic effect between water/oil soluble sunscreen agents.
2.3.1 Solvent Effect
Literature has reported that the extinction coefficient ? of most sunscreen agents rose in polar and non-polar solvents,which meant increased uv absorption capabilities,while fell in solvents with semi-polarity[16].Formulas with 10% BEMT or/and 10% MBBT were selected to study their uv absorption capabilities,indicated by Aλmaxat UVB range,in ethanol,a typical semi-polarity solvent and isooctane,a non-polar solvent correspondingly.Data were summarized in Table 5 with synergistic efficiency in isooctane listed in No.1~3 and in ethanol listed in No.4~6 correspondingly.
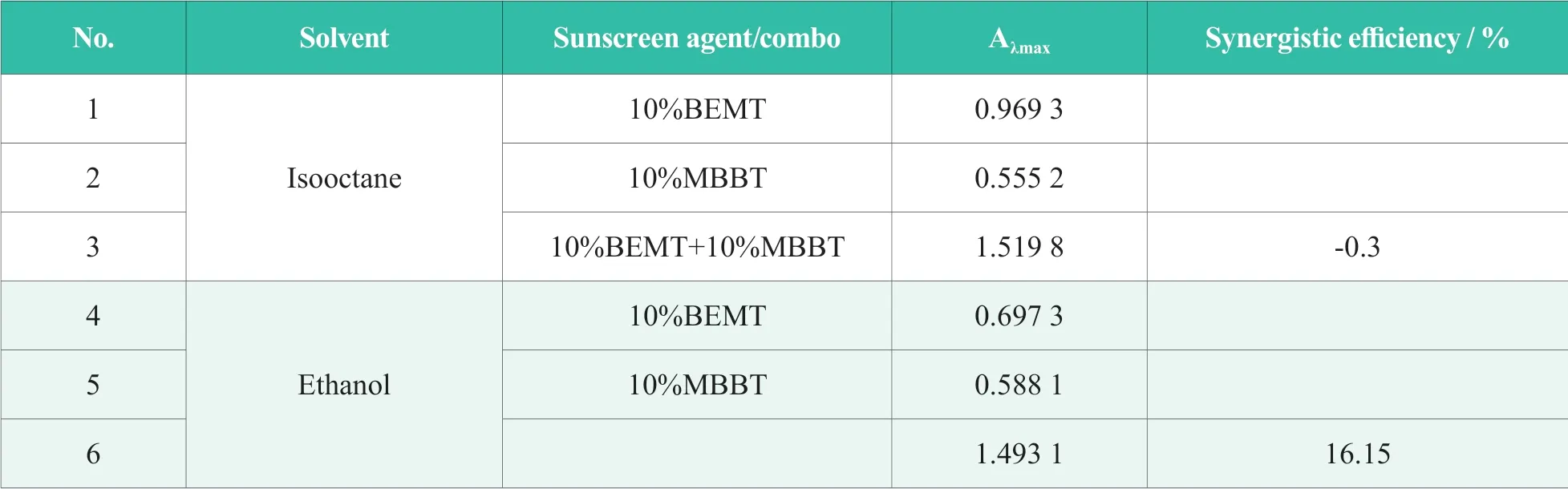
Table 5.UV absorption and synergistic efficiency of BEMT and MBBT in ethanol or isooctane

Table 6.SPF in vitro of BEMT in different emollients
The most common solvents in sunscreen products are emollients.Myriam etc.[12,17]have found that increased polarity of emollients resulted to better UVA protection,thus enhanced SPF value of the system.Three commonly used emollients in sunscreen products,C12-15 alkyl benzoate,caprylic/capric triglyceride and dicaprylyl carbonate,with surface tension coefficient of 55,50 and 39 (mN/m)accordingly,were chosen to dissolve BEMT and MBBT.Six samples were prepared as Fig.6 shown.In vitro test results of them indicated that in 6% BEMT solution,SPF increased as the polarity of emollient rose.While after the addition of MBBT,the polarity of emollient had little impact on SPF value.This phenomenon indicated that the addition of MBBT had reduced the influence of solvent polarity,showing a synergistic effect between BEMT and MBBT.
To summarise,test results of both UV-visible spectrophotometric analysis and in vitro test by UV-2000s indicated that solvent polarity had impacts on UV absorption capability of BEMT.While the addition of MBBT could offset this influence,showing a synergistic effect between them.This finding was also quite instructive for formula designers.
2.3.2 The reflection and scattering effect of MBBT
When the particle size of MBBT is larger than 200 nm,it shows weak UV absorption capability.Given the fact that its nanoparticles exhibit strong absorption of middle-and-long-wave ultraviolet[18],its non-aqueous-non-oleaginous particle dispersion with the particle diameter of less than 200 nm(active content around 50%) has been widely used in cosmetic industry.
Particles can reflect and scatter lights.Reflection effect of particles in sunscreen product means the extension of optical path,and therefore UV absorption is increased.So the size of particles plays an important role in the efficiency of reflection and scattering effect[19].A sample with 4% MBBT whose UV absorption wavelength is less than 440 nm was scanned by UV-2000s at 290~450 nm.The spectrum indicated that the sample could absorb light with wavelength higher than 440 nm (shown in Figure 4).This result suggested that MBBT could reflect lights with common dosage in formulas.Bernd etc.[20]added MBBT into a dye with Aλmaxof 638 nm and found that the light absorption at 638 nm increased as the dosage of MBBT rose,proving the scattering effect of MBBT.This effect might also help enhance UV absorption of combos with MBBT.
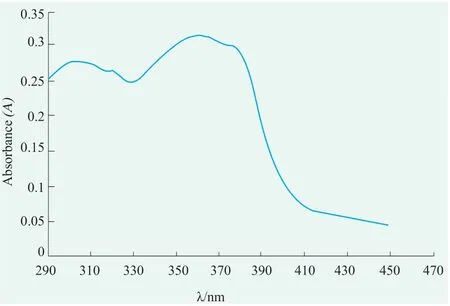
Figure 4.UV-2000s spectrum of 4%MBBT(290~450 nm)
2.3.3 The application of both water soluble and oil soluble sunscreen agents
Currently,the most majority of sunscreen agents are oil soluble,and the kinds of water soluble sunscreen agents is quite limited,which means that sunscreen agents mainly stay at oil phase in sunscreen products.So after applying O/W sunscreen products on skin,water evaporates,leaving oil soluble agents and some involatile agents in water phase on skin.If there is no water soluble sunscreen agent in product,‘UV window’ will form and UV protection efficiency will be weakened.Computer simulation indicates that the combo of water soluble and oil soluble sunscreen agents has higher synergistic efficiency than that of water or oil soluble sunscreen agents only[21].
Small particles of MBBT pretreated as a water dispersed agent is widely used in cosmetic industry.BEMT is an oil soluble sunscreen agent,and BEMT(aqua) is a light yellow dispersion with 20% BEMT,which is prepared by encapsulation technology.Scanning of BEMT by UV-2000s at 290~450 nm shown that there was little light absorption at the wavelength of larger than 400 nm,indicating the weak reflection and scattering capability of BEMT.Eight combos of BEMT/BEMT (aqua) and MBBT were prepared to study the synergistic effect of water soluble and oil soluble sunscreen agents.Among them,four combos of BEMT and MBBT represent oil/water sunscreen systems,and other four combos of BEMT (aqua) and MBBT are water/water sunscreen systems.Their in vitro SPF values tested by UV-2000 s were listed in Table 7.The synergistic efficiency was calculated by following formula:synergistic efficiency(%)=(SPFBEMT+MBBT-SPFBEMT(aqua)+ MBBT)×100/ SPF BEMT(aqua) + MBBT.Synergistic efficiency data shown that in vitro SPF values of 2%BEMT+4% MBBT and 10% BEMT (aqua) +4% MBBT (No.1) were almost the same,suggesting no synergistic effect in these systems.Then,dosages of sunscreen agents were increased 6%BEMT and 30% BEMT (aqua) respectively (No.2),and a significant synergistic efficiency of 23.26% was observed.Further increasing the dosage of MBBT to 8%and 12% correspondingly shown synergistic efficiencies of 39.95% and 138.75%,indicating that the higher the dosages of sunscreen agents might result to more significant synergistic effect again.
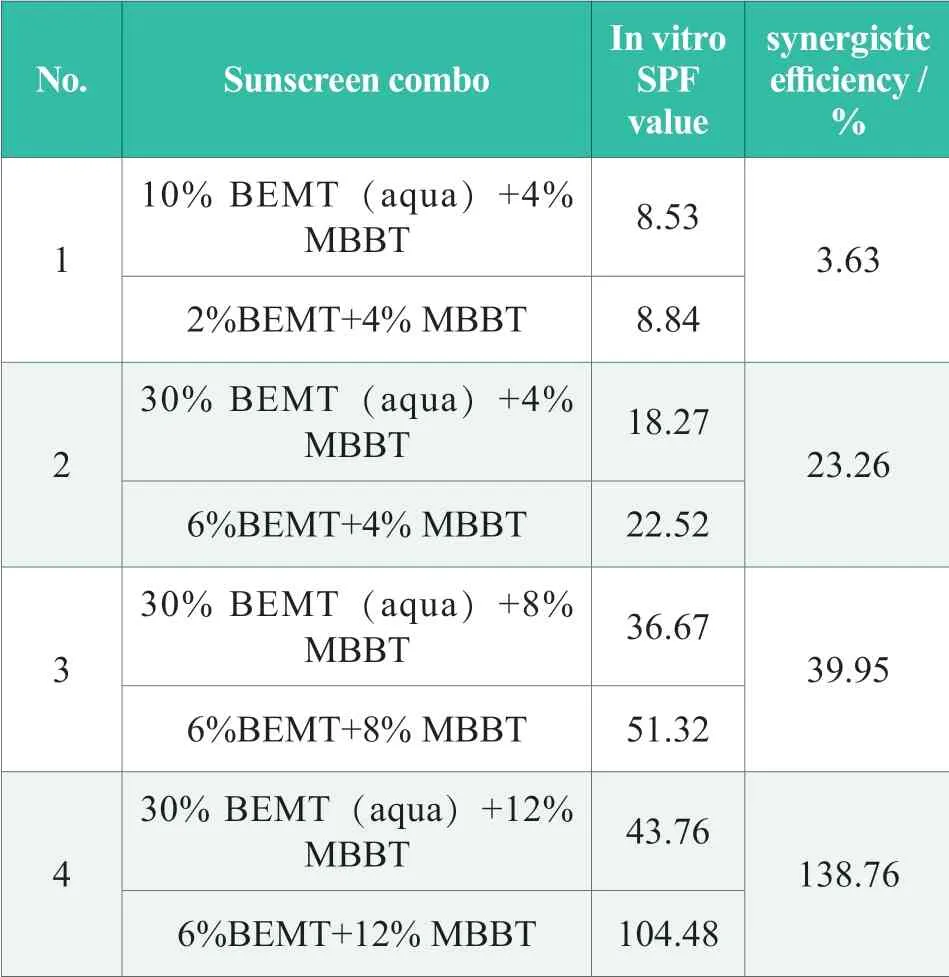
Table 7.SPF in vitro and synergistic efficiency of BEMT/BEMT (aqua) and MBBT
3 Conclusion
Investigations above shown that there was a remarkable synergistic effect between BEMT and MBBT.UV-visible spectrophotometric analysis demonstrated that solvent polarity might have an impact on Aλmax of BEMT and MBBT.The increase of solvent polarity could weaken UVB absorption capability of BEMT,while the addition of MBBT might offset this influence.Test results of UV-2000s indicated that polarities of emollients might also have impacts on in vitro SPF values of BEMT,while the addition of MBBT might offset this influence.MBBT shown a reflection effect at a common dosage in cosmetic products.Synergistic effect was observed when combining MBBT with other sunscreen agents because of its scattering capability.Both BEMT and MBBT can be dispersed in water phase after some kinds of pretreatment.And combos of water soluble and oil soluble sunscreen agents exhibited significant synergistic efficiencies,although this effect was not so remarkable at low dosages of sunscreen agents.
To conclude,the application of particles with reflection and scattering capability,and combing both water soluble and oil soluble sunscreen agents in sunscreen products can greatly increase systems’protection capability,and thus better balance product efficacy and aesthetics.

 China Detergent & Cosmetics2021年4期
China Detergent & Cosmetics2021年4期
- China Detergent & Cosmetics的其它文章
- Progress of Study on Cistanche Application in Cosmetics
- Experimental Method Of Cosmetics Human Efficacy Evaluation—Delaying Skin Aging Efficacy
- Research Progress and Cosmetic Application of Fibronectin
- Mechanism of Novel Natural Products Maintaining Skin
- Discussion on the Efficacy Evaluation Method of Skin Repair Cosmetics
- Interpretation of China’s Cross-border E-commerce Regulation
