Blepharis persica increases testosterone biosynthesis by modulating StAR and 3 β-HSD expression in rat testicular tissues
Nilesh Gaikar, Nishit Patel, Samir Patel , Priyal Patel, Piyush Chudasama, Manan Raval?
1Department of Pharmacognosy, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, CHARUSAT Campus, Changa,Gujarat, India
2Department of Pharmaceutical Chemistry, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, CHARUSAT Campus, Changa, Gujarat, India
3Department of Pharmacology, Ramanbhai Patel College of Pharmacy, Charotar University of Science and Technology, CHARUSAT Campus, Changa,Gujarat, India
4Research Scientist, R&D Unit, Sat-Kaival Hospital Pvt. Ltd, Anand, 388001 Gujarat, India
ABSTRACT
Objective: To evaluate the effect of methanolic extract and ethyl acetate fraction of methanol extract prepared from the seeds of Blepharis (B.) persica on testosterone biosynthesis and also to elucidate the underlying mechanism.
Methods: Forty-eight male Wistar rats were divided into eight groups (n=6 per group). GroupⅠ received 0.3% w/w gum acacia suspension p.o. and served as the normal control group. GroupⅡwas administered testosterone propionate in arachis oil i.m.as the positive control group. Group Ⅲ to Ⅴ received B. persica methanolic extract p.o. at doses of 50, 100 and 200 mg/kg body weight. Group Ⅵ to Ⅷ received B. persica ethyl acetate fraction p.o. at doses of 50, 100 and 200 mg/kg body weight. The testis was used for biochemical estimation and histological studies. The effects of methanolic extract and ethyl acetate fraction of B. persica on testicular testosterone, mRNA expression corresponding to steroidogenic acute regulatory protein (StAR) and 3β-hydroxysteroid dehydrogenase (3β-HSD) along with 3β-HSD enzyme assay were evaluated in testicular tissues and sperm concentration. Ethyl acetate fraction of B. persica was subjected to column chromatography. Invitro studies were performed using TM3 cell line at three dose levels (50, 100, 200 μg/mL), each for methanolic extract, ethyl acetate fraction and 2-benzoxazolinone for evaluation of their comparative effect on testosterone production.
Results: Ethyl acetate fraction and methanolic extract of B. persica could elevate the testicular testosterone content compared to the normal control group. The treatment with methanolic extract and ethyl acetate fraction of B. persica increased the expression of mRNA corresponding to StAR by 6.7 fold and 10.6 fold, respectively,whereas the mRNA expression of 3β-HSD increased by 5.7 fold and 7.3 fold, respectively. Moreover, fraction and extract treatment exhibited increased 3β-HSD activity in the testicular tissues and were found to elevate sperm concentration in seminal fluid.The spermatogenic potential was further ensured by histological observations. 2-benzoxazolinone was isolated from ethyl acetate fraction and identified using spectral studies. It showed the ability to increase the testosterone content in the TM3 Leydig cells.
Conclusions: Methanolic extract and ethyl acetate fraction of B.persica are able to increase the testicular testosterone in rats by elevating mRNA expression of StAR and 3β-HSD in testicular tissues, leading to increase the sperm concentration.
KEYWORDS: Blepharis persica; Testosterone; Spermatogenic;Leydig cells; 3β-hydroxysteroid dehydrogenase; Steroidogenic acute regulatory protein; mRNA expression; TM3 cell line
Significance
Testosterone is produced by the testis through steroidogenic process catalyzed by the enzymes. The extract and fraction of Blepharis persica seeds stimulated mRNA expression of steroidogenic enzyme and increased the activity of 3β-HSD,and led to upregulation of testosterone biosynthesis and elevated sperm concentration. 2-benzoxazolinone isolated from the fraction showed ability to elevate testosterone level in TM3 cells, which provided a potential scaffold for developing new drug molecules affecting testosterone biosynthesis.
1. Introduction
The steroidogenic enzymes are essential for testosterone biosynthesis and the normal function of the male reproductive system. Testosterone produced by testicular tissues found crucial for assembly and maturation of sperms in the testes[1]. Testosterone is produced by the Leydig cells of the testes through steroidogenesis[2].The critical step involved in steroidogenesis is the transfer of substrate cholesterol through steroidogenic acute regulatory protein(StAR), influenced by the binding of luteinizing hormone (LH) to the androgenic receptors expressed on Leydig cells. Conversion of pregnenolone to progesterone is considered to be a rate limiting step in testosterone biosynthesis, catalyzed by 3β-hydroxysteroid dehydrogenase enzyme (3β-HSD)[3-6]. The steroidogenesis process may be debilitated by alteration in the expression of steroidogenic enzymes, which is attributed to male factor infertility due to inadequacy of testosterone production in the testes, thereby affecting the process of spermatogenesis[1,7].
Blepharis persica (Burm f.) O. Kuntze, (Family: Acanthaceae) (B.persica) is a basally branched herb, found in India, Afghanistan,Iran, Iraq, and Egypt[8,9]. The seeds of B. persica are known as Utanjan in the Indian system of medicine and are traditionally considered aphrodisiac[9,10]. B. persica seeds are indicated in the treatment of male reproductive system disorders and infertility[11,12].The chemical investigations on B. persica revealed the presence of benzoxazinoid class of compounds, flavonoids, and phenolic acid derivatives[13-16]. Flavonoid compounds were isolated from ethyl acetate fraction prepared from methanolic extract of B. persica seeds.Moreover, the extract and fraction of B. persica showed the ability to increase the testosterone level in culture media containing TM3 cells[17]. Ethanolic extract prepared from the seeds of B. persica showed aphrodisiac activity in male mice and increased serum testosterone content[18]. The literature review indicated that the seeds of B. persica could upregulate testosterone biosynthesis and promote spermatogenesis; however, the underlying mechanism has not been proposed.
The studies thus were aimed to investigate the effects of methanolic extract and ethyl acetate fraction of B. persica administration on testicular testosterone content, expression of mRNA corresponding to StAR and 3β-HSD in testicular tissues, activity of 3β-HSD in testicular tissues, sperm concentration and testicular histology in rats. Additionally, isolation of chemical constituent from bioactive ethyl acetate fraction and its effect on testosterone biosynthesis was evaluated in-vitro.
2. Materials and methods
2.1. Chemicals
All solvents, chemical reagents and silica gel (200-400 mesh) for column chromatography were purchased from Loba Chemie, India.The solvents used for extraction and chromatographic purposes were of analytical grade. The solvents used were distilled immediately before use. Media and different reagents for biochemical studies were procured from Himedia, India. Dehydroepiandrosterone(DHEA) was procured from Sigma Aldrich, USA. Primers used in mRNA expression studies were procured from Europhins Genomics Pvt. Ltd., Bangalore, India.
2.2. Identification and authentication of the plant material
B. persica seeds were received from Yucca Enterprises, Wadala (E),Mumbai, India. The plant was authenticated by Dr. A. S. Upadhye,Scientist, Plant Science Division, Agharkar Research Institute, Pune.A voucher specimen was deposited at their herbarium (voucher specimen No. 17–218). The seeds were separated and dried under a shed for 15 days. Dried seeds were powdered using a laboratory grinder to a coarse powder and stored in an airtight glass container.
2.3. Preparation of extract and fraction
The coarse powder of B. persica seeds (900 g) was defatted using petroleum ether 60 ℃–80 ℃ (2 000 mL) in a soxhlet apparatus(Dolphin Labware, India) (3 h). The dried defatted powder was extracted using methanol in a soxhlet apparatus (8 h). The filtered methanolic extract was concentrated using a rotary vacuum evaporator (R-215Ⅴ, Heidolph, Germany), and the concentrated mass was then dried using a hot water bath. Methanolic extract (20 g)was suspended in water (100 mL) and partitioned with ethyl acetate(100 mL). The procedure was repeated three times, and the organic layers were collected and pooled. The pooled ethyl acetate fractions were concentrated using a rotary vacuum evaporator and further evaporated to dryness using a water bath to obtain ethyl acetate fraction. The dried fraction was stored in a desiccator.
2.4. Column chromatography
Ethyl acetate fraction (7 g) was subjected to silica gel column chromatography (600 mm length × 40 mm internal diameter).The subfractions A-D were obtained by eluting the column using a solvent mixture containing chloroform and methanol. Subfraction A obtained from chloroform methanol gradient system(1% to 7% methanol in chloroform) was loaded on silica gel column (290 mm length × 15 mm internal diameter) and eluted with chloroform initially and then by various proportions of methanol in chloroform (0.1% to 5.0% methanol in chloroform)leading to the isolation of one compound (2-benzoxazolinone).The isolated compound was subjected to purity analysis on silica gel G60 F254coated aluminum sheets (Merck, India) using thin layer chromatography method.
2.5. Spectral characterization
The infrared spectroscopy (IR) spectrum was recorded using FTIR‐Eco‐ATR (Bruker, Optic, Model ALPHAⅡ).1H and13C‐NMR spectrum were recorded by dissolving the compound in deuterated chloroform (CDCl3) with the help of Bruker Biospin 800 MHz instrument (Avance AV800, Switzerland). The mass spectrum was recorded using 1290 Agilent InfinityⅡ1300 bar and 6470 QQQ mass spectrometer (Agilent Technologies Pvt. Ltd, USA).
2.6. Animals
Forty-eight male Wistar rats (6-8 weeks old) weighing (200-250) g were used for the set of experiments. The animals were obtained from the Animal House of National Institute of Biosciences,Pune, India. The rats were housed in separate polypropylene cages.The rats were acclimatized to standard laboratory conditions[temperature: (25±2) ℃, humidity: (75±5)%], under 12 h light/dark cycle for 7 days.
2.7. Experimental design
Male Wistar rats were divided into eight groups with six animals per group. Group Ⅰ served as the normal control group and was administered with 0.3% w/w gum acacia suspension (0.2 mL)prepared in distilled water, p.o. daily for 28 days. Group Ⅱ was administered with 500 μg/kg body weight testosterone propionate in arachis oil, i.m. [Testo (500 μg/kg i.m.) treated] twice a week for 28 days, and served as a positive control group. Group Ⅲ, Ⅳ and Ⅴwere administered 50 mg/kg (methanolic extract 50), 100 mg/kg (methanolic extract 100), and 200 mg/kg (methanolic extract 200) body weight of B. persica methanolic extract in 0.3% w/w gum acacia, p.o. daily for 28 days. Group Ⅵ, Ⅶ and Ⅷ were administered 50 mg/kg (ethyl acetate fraction 50), 100 mg/kg (ethyl acetate fraction 100), and 200 mg/kg (ethyl acetate fraction 200) body weight of B. persica ethyl acetate fraction in 0.3% w/w gum acacia, p.o. daily for 28 days. All the oral dose administrations were done by using an oral gavage syringe. As per toxicity studies, the dose 2 000 mg/kg body weight was tolerated by the animals. 1/10th of this dose was selected as the highest dose for the set of the studies (200 mg/kg body weight).
2.8. In-vivo studies
2.8.1. Determination of testicular testosterone
Animals were sacrificed using a high dose of pentobarbitone sodium (Sigma Aldrich, USA) and the testes were dissected out.Testicular tissue was minced and transfered in a homogenizer to obtain 10% homogenate solution using 10 mL phosphate buffer saline (pH 7.4). The suspension containing minced tissue was centrifuged at 15 432 ×g for 10 min at 6 ℃. The supernatant was collected and used to measure testicular testosterone level using the commercially available kit (Vidas TestosteroneⅡ, Biomerieux, France, Catalogue No. VIDAS414320).
2.8.2. Real time quantitative polymerase chain reaction (qPCR)
The reported methodology was adopted to carry out the studies[19].1 μg of total RNA was subjected to reverse transcription to obtain the first strand cDNA template using gene specific primer corresponding to StAR and 3β-HSD (Table 1). GADPH was used as an internal control. qPCR was performed in a Rotor-Gene-Q using TAKARA exTaq SYBR Green Kit.
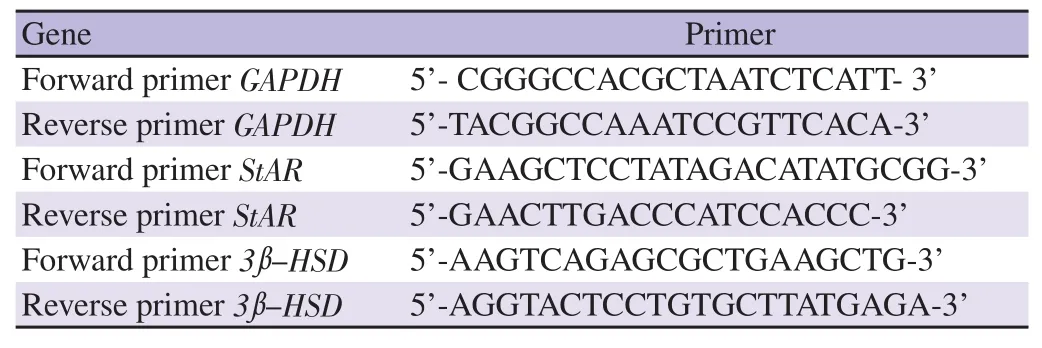
Table 1. Details of gene specific primers used for real time-qPCR.
2.8.3. Determination of testicular 3β-HSD activity
The reported methodology was adopted to determine the 3β-HSD activity in testicular tissue[20]. Enzyme activity was determined from the reduced nicotinamide adenine dinucleotide (NAD) standard curve and expressed as nmol of nicotinamide adenine dinucleotide reduced/min/mg protein. The amount of protein in the testicular tissue was determined using the Lowry method[21].
2.8.4. Sperm concentration
Sperm concentration was assessed using an improved Neubauer hemocytometer at 1: 19 dilution and sperms were counted using an optical microscope (AxioLabA1, Carl Zeiss, Germany)[22].
2.8.5. Histological studies
The specimens containing testicular tissues were preserved initially in buffered formalin (10%) individually (24 h). The wax block of testicular tissue was prepared by denaturing process. The ribbon of the section (thickness 5 μm) was prepared using a microtome (Leica RM2265). Hematoxylin and eosin dyes were used for staining the section. The histoarchitecture of tissue was observed under the optical microscope (20×) (AxioLabA1, Carl Zeiss, Germany)[22].
2.9. In-vitro studies using TM3 cell line
2.9.1. Cell culture maintenance
The TM3 Leydig cell line (Mus musculus) was procured from the National Centre for Cell Science, Pune, India. The Leydig cell line was cultured at 37 ℃ in a humidified atmosphere of 5% carbon dioxide incubator with a medium consisting of 5% fetal bovine serum (Himedia, India) supplemented in Dulbecco’s Modified Eagle Medium (pH 7.2) (Himedia, India).
2.9.2. Preparation of test solutions
The test samples: methanolic extract, ethyl acetate fraction,2-benzoxazolinone were weighed accurately (10 mg), individually,and dissolved in a minimum amount of dimethyl sulfoxide (DMSO).DHEA was weighed accurately (10 mg), and dissolved in a minimum amount of DMSO. The final volume was made (up to 10 mL) using culture media. The test solution concentrations(50 μg/mL, 100 μg/mL, 200 μg/mL) were prepared from the stock solutions using different aliquots[17]. Media containing 0.1% DMSO were used as a control for all experiments.
2.9.3. Effect of test solutions on testosterone concentration using TM3 cells
TM3 cells (1.5×104cells/well) were seeded in 96-well plates and incubated at 37 ℃ for 24 h in 5% carbon dioxide and 95% air. The cells were then incubated with selected concentrations (50, 100,and 200 μg/mL) of test solutions containing methanolic extract,ethyl acetate fraction, 2-benzoxazolinone, and DHEA for 24 h.The control experiments were run parallel to test experiments. The testosterone content was determined from the supernatant at the end of the studies using the commercially available kit (Immulite 1 000, Siemens, Catalog number: LKTW1). Each experiment was performed three times.
2.10. Statistical analysis
Data were analyzed using GraphPad Prism software (GraphPad software, version 8.4.2, Trial version, San Deigo, USA). All data were expressed as mean±standard deviation (mean±SD). Means were statistically analyzed using one-way analysis of variance followed by Dunnett’s test. The values were considered to be statistically significant if P<0.05.
2.11. Ethics statement
The protocols to execute the set of studies were approved by the Institutional Animal Ethics Committee (Registration number 940/PO/Re/S/06/CPCSEA) constituted as per “The Committee for the Purpose of Control and Supervision of Experiments on Animals”.The protocol for a set of studies to be performed experimentally was approved with certificate No. RPCP/IAEC/2020-21/R14.
3. Results
3.1. Spectral characterization of the isolated compound
2-benzoxazolinone isolated from ethyl acetate fraction of B. persica using repetitive column chromatography showed a single spot on thin layer chromatography and confirmed the purity. The compound was subjected to spectral studies to evolve the probable chemical structure. Infrared spectroscopy (IR) spectrum (Supplementary Figure 1A), mass spectrum (Supplementary Figure 1B),1H NMR(Supplementary Figure 2A), and13C NMR (Supplementary Figure 2B) spectra were interpreted to characterize the isolated compound,which was found as 2-benzoxazolinone.
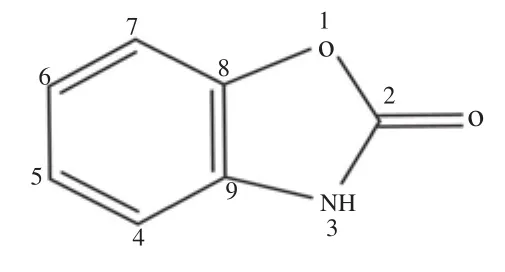
Chemical structure of 2-benzoxazolinone
3.2. Testicular testosterone estimation
B. persica methanolic extract and ethyl acetate fraction treated animals showed statistically significant and dose dependent increase in testicular testosterone compared to the normal control group(P<0.01) (Figure 1). The amount of testicular testosterone measured in methanolic extract (200 mg/kg body weight) [(6.9±1.9) ng/g testis]and ethyl acetate fraction (200 mg/kg body weight) [(13.2±3.7) ng/g testis] treated groups was found to be significant higher compared to the normal control group [(0.8±0.2) ng/g testis] (P<0.01).The increase in content of testicular testosterone was found dose dependent. Testo (500 μg/kg, i.m.) treated group [(4.33±0.3) ng/g testis] (P<0.05) showed statistically significant increase in testicular content compared to the normal control group. Ethyl acetate fraction treated animals showed comparatively higher testosterone content compared to methanolic extract and Testo treated groups of animals.
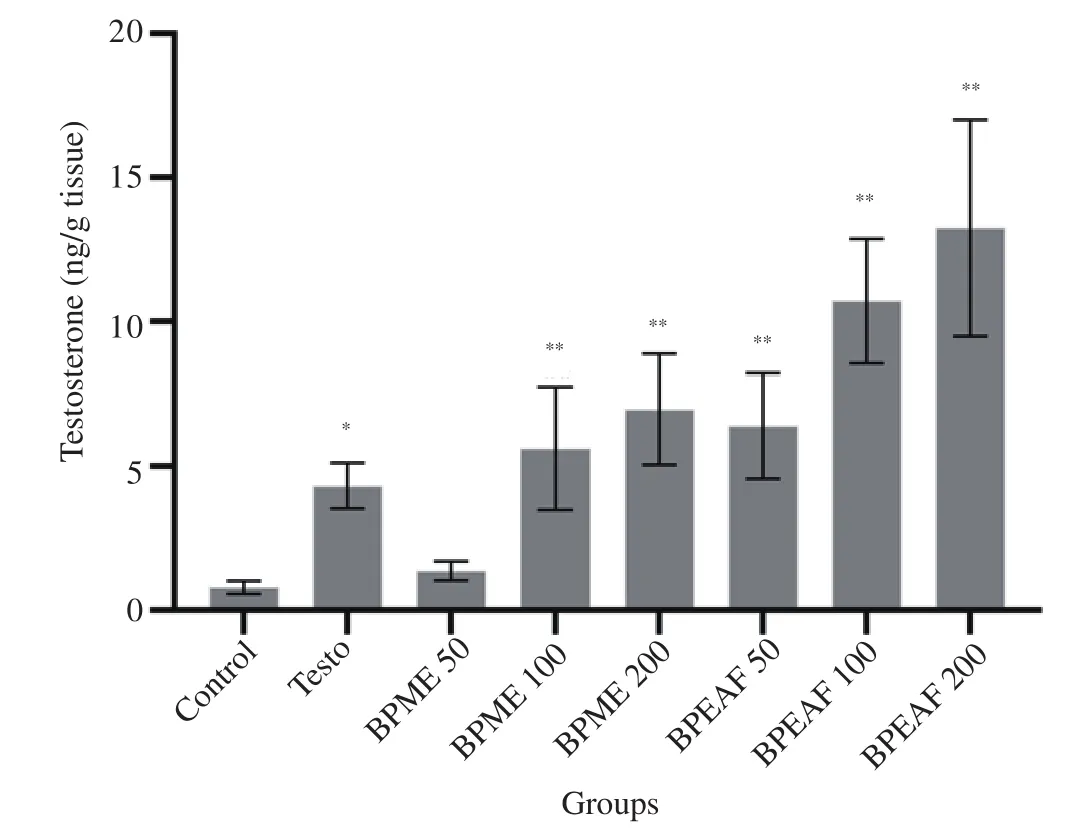
Figure 1. Effect of BPME and BPEAF on testicular testosterone level (n=6 in each group). Values are expressed as mean±SD. *P<0.05 and **P<0.01 compared to the normal control group. Testo: testosterone propionate;BPME: Blepharis persica methanolic extract; BPEAF: Blepharis persica ethyl acetate fraction.
3.3. mRNA expression studies corresponding to StAR and 3β-HSD
B. persica methanolic extract (200 mg/kg body weight) and ethyl acetate fraction (200 mg/kg body weight) treated animals in comparison to the normal control group showed 6.7 fold and 10.6 fold increase in expression of mRNA corresponding to StAR, respectively (P<0.01), whereas the expression of mRNA corresponding to 3β-HSD was increased by 5.7 fold and 7.3 fold(P<0.01), respectively (Figure 2). The results also showed that the increase in mRNA expression was dose dependent increase and statistically significant. Ethyl acetate fraction showed higher amplitude of the action on mRNA expression of StAR and 3β-HSD compared to the methanolic extract and Testo treated groups.
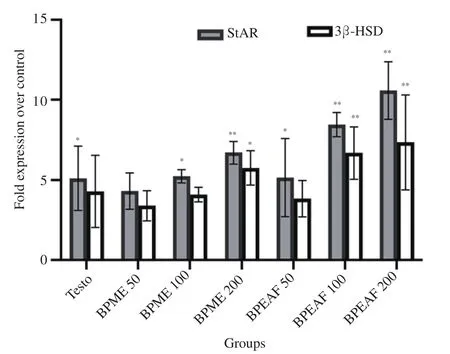
Figure 2. Effect of BPME and BPEAF on mRNA expression corresponding to 3β-HSD and StAR in testicular tissues (n=6 in each group). Values are expressed as mean±SD. *P<0.05 and **P<0.01 compared to the normal control group. 3β-HSD: 3β-hydroxysteroid dehydrogenase; StAR:steroidogenic acute regulatory protein.
3.4. Activity of 3β-HSD
The treatment of animals with methanolic extract (200 mg/kg body weight) (1.22±0.11 NAD reduced/min/mg protein) and ethyl acetate fraction (200 mg/kg body weight) (1.46±0.13 NAD reduced/min/mg protein) showed dose dependent and statistically significant increase in 3β-HSD activity in comparison to the normal control group(0.73±0.12 NAD reduced/min/mg protein) (P<0.01). Testo(500 μg/kg i.m.) treated group of animals showed no significant alteration compared to the normal control group in 3β-HSD activity.3β-HSD activity was found higher in ethyl acetate fraction treated animals as compared to the animals treated with methanolic extract and the Testo treated group (Figure 3).
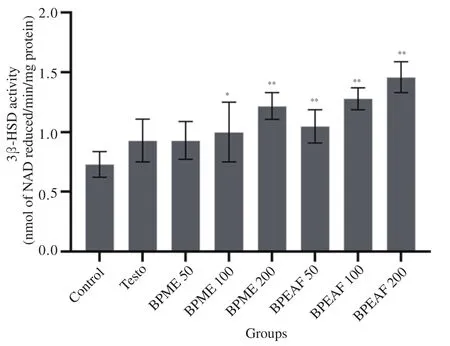
Figure 3. Effect of BPME and BPEAF on 3β-HSD activity (n=6 in each group). Values are expressed as mean±SD. *P<0.05 and **P<0.01 compared to the normal control group.
3.5. Sperm concentration
The sperm concentration was found to be increased significantly in the treated groups of methanolic extract (200 mg/kg body weight)[(43.10±2.64)×106/mL] and ethyl acetate fraction (200 mg/kg body weight) [(46.50± 2.43)×106/mL] in comparison to the normal control group [(28.60±2.16)×106/mL] (P<0.01), while Testo (500 μg/kg i.m.) treated animals did not show significant alteration in sperm concentration (Figure 4). Ethyl acetate fraction treated animals showed comparatively higher sperm concentration compared to methanolic extract treated group of animals.
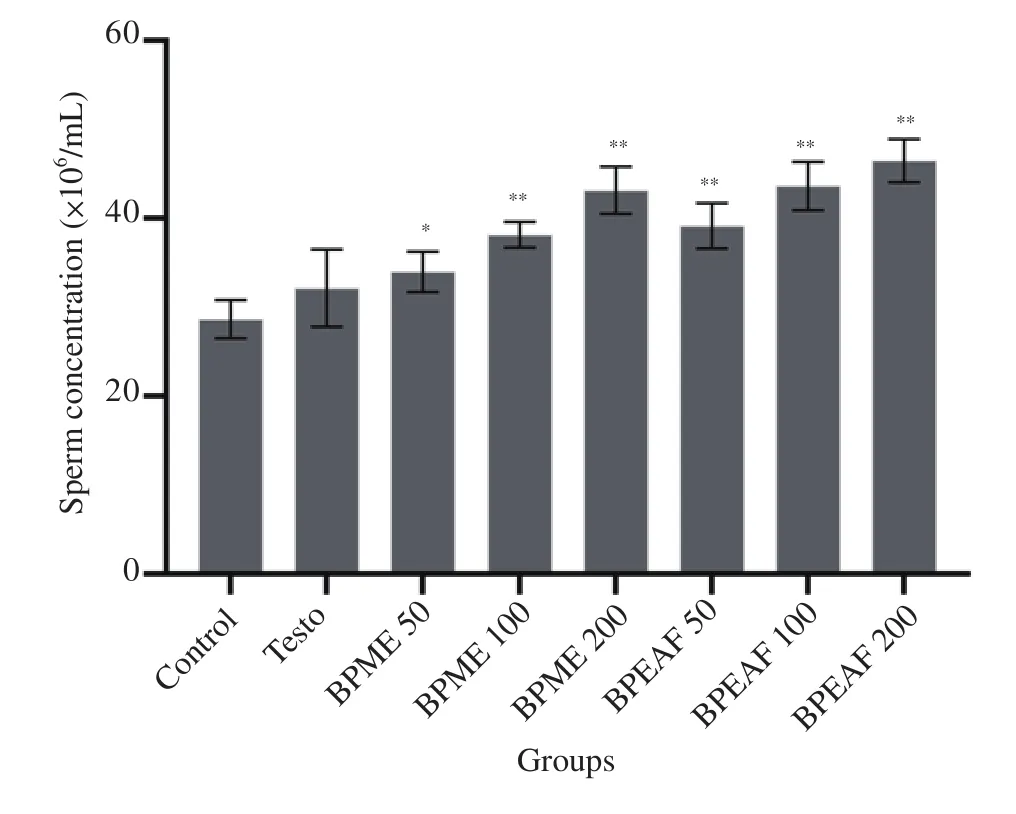
Figure 4. Effect of BPME and BPEAF on sperm concentration (n=6 in each group). Values are expressed as mean±SD. *P<0.05 and **P<0.01 compared to the normal control group.
3.6. Histology of the testis
Testis histoarchitecture of the normal control group (Figure 5A)showed normal features of germinal epithelium and the lumen of the tubules filled with an optimal number of spermatozoa. Sertoli cells observed near the basement membrane of the seminiferous tubules appeared as less dense mass. Leydig cells present in the interstitial spaces were found normochromic. Histoarchitecture examination of testis in the treated groups of methanolic extract (Figure 5B-5D) and ethyl acetate fraction (Figure 5E-5G) showed that the seminiferous tubules were enlarged in shape as well as the lumen was filled with an abundant number of spermatozoa. The cytoplasm of Sertoli cells appeared dark in color near the basement membrane, while the Leydig cells in the interstitial spaces appeared as dense mass. The similar alterations were also seen in the animals treated with Testo(500 μg/kg i.m.) treated group (Figure 5H).
3.7. In-vitro studies using TM3 cell line
The dose-dependent increase was found in testosterone content after incubation of TM3 cells with test solutions (Figure 6).TM3 cells treated with test solutions containing methanolic extract (200 μg/mL) [(39.3±2.5) ng/dL], ethyl acetate fraction(200 μg/mL) [(45.9±4.5) ng/dL] and 2-benzoxazolinone (200 μg/mL)[(88.6±4.7) ng/dL] showed a statistically significant increase in the amount of testosterone compared to the control group[(17.6±0.5) ng/dL] (P<0.01). 2-benzoxazolinone (200 μg/mL)showed higher the amplitude of action on TM3 cells as compared to methanolic extract and ethyl acetate fraction. TM3 cells treated with DHEA (200 μg/mL) showed a significant increase in testosterone content [(118.6±7.1) ng/dL] (P<0.01), which confirmed that the cells could consume the substrate DHEA and produce testosterone.
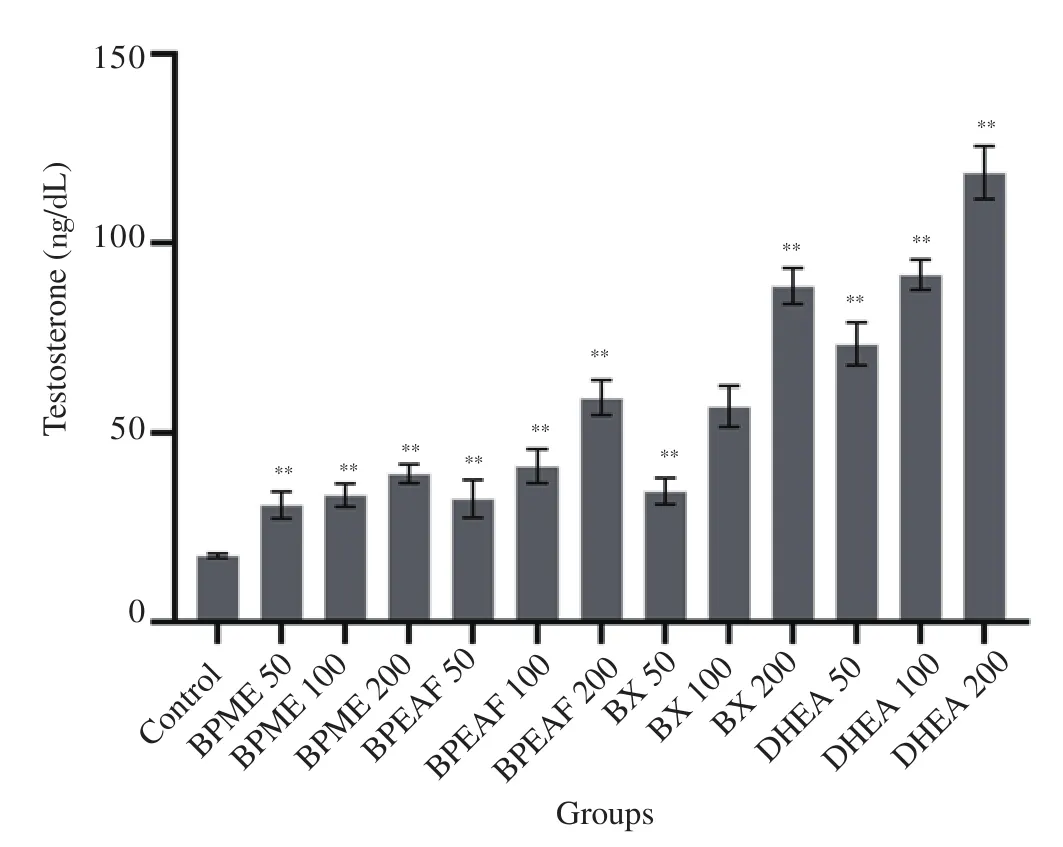
Figure 6. The comparative effect of extract, fraction and isolated compound on testosterone content using TM3 Leydig cells (n=3 in each group).Values are expressed as mean±SD. **P<0.01 compared to the normal control group. BX: 2-benzoxazolinone. DHEA: dehydroepiandrosterone(served as the positive control).
4. Discussion
The seeds of B. persica showed the ability to stimulate testosterone biosynthesis, in-vitro[17]. The present study was planned to evaluate the effect of methanolic extract and ethyl acetate fraction prepared from the seeds of B. persica on testicular testosterone biosynthesis and also aimed to evolve probable mechanism.
The animal studies showed the ability of the constituents of methanolic extract and ethyl acetate fraction to upregulate the steroidogenesis process in the Leydig cells, which were comparable to testosterone-treated group which was selected as positive control for comparison purposes[19,22]. The translocation of substrate cholesterol to the inner mitochondrial membrane is an essential step in steroidogenesis facilitated by StAR. Moreover, it has been reported that the reduction in StAR gene expression in the aging population led to reduced testosterone biosynthesis in the Leydig cells. This resulted in reduced testosterone production, affecting the fertility[23]. Methanolic extract and ethyl acetate fraction treated animals showed elevated mRNA expression corresponding to StAR.Stimulation to StAR gene expression was reported to be mediated through cyclic adenosine monophosphate response element-binding protein transcription factors and cytosine-cytosine-adenosineadenosine-thymidine/enhancer-binding protein, involved in the steroidogenesis process[23-25]. The conversion of the cholesterol(C27) to progesterone (C21) catalyzed by 3β-HSD is a rate limiting step in the process of testosterone biosynthesis in the testicular tissues. 3β-HSD is an enzyme of the aldo-ketose reductase family,required for dehydrogenation of hydroxysteroids and found to catalyze the conversion of pregnenolone to progesterone[26,27]. The results of the studies showed that mRNA expression corresponding to 3β-HSD activity was found higher in the testicular tissues of animals treated with methanolic extract and ethyl acetate fraction, accompanied by the elevated activity of the 3β-HSD in the testicular tissues. The binding of the LH to LH receptors on the Leydig cells was reported to elevate the expression of mRNA corresponding to 3β-HSD[26]. This led to propose that the chemical present in methanolic extract and ethyl acetate fraction might bind with LH receptors and elevated expression of mRNA corresponding to 3β-HSD[5].
Spermatogenesis is the multistep process whereby germ cells are converted into the sperm within the seminiferous tubules of the testis and this process largely determines the male fertility.Testosterone is required for germ cell to progress beyond the meiosis, and necessary for the release of mature spermatids during the stage Ⅷ of spermatogenesis[1]. The set of the studies showed that the administration of methanolic extract and ethyl acetate fraction elevated sperm concentration in seminiferous fluid, concurrently with elevated testicular testosterone content. This suggested that the elevated testicular testosterone might influence the process of spermatogenesis in test group animals, and was responsible for elevated sperm concentration[22,28,29]. The histo-architecture of testis obtained from the animals treated with methanolic extract and ethyl acetate fraction showed that the seminiferous tubules were enlarged in size, appeared oval in shape and the lumen was densely populated with spermatozoa. The Sertoli cells and Leydig cells appeared densely stained in treated animals which indicated the elevated metabolic activity. This was in line of elevated mRNA expression of steroidogenic enzymes and 3β-HSD activity. The results showed that methanolic extract and ethyl acetate fraction could upregulate testosterone biosynthesis by affecting mRNA expression of selected steroidogenic enzymes, influencing the activity of 3β-HSD in testicular tissues. This might influence the process of spermatogenesis in the treated animals and elevated sperm concentration.
The bioactive ethyl acetate fraction subjected to column chromatography yielded one compound, identified as 2-benzoxazolinone through spectral studies[30,31]. The up-regulation of testosterone biosynthesis in-vitro using TM3 Leydig cells with dose-dependent increase in testosterone content suggested the bioactive potential of the methanolic extract,ethyl acetate fraction, and 2-benzoxazolinone. Benzoxazinoid and its derivatives were reported to possess stimulatory effects on the central nervous system and showed antimicrobial, immunoregulatory,reproductive system stimulatory and weight-reducing activities[32,33].The biological effect of the benzoxazinoid class of compound is attributed to the presence of nitrogen containing ring structure[33,34].2-benzoxazolinone isolated from the ethyl acetate fraction might act by altering the expression of StAR and 3β-HSD. Though this is an assumption only, this needs to be verified by performing detailed investigations.
The results of the studies showed the effect of methanolic extract and ethyl acetate fraction on testicular testosterone and sperm concentration in rats, involving the assessment of testicular testosterone level, mRNA expression studies corresponding to StAR and 3β-HSD along with activity of 3β-HSD in testicular tissues.The studies did not consider the assessment of LH and folliclestimulating hormone which might be affected by the administration of methanolic extract and ethyl acetate fraction and capable of influencing the process of spermatogenesis.
In conclusion, the set of studies report the ability of methanolic extract and ethyl acetate fraction prepared from the seeds of B.persica in elevating testicular testosterone content in rats. Methanolic extract and ethyl acetate fraction elevate the expression of mRNA corresponding to StAR and 3β-HSD as well as are found to increase the activity of 3β-HSD in testicular tissues, which elevate the amount of testicular testosterone. The stimulation of testosterone biosynthesis in the testes promotes the process of spermatogenesis,as evident from testicular histology and increased sperm concentration.2-benzoxazolinone isolated from ethyl acetate fraction shows the ability to increase testosterone content in-vitro, providing a potential scaffold for the development of novel therapeutic agents affecting testosterone biosynthesis.
Conflict of interest statement
All authors declare that there is no conflict of interest.
Funding
The study was financially supported by Charotar University of Science and Technology through CHARUSAT Research Grant sanctioned to Dr. Manan Raval [CHARUSAT SEED RESEARCH GRANT/RPCP/MAR/12].
Acknowledgments
The authors are thankful to Ramanbhai Patel College of Pharmacy-CHARUSAT for providing the necessary research facilities to carry out the work. We would also like to express thanks to the staff of Tata Institute of Fundamental Research, Mumbai, for the NMR facility.
Authors’ contributions
Manan Raval was involved in conceptualization and designing of experiments, data analysis and verification, review and editing of the manuscript. Nilesh Gaikar performed phytochemical analysis,animal experimentation, and manuscript draft preparation. Nishit Patel contributed to performing in-vitro studies. Priyal Patel was involved in performing animal experimentation. Piyush Chudasama perfomed mRNA expression studies. Samir Patel contributed to analysis and interpretation of the data (phytochemical analysis).
 Asian Pacific Journal of Reproduction2022年1期
Asian Pacific Journal of Reproduction2022年1期
- Asian Pacific Journal of Reproduction的其它文章
- Successful live birth after intracytoplasmic sperm injection using testicular sperm in non-mosaic Klinefelter syndrome
- Effects of enzymatic and non-enzymatic antioxidants in diluents on cryopreserved bull epididymal sperm
- Taurine in semen extender modulates post–thaw semen quality, sperm kinematics and oxidative stress status in mithun (Bos frontalis) spermatozoa
- Recurrent ovarian endometrioma after conservative surgery: A retrospective study
- Uptake of in-vitro fertilization among couples attending fertility clinic in a tertiary health institution
- An implementation study of barriers to universal cervical length screening for preterm birth prevention at tertiary hospitals in Thailand: Healthcare managers’ perspectives
