Systematic review and meta-analysis of Bupi Yichang pills for ulcerative colitis
Xiao-Xiao Xing,Jian-Bo Guo,Hua-Shan Li
1.Beijing University of Chinese Medicine,Beijing 10029,China
2.Guang'an Men Hospital,China Academy of Chinese Medical Sciences,Beijing 10053,China
Keywords:Bupi Yichang pills Ulcerative colitis Systematic review Meta-analysis
ABSTRACT Objective:To evaluate the clinical efficacy and safety of Chinese patent medicine Bupi Yichang Pill in the treatment of ulcerative colitis.Method:Use a computer to retrieve English and Chinese database(PubMed,EMBASE,CNKI,VIP,and Wanfang) from the establishment of the database to May 2020.The literature of clinical randomized controlled trials of Chinese patent medicine Bupi Yichang Pill for the treatment of ulcerative colitis were collected.Two researchers independently extracted the data and evaluated the quality of the included studies and meta-analysis was conducted by Using Stata 16.0 software,and the results were graded and evaluated by GRADE.Result:Eight studies with 835 patients were included.The overall analysis showed that Bupi Yichang Pill was more effective than mesalazine sustained-release granules both in mucosal score under colonoscopy (SMD=-0.93,95% CI [-1.20,-0.67]) and total efficiency (RR=1.26,95% CI [1.11,1.43]).Compared with sulfasalazine,Bupi Yichang Pill produced a more significant effect in terms of total efficiency (RR=1.13,95% CI [1.05,1.21]).In the comparison of total symptom score,Bupi Yichang Pill group was lower than mesalazine sustained-release granules group(SMD=-0.94,95% CI [-1.16,-0.72]).The more adverse effect occurred in the mesalazine group when comparing with the Bupi Yichang Pill group (RR=0.46,95% CI [0.16,1.28]).Conclusion:Bupi Yichang Pill with great safety can effectively improve the clinical effectiveness of ulcerative colitis but the conclusions above are still needed a further study through the higher quality of evidence.
1.Introduction
Ulcerative colitis (UC),one of the inflammatory bowel disease,which mainly occurs in the rectum and left colon.The main clinical manifestations include abdominal pain,diarrhea,stool with mucus pus and blood,and tenesmus.Intestinal mucosal ulcers can be seen under the colonoscopy,and neutrophils and lymphocytes can be found in the pathological specimen.As one of the global diseases with high incidence rates,the number of patients with ulcerative colitis is still increasing [1],often accompanied by mental impacts.The gut-brain axis and psychological disorders can easily bring a two-way impact on patients with ulcerative colitis,forming a vicious circle [2].Furthermore,long-term ulcerative colitis more likely leads to colorectal cancer [3].At present,western medicine and colectomy are the most common treatments for ulcerative colitis[4].Even though some advanced western medicine like intestinalspecific anti-integrin,anti-interleukin-12,and anti-interleukin-23 are developed,there are still some patients who have to undergo colectomy,which means that they need to bear a huger mental and economic burden [5].Therefore,it is extremely vital to find a treatment for ulcerative colitis with better effectiveness,low side effects,and wide patient acceptance.
Traditional Chinese medicine has achieved a significant clinical effect through the differentiation of symptoms and signs,and as a convenient dosage form,Chinese patent medicine has been widely used in clinical practice.The pathogenesis of ulcerative colitis mainly contains spleen deficiency and mixed deficiency and excess.Chinese patent medicine Bupi Yichang Pill has the effects of nourishing the middle and nourishing qi,invigorating the spleen and stomach,and astringent intestines to relieve diarrhea.Although the clinical efficacy of Bupi Yichang Pill in the treatment of ulcerative colitis has been reported successively [7-14],there is still a lack of support of evidence-based medicine.Therefore,through the systematic reviews and meta-analysis,the clinical efficacy and safety of Bupi Yichang Pill will be explored in this study.
2.Methods
2.1.Search strategy
A literature search was conducted (using PubMed,the Chinese Science and Technology Periodical Database (Embase),the Chinese National Knowledge Infrastructure Database (CNKI),Wanfang Database,and China Scientific Journal Database (VIP).The retrieval time was from the establishment of the database to May 8,2020.The search method combined MeSH subject words and free search words as follows:“Ulcerative Colitis Type OR Ulcerative Colitis OR Inflammatory Bowel Disease OR Colitis Gravis”AND“Medicine,Chinese Traditional OR Drugs,Chinese Herbal OR traditional Chinese medicine OR traditional Chinese herbal medicine OR traditional Chinese herb OR Chinese herb OR Chinese herbal medicine OR Chinese medicine OR TCM”AND“randomly”AND“controlled”.
2.2.Inclusion and Exclusion criteria
The literature included in the study met the following requirements:(1) Study type:clinical randomized controlled trials of Bupi Yichang Pill treatment for Ulcerative Colitis,blinded or non-blinded,written in Chinese or English,and available online before May 8,2020;(2)Participants:All patients aged at least 18 years old,with unlimited gender,who were definitely diagnosed with Ulcerative Colitis[6];(3)Intervention measures:The treatment group was treated with Bupi Yichang Pill or combined with the control group,and the control group was treated with conventional western medicine;(4) Outcome indicators:mayo endoscopy score,total efficiency,recurrence rate,total symptom score,and adverse effect.The exclusion criteria were as follows:(1) studies irrelevant to Ulcerative Colitis cases;(2)conference papers;(3) literature published multiple times;(4) the intervention measures of the treatment group without Bupi Yichang Pill;(5) the treatment group contains other traditional Chinese medicine besides Bupi Yichang Pill;(6) literature with western medicine or other therapies as the main research objective;(7)animal experiments.
2.3.Literature Quality Assessment
According to the Cochrane criteria,we evaluated the quality of the included studies:(1) random treatment assignment;(2) treatment assignment concealment;(3) treatment blinding (blinding for patients,study implementers,and study outcome assessors);(4)data integrity of the study results;(5) selective reporting in the study;(6) other biases.From the above standards,researchers (X.X) assessed the risk of bias in the included literature according to the three criteria of "low risk","unknown risk" or "high risk".If there was any ambiguity,the decision was made via consultation or discussion with other researchers (J.G and H.L).GRADE (grades of recommendation,assessment,development,and evaluation) was used to grade and evaluate mayo endoscopy score,total efficiency,recurrence rate,total symptom score,and adverse effect analysis results.
2.4.Data Extraction and Analyses
Data extraction included:(1) basic information of the literature including the first author,intervention cycles,sample size,mean age,and year of publication;(2) treatment information of the study which included treatment methods,outcome indicators,and adverse effects.If the data included in the study were incomplete,we would try to contact the original author for supplementation.
2.5.Stata 16.0 software was used for this meta-analysis
The therapeutic effect might be affected by different western medicine or intervention cycles in each study,therefore a randomeffect model was used.Cohen's D and 95% confidence interval (CI)were used for continuous variables,and RR (relative risk) was used for secondary variables.Q statistics and I2 were used to judge the heterogeneity of the study (i.e.when the p-value of Q statistics <0.1 or I2 >50%,there is a large heterogeneity between the studies).A L'Abbe chart was used to test the heterogeneity of binary variables.A funnel graph and an Egger test were used to evaluate publication bias.If there was significant heterogeneity between studies,a sensitivity analysis would be conducted,and then meta-analysis was conducted by excluding the studies that inducing the heterogeneity.
3.Results
3.1.Literature Selection
Altogether,391 documents were retrieved,290 of which were obtained after removing duplicate literature or publications with the same data,29 of which were left after reading the title and abstract.After reading the full text,8 studies met the inclusion standards and were eventually included,all of which were published in journals.Figure 1 shows the inclusion and exclusion flow chart.
3.2.Literature Characteristics
Among the 8 studies [7-14] included,2 studies [8,10] used the allocation hidden method,which was evaluated as low risk;1 study[7] used the random allocation method,which was evaluated as high risk and the rest of included studies did not clearly describe the method of random sequence,evaluated as unknown risk;2 articles [12,14] mentioned the follow-up recurrence and 2 articles [7,13]reported the adverse reactions,which were evaluated as low risk or unknown risk.Table 1 shows the basic information of the included studies,and Figure 2 provides a risk of bias graph of the included literature.Table 2 presents the results of GRADE:recurrence rate,total efficiency,mayo mucosal score under colonoscopy,adverse reactions,total symptom score.

Table 1 Basic information of the included studies
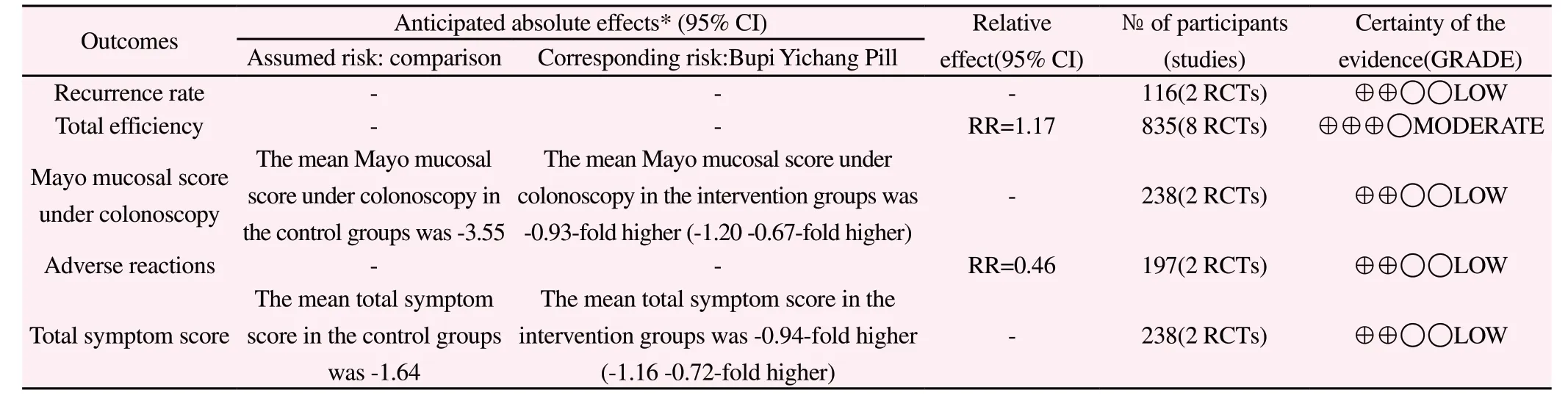
Table 2 GRADE summary of comparing Intervention groups with Comparison groups
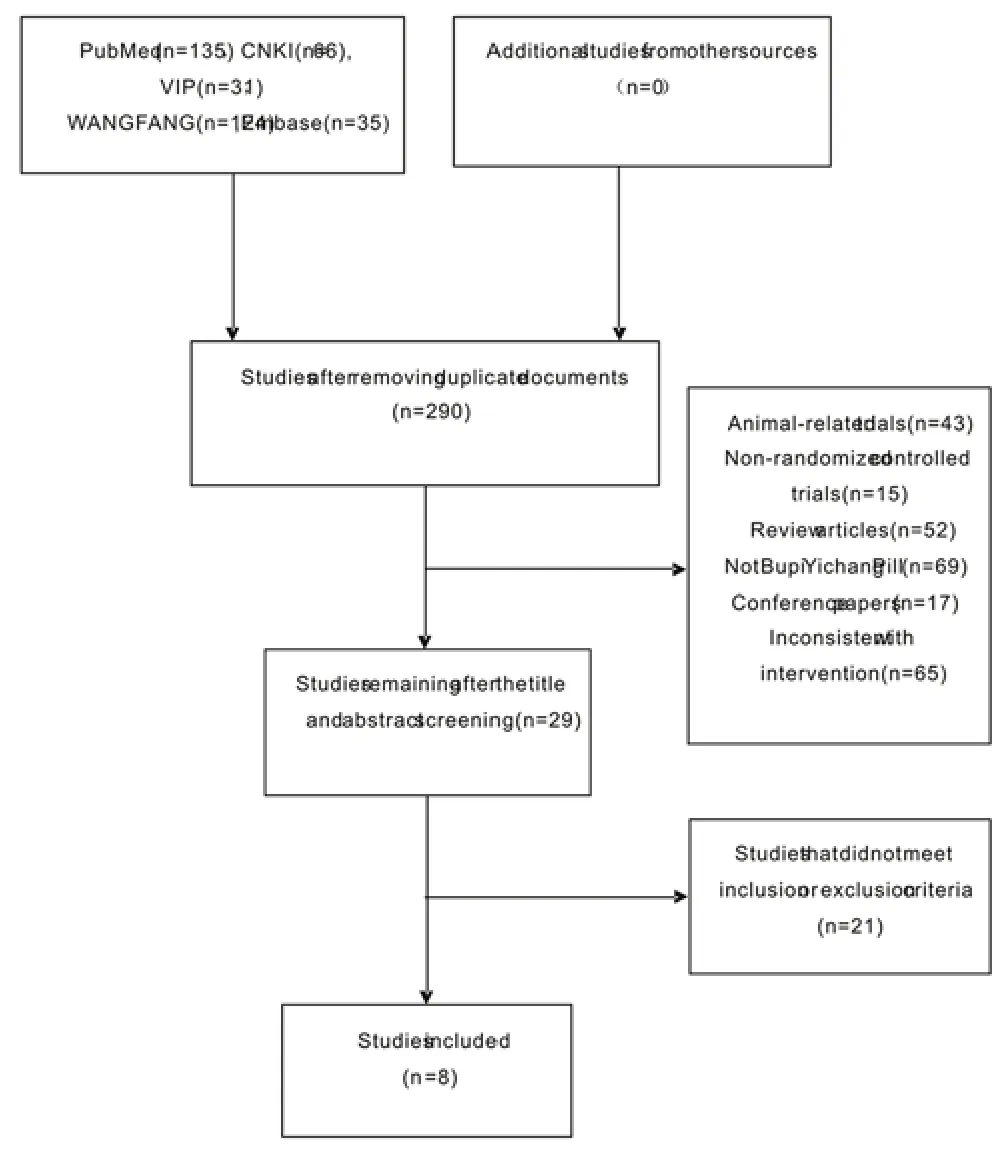
Figure 1 Flow chart of literature search
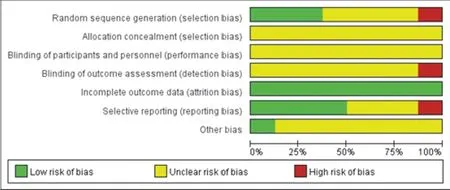
Figure 2 Risk of bias graph
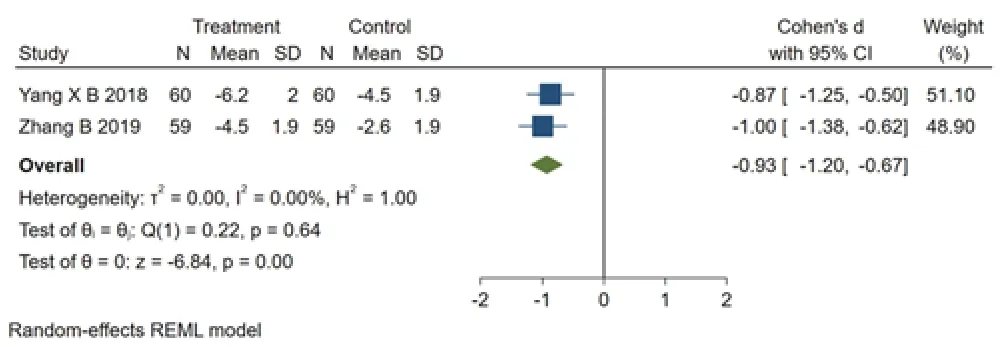
Figure 3 Forest plot of comparison of Mayo mucosal score under colonoscopy between the Bupi Yichang Pill group and Mesalazine sustained-release granules group
4.Results
4.1.Mayo mucosal score under colonoscopy
Two studies [8,9] reported Mayo mucosal score under colonoscopy.The intervention methods of control groups were all mesalazine sustained-release granules.Shown in Fingure3,the results of heterogeneity test demonstrated that there was no a significant heterogeneity between the studies (Q (1)=0.22,P=0.64,I2=0.00%).And a statistical difference between studies exists (SMD=-0.93,95% CI [-1.20,-0.67],p=0.00).
4.2.Total efficiency
Eight studies [7-14] reported total efficiency.Because of the different intervention methods of the control groups,we compared and analyzed some of the studies [8,9,10,11,12] through the subgroup.The results showed that there was no a significant difference in comparing Bupi Yichang Pill with Sulfasalazine (RR=1.13,95%CI [1.05,1.21],p>0.05),Bupi Yichang Pill with Mesalazine sustained-release granules (RR=1.26,95% CI [1.11,1.43],p >0.05).And there was no obvious heterogeneity between these studies(Q(4)=3.42,P=0.49,I2=17.67%).Figure 4 presents this data in a forest plot.Combined with shear complement analysis,Egger test results showed that there is no published bias (β1=1.82,SE of β1=1.113,z=1.63,P=0.1028).The L'Abbe plot of the heterogeneity test and funnel plot are presented in Figure 5.
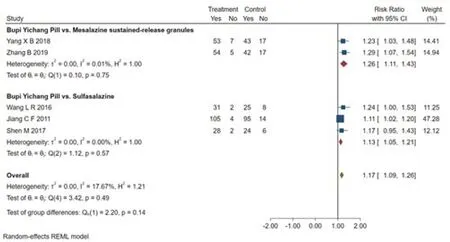
Figure 4 Forest plot of subgroup analysis of total efficiency
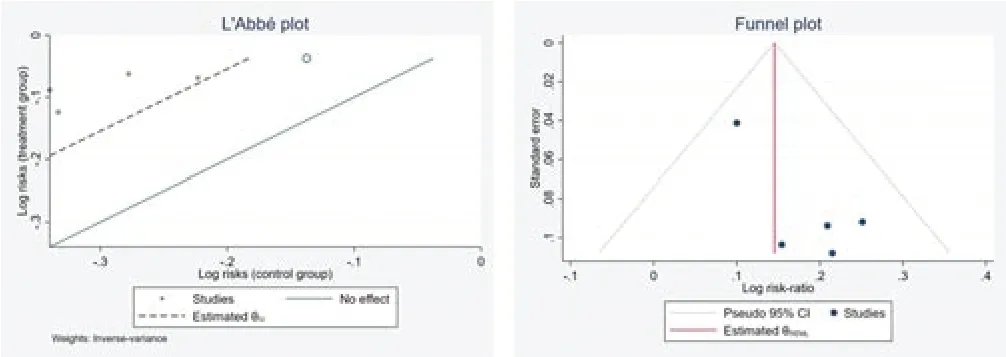
Figure 5 L'Abbe and funnel plots of total efficiency
4.3.Total symptom score
Two studies [8,9] reported the total symptom score.Figure 6 presents a forest chart which shows the statistical differences among studies (SMD=-0.94,95% CI [-1.16,-0.72],p=0.00),while an obvious heterogeneity (Q(9)=29.73,P=0.00,I2=70.71%) exists among the different groups.Across the studies,the total score of symptoms including fatigue,abdominal pain,tenesmus,diarrhea,bloody purulent stool in the treatment group was lower than that in the control group.
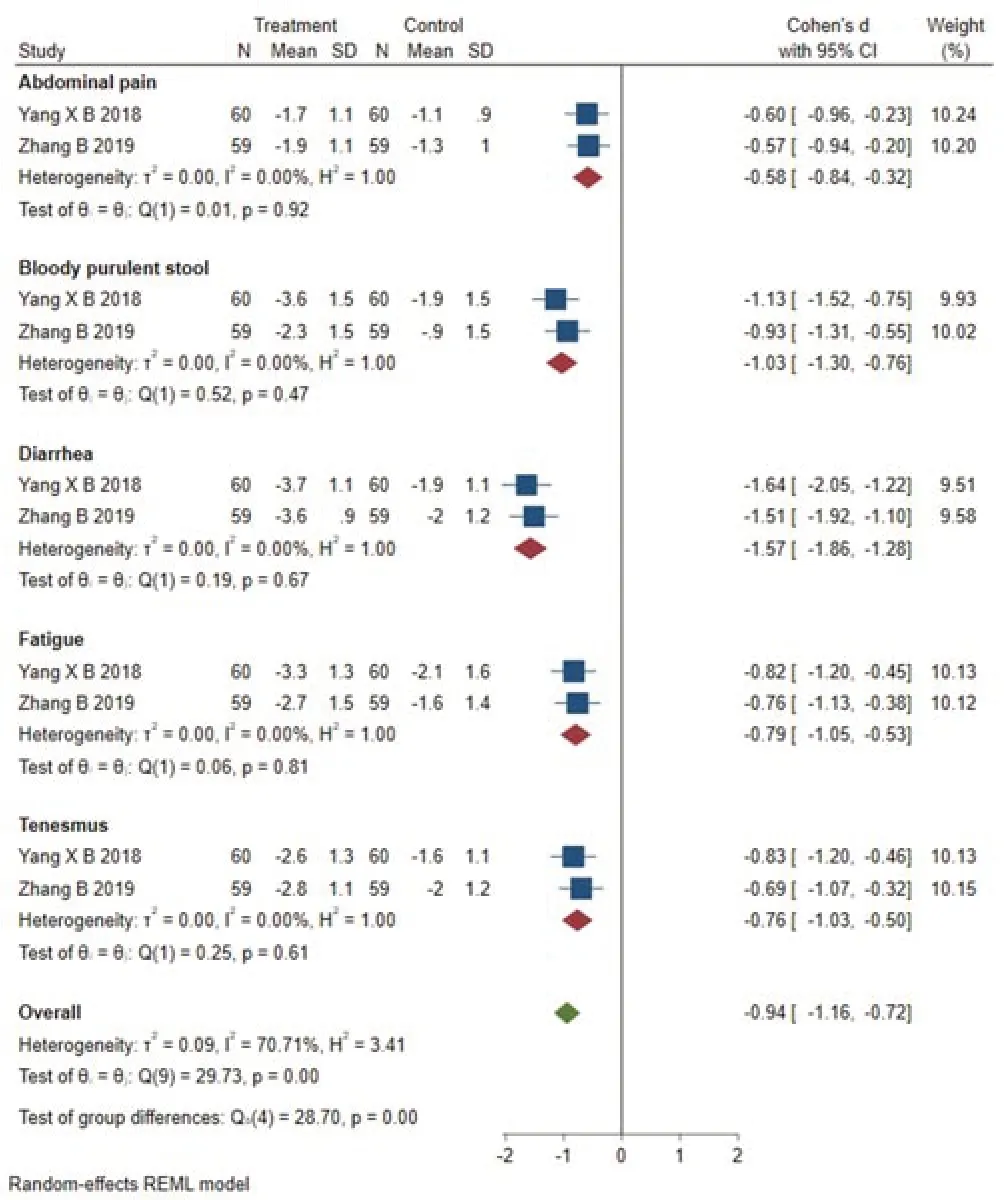
Figure 6 Forest plot of subgroup analysis of total symptom score
4.4.Adverse effect
Adverse events were reported in 2 studies [7,13].Figure 7 shows there is no significant statistical difference in comparing Bupi Yichang Pill group with mesalazine group (RR=0.46,95% CI[0.16,1.28],p>0.05) and no heterogeneity among studies (Q (1)=0.34,P=0.56,I2=0.00%).There were fewer adverse events in the treatment group than in the control group.
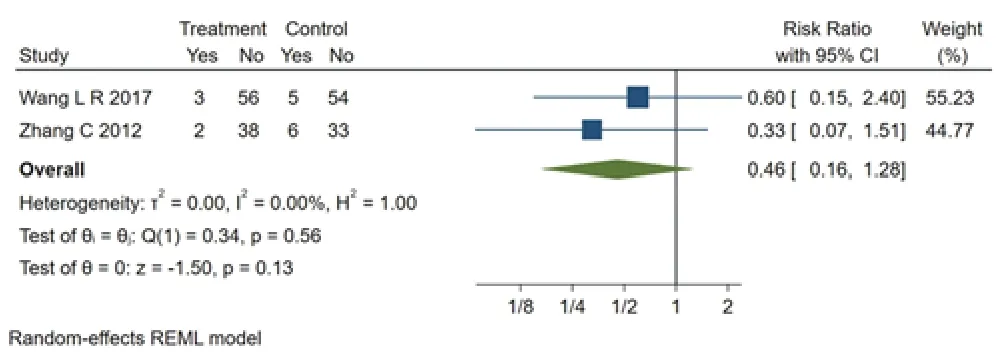
Figure 7 Forest plot of comparison of adverse effect
5.Discussion
In this systematic review and meta-analysis,the efficacy and safety of 8 Bupi Yichang Pill concerned studies for patients with ulcerative colitis were evaluated.We found that Bupi Yichang Pill can effectively improve the clinical symptoms and colonic mucosal symptoms in ulcerative colitis patients,with less adverse effect and more safety.Especially in the Grade summary,the quality grade of total efficiency was relatively high,reflecting that Bupi Yichang Pill could significantly improve the overall efficacy.The heterogeneity of the total symptom score might be derived from the different diarrhea assessment criteria in the two studies.Meanwhile,in terms of the recurrence rate,the two studies [12,14] showed that Bupi Yichang Pill was better to stable the patients' condition and reduce the recurrence rate,which could be used as preliminary evidence for clinical drug recommendation.However,there were still some limitations in this study like in some literature,the outcome evaluation indexes were not comprehensive enough,and objective outcome indexes such as erythrocyte sedimentation rate and C-reactive protein were not included as well as in some literature,recurrence,adverse effects and follow-up time were not mentioned.Heterogeneity might be enhanced by differences of other diseases in patients.
According to previous studies on ulcerative colitis [15],it has been found that the probability of colorectal cancer 10 years after the diagnosis of ulcerative colitis is 2%,and 30 years later is 18%,which should not be neglected by us.Currently,5-amino-salicylic acid,corticosteroids,and immunosuppressants are commonly used in the clinical treatment of ulcerative colitis,but problems such as hepatorenal toxicity,drug resistance,or allergic reaction still occur from time to time,which does not substantially improve the quality of life of patients.At the same time,in the cases of initial corticosteroid treatment,patients relapsed soon after drug withdrawal or a dosage reduction,and even up to 20% of patients develop steroid-dependent ulcerative colitis [16].Also,immunosuppressants like methotrexate were usually used for patients with moderate or severe ulcerative colitis,although it could alleviate the clinical symptoms of patients with ulcerative colitis,intestinal mucosal inflammation under the endoscopic did not get improved,and even in the aspect of preventing recurrence of ulcerative colitis,it was not superior to placebo [17].It has been proved that about 75% of patients who used methotrexate relapsed within five years after the drug withdrawal [18];Moreover methotrexate is likely to reduce DNA integrity in sperm and cause damage to the reproductive system through oxidative stress [19].At present,Chinese patent medicine has been widely used in clinical practice,which has a significant effect on improving the symptoms of ulcerative colitis,clinical efficacy,and reducing recurrence.Relevant studies have also confirmed that Chinese patent medicine can intervene in the development of ulcerative colitis through anti-inflammatory,immunological regulation,mucosal repair,and other ways [15,20-33].
The main pathogenesis of ulcerative colitis is based on spleen deficiency,a mix of deficiency and excess.Therefore,invigorating qi and invigorating spleen,and clearing dampness are the primary treatment for ulcerative colitis.Bupi Yichang Pill can nourish the middle and nourish qi,invigorate the spleen and stomach,and relieve diarrhea.The composition of Bupi Yichang Pill includes Codonopsis,astragalus,Fructus amomi,Paeoniae,Angelicae Sinensis,atractylodes,cinnamon,Rhizoma Corydalis,Litchi Semen,dried ginger,licorice,aucklandiae,Fructus psoraleae,Halloysitum Rubrum and Saposhnikoviae,which are verified to meet the standards set by the National Food and Drug Administration [34].Codonopsis,astragalus,and atractylodes are the main medicine for invigorating the spleen and qi,at the same time astragalus also has the effect of reducing swelling and discharging pus,modern pharmacological studies have shown that [35],astragalin (ASI) is the main active ingredient of astragalus,which can induce the activation of T cell,modify the balance of T cells,enhance the activity of CD45 phosphatase and inhibit pro-inflammatory cytokines.Fructus amomi has the effect of removing dampness and regulate the function of the stomach;Cinnamon,dried ginger,Fructus Psoralea can tonify the spleen and kidney.And as the main active compounds of cinnamon,cinnamaldehyde (CA) has the function of antibacterial and anti-inflammatory and reducing the number of pro-inflammatory cytokines and NLRP3 inflammatory,miR -21 and miR -155 in colon tissue,which can improve the ulcerative colitis [36].Some scholars have also found [37] that dried ginger has an obvious effect on anti-inflammatory for ulcerative colitis mice,which is similar to the cinnamon with the effect of relieving symptoms like weight loss,higher disease activity index (DAI),colon length shortening,inflammatory cells infiltration and so on.Paeoniae,Angelica Sinensis,Rhizoma Corydalis can harmonize qi and blood,promote blood circulation,and relieve pain.Litchi Semen and aucklandiae can promote qi to relieve pain,which reflects that "after regulating qi,tenements will be removed,and after smoothing the blood flow,the bloody purulent stool will diminish".Halloysitum Rubrum with the efficacy of inducing astringency lower jiao can improve chronic diarrhea.Saposhnikoviae called as the "Run agent in Feng medicine" can improve the symptoms like abdominal pain,diarrhea,and bloody purulent stool.Therefore,no matter from the perspective of traditional Chinese medicine theory or modern pharmacological research,Bupi Yichang Pill has a significant therapeutic effect on ulcerative colitis.
In conclusion,our systematic review and meta-analysis can preliminarily prove the efficacy and safety of Bupi Yichang Pill in the treatment of ulcerative colitis,but it still needs to be verified by clinical large sample trials.
Conflict of Interest
All authors declare that they have no conflict of interests.
Authors’ Contribution
Xiaoxiao Xing analyzed the data and wrote the draft.Jianbo Guo and Huashan Li designed the study.All authors read and approved the final manuscript.
 Journal of Hainan Medical College2022年1期
Journal of Hainan Medical College2022年1期
- Journal of Hainan Medical College的其它文章
- Research progress of pattern recognition receptors and chronic periodontitis
- Research progress on the correlation between TCM syndromes of coronary heart disease and blood biochemical indexes
- Rhegmatogenous retinal detachment after intraocular lens implantation in high myopia:A case report and literature review
- Analysis on the law of differentiating and treating insomnia by physicians based on cluster analysis of drug syndrome
- Study on the mechanism of Yupingfeng Powder in the treatment of non-small cell lung cancer based on network pharmacology and molecular docking method
- Clinical effect of Chinese medicine aerosol fumigation on demodex infection related meibomian gland dysfunction
