Ignition of nanothermites by a laser diode pulse
Alexnder Yu.Dolgoorodov , Vldimir G.Kirilenko , Michel A.Brzhnikov ,Leonid I.Grishin , Michel L.Kuskov , Georgii E.Vlyno
a Joint Institute for High Temperatures, Russian Academy of Sciences, Russia
b N.Semenov Federal Research Center for Chemical Physics, Russian Academy of Sciences, Russia
c National Research Nuclear University MEPhI Moscow, Russia
Keywords:Nanothermites Laser ignition Burning rate Ignition delay
ABSTRACTExperimental investigation has been carried out for laser ignition and combustion of nanothermites based on aluminum and oxides of copper, bismuth and molybdenum.Ultrasonic mixing of nanosized powders was used to produce compositions.For thermite ignition,initiating laser pulse with a maximum intensity of 770 W/cm2 was generated by a laser diode with a wavelength of 808 nm.The ignition delay times,the minimum initiation energy density,and the average burning rate at various thermite densities and mass fractions of components were determined by recording the emission of radiation of the reaction products using a multichannel pyrometer jointly with a high-speed video camera.The effect of adding carbon black on the threshold parameters of a laser pulse was also studied.Based on the obtained results, certain assumptions were put forward with regard to the mechanism of nanothermites’ ignition by laser radiation and their burning.In particular,the assumptions were made on the two-stage process of the reaction initiation and jet burning mechanism of porous nanothermites.
1.Introduction
Thermite compositions prepared using aluminum mixed with metal oxides provide a high exothermic effect during combustion(more than 20 kJ/cm).However, common mixtures based on micron-sized particles have low combustion rates (about tens of millimeters per second) and high ignition temperatures, which limit the scope of application of such thermites.The rate of propagation of reactions in solid oxidizer-fuel mixtures predominantly depends on the area of the effective contact surface of the reactants.The usage of nanosized components significantly increases the reactivity of thermites due to larger surface area of the components.Energetic compositions based on mixed nanoparticles of metals and solid oxides are comparable in terms of energy release rate to monomolecular energetic materials and are, thus, capable of reacting in an explosive combustion mode at speeds of more than 1 km per second [1].These compounds are often called nanothermites or nano energetic materials (NEM).The descriptions of the various manufacturing techniques and the characteristics of NEM produced can be found in the literature [1,2].The most widespread method for production of NEM is via ultrasonic mixing of nanosized powders of aluminum and solid oxides in various mass ratios.
The high reactivity of NEM allows them to be used as components in various initiating and incendiary devices.In particular, a number of works has demonstrated the possibility of using NEM in laser initiation systems.Unlike other methods of initiating a reaction in energetic materials,laser radiation has a level of high noise immunity and stability for the excitation of fast chemical reactions[3].With the reduction in the size of modern laser systems, it became possible to create for mobile devices remote laser initiation tools.Currently, researches are in active search for optimal compositions for laser initiation [4-12].
This paper presents the results of studies of initiation of NEM Al/CuO, Al/BiOand Al/MoOby a pulse of a small-sized laser diode with a maximum power of 4 W(the maximum intensity of 770 W/cm).Ignition delay time, minimum energy density and burn rate were determined as a function of porosity and stoichiometry of composions using the technique described in Ref.[13](see Section 2 of the present paper).Comparison with the results[13]for a laser beam with an intensity of 207 W/cmand a cross section of 1.5 mmis shown in Section 3.1, Table 3.
As demonstrated below the addition of nanosized black carbon has a significant effect on the sensitivity of NEM to laser radiation.
2.Experiment
2.1.Materials
To prepare NEM, we used nanosized powders of Al, CuO, BiOand MoO.Nano-Al powder consisted of spherical particles with an average particle size of 100-120 nm and was obtained by the Gen-Miller Flow-Levitation evaporation-condensation method[14]using a modified MIGEN installation[15].The active aluminum content in the powder was 90-93%.Nano-CuO powder with particle size of 50-80 nm and specific surface area= 20 m/g was procured from “Advanced Powder Technology”, Tomsk.Nanoparticles of BiOand MoOpowders were obtained by grinding in an energy-intensive planetary mill“Activator-2SL”.Initial chemical materials powders were 20-200 μm in size.After grinding, the powders were submicron agglomerates consisting of fragmented particles with sizes ranging from 20 to 200 nm.SEM images of the grinded powders are shown in Fig.1 (a, b).
The obtained powders were placed into hexane in the container of MEF93-T ultrasonic disperser.They were subsequently mixed in several stages.Firstly, oxidizer powder was placed in a container and dispersed for 8 min to break up conglomerates.Then nano-Al was added to the oxide/hexane mixture and sonicated for another 24 min with water cooling.The powders of metal oxide(which would play the role of oxidizer interacting with Al) and aluminum mixed in a certain mass ratio are characterized by the so-called equivalence ratio.The latter is determined as, φ = (Al/Ox)/(Al/Ox)where Al is mass content of aluminum, Ox is content of oxidizer, subscripts “act” and “st” indicate the actual and stoichiometric ratios (this equation considers the actual active Al content in nanoparticles).So, φ = 1 corresponds to the stoichiometric ratio, φ < 1 indicates the lean fuel composition, φ > 1 indicates the rich fuel composition.
The mixtures under study were prepared with the following mass ratios Al/CuO(19/81,φ=0.96),Al/BiO(11/89,φ=0.99),Al/BiO(13/87,φ = 1.20), Al/BiO(15/85,φ = 1.41), Al/BiO(30/70,φ=3.43)and Al/MoO(30/70,φ=1.04).In some tests with Al/CuO in order to produce additional hotspots at laser ignition,nanosized powder of carbon black was added (1-5 wt %)
After ultrasonic stirring, the resulting suspension was dried,kept in a vacuum for 24 h, and then sieved through a sieve with a 0.2 mm mesh to break up the agglomerates.All operations with the compositions were carried out with great care due to the high sensitivity of freshly prepared nanothermites to mechanical, thermal and electrical influences.
For the mixtures, the aging effect was observed.Freshly prepared mixtures were found to be very active due to the partial destruction of the aluminum oxide film under ultrasonic exposure but after having been kept in an open air for at least two weeks,their properties became has been stabile.
Fig.1 shows the SEM images of grinded powders BiOand MoO, and NEM Al/CuO, Al/BiO, and Al/MoOwhich show the characteristic sizes and mutual arrangement of nanoparticles.
The produced NEM underwent preliminary tests in order to measure the temperature of ignition of the mixtures,when heated by the hot surface of a massive copper plate according to the techniques described in Ref.[16].The heating of the copper plate was carried out in a muffle furnace.A weighed amount of the powder(30±1 mg)was quickly input into a furnace and placed on a heated copper plate.The temperature control with an accuracy of 1C was performed by a copper-constantan thermocouple.The ignition delay was determined by a stopwatch with an accuracy of 0.5 s.Table 1 shows the values of the minimum temperature of the copper plate Tat which the nanothermites ignited in 3 consecutive experiments.
Special characteristics of ignition of Al/CuO nanothermites should be noted.In contrast to the previously investigated mechanoactivated mixtures [16], nano-Al/CuO ignites in a very narrow temperature range.The ignition of Al/CuO(φ=0.96)occurs with a delay of less than 0.5 s at a surface temperature T≥460C.However, with a slight decrease in temperature to 454C,ignition does not occur at all within 2-3 min,even if is the temperature is subsequently increased to 500C.This indicates fundamentally different scenarios for the development of the reaction, depending on the intensity of energy input.It can be assumed that on one hand heating the mixture to the temperature below,but close to critical is insufficient for its autoignition,and on the other that under such conditions the active ignition centers of the reaction “burn out”, so that the subsequent increase in the temperature above the critical value (additional energy supply)does not result in combustion.
2.2.Sample preparation
In order to carry out tests for laser ignition, the mixtures were loaded in portions into the cylindrical duct (5-7 mm in diameter)of the target (textolite plate 4-8 mm of thick).Both sides (frontal side,facing the laser and back one)of the duct were covered by thin(0.1 mm) cover glasses.The porosity of samples ε is calculated by the formula: ε = (1 - ρρ) × 100%, where ρρis the relative composition density (TMD - theoretical maximum density).The porosity of loose-packed samples ε ranged from 60 to 90% and samples with a porosity of 50% were prepared by hand pressing.
Photographs of the frontal surface of samples of Al/CuO mixture of different porosity obtained using optical digital microscope Levenhuk DTX-90 are shown in Fig.2.For the sample with a porosity of 89%shown in Fig.2a,one can see a mixture of granules(10-150 μm)with macroscopic pores(50-60 μm).The granules are produced by sieving the agglomerated dried mixture.They can be easily destroyed by application of weak pressure and thus the large pores of tens microns in size disappear and the structure of the sample changes noticeably (Fig.2b).
In some tests with Al/CuO(φ=1)in order to produce additional hot spots at laser ignition, carbon black nanosized powder was added (1-5 wt %).
2.3.Experimental set-up
The scheme of the experimental set-up is given in Fig.3.Laser radiation was generated by a laser diode module with a nominal power up to 4 W.The laser beam passed through an optical focusing system and protective glasses,and the actual power of the laser beam was measured by the optical power meter(FieldBest II,Fastlaser Tech, China).The effective characteristics of the laser beam are shown in Table 2.

Table 1Ignition temperature.

Table 2The actual characteristic of the laser beam at the sample surface.
The effective cross-sectional areaof the beam with a“Gaussian” radiation intensity distribution over the cross-section was determined using the level of the intensity decay by a factor of.was determined experimentally.The power of laser radiation () passed through holes of different sizes.The area of the holes () varied from 0.30 mmto 2.92 mm.This way, the dependence ofversus reciprocal area of the holes(1/)was found to be approximated by the function:= 3.376-0.222, whereandare respectively given in W and mm.
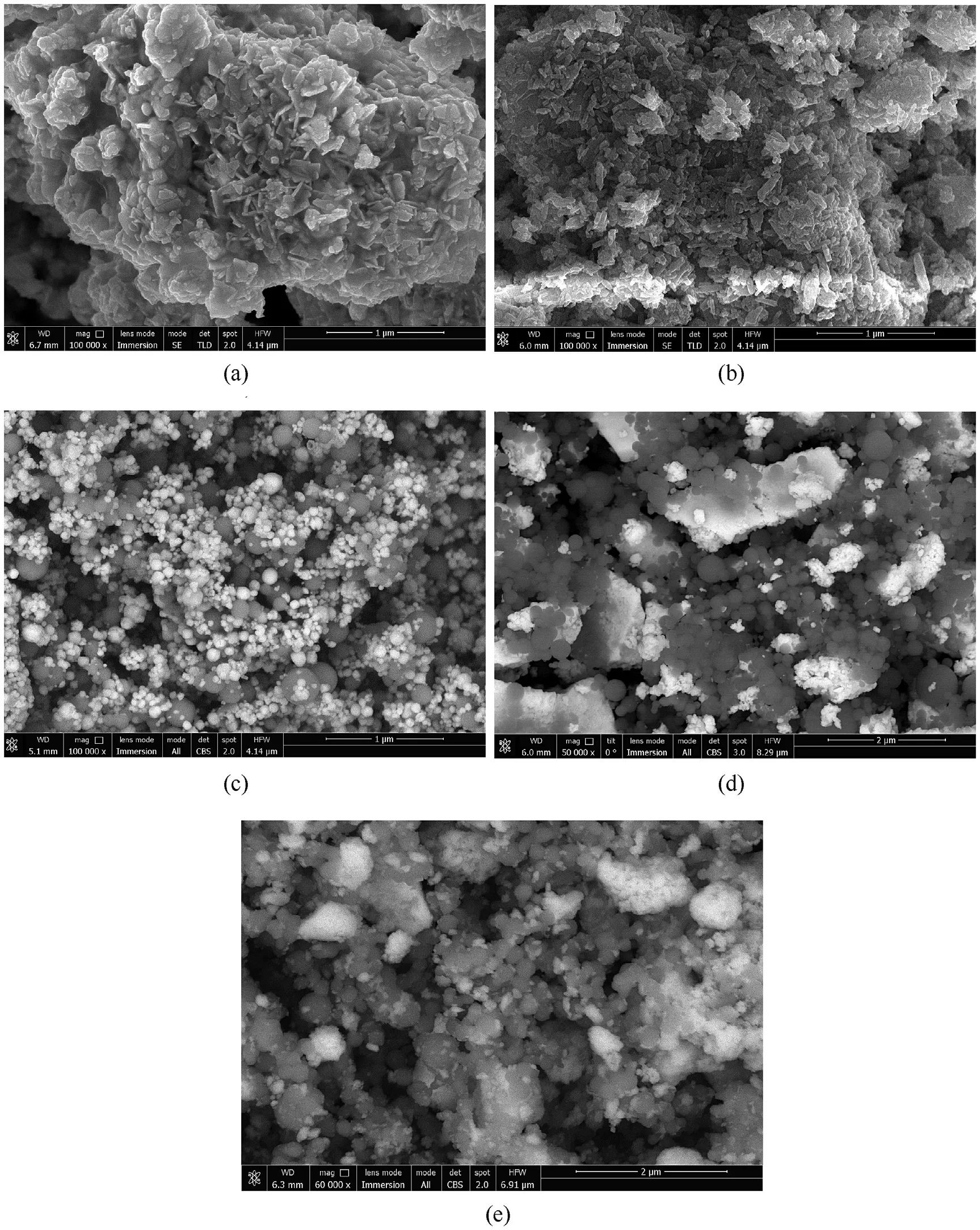
Fig.1.SEM images of nano- Bi2O3 (a), MoO3 (b), Al/CuO 19/81 (c), Al/Bi 2O3 15/85 (d), and Al/MoO3 30/70 (e), nano-Al particles are gray.
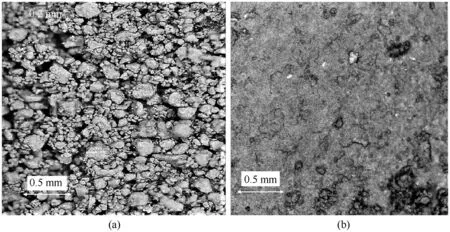
Fig.2.Microphotographs of frontal surfaces of Al/CuO samples of different porosity ε = 86% (a) and ε = 78% (b).
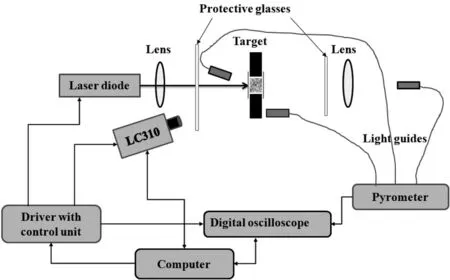
Fig.3.Experimental set-up.
To control the operating of the experimental set-up,a computer program was used.The computer set duration of laser pulses and controlled the settings of both the digital oscilloscope and highspeed video camera Phantom Miro LC310.After having received the command from the computer,the control unit generates a TTLpulse of a certain length, the leading edge of which triggers the oscilloscope,video camera trigger and laser(with a delay of 100 μs).Subsequently the falling edge of TTL-pulse turns off the laser(with a delay of 33 μs).The lens in front of the target is responsible for additional focusing of the laser beam,while the lens located behind the target focuses the light from the backside at the input of the optical fiber.Typical oscillograms of a laser pulse(1,vertical scale is 500 mV/div)and the corresponding TTL pulse(2,the vertical scale is 2 V/div)that controls the laser diode driver are shown in Fig.4.

Fig.4.Oscillograms of a laser pulse (1) and TTL pulse (2).
The intensity of light emission from the reaction products was recorded from the front and rear surfaces of the sample by a multichannel pyrometer using optical fibers with a quartz core diameter of 0.4 mm.In some experiments using an additional light guide,the rate of combustion wave propagating in the radial duct of the target was measured.
To exclude“failures”in initiation,the duration of the laser pulsewas selected in such a way as to exceed the ignition delayof the mixture studied.The consequent attempt of successful ignition of the“failed”samples requires a significant(up to 2-fold)increase in the pulse duration,and hence the energy of the laser pulse.It can be assumed that in this case “annealing” occurs, i.e., the burnout and degradation of active ignition centers on the sample surface.Thus,in such“failed”samples the number of“ignited”centers(hot spots) is insufficient for the development of a self-sustaining process during the first attempt to initiate.In our opinion,taking into account all the difference in the energy supply to the sample,such situation is similar to the heating the mixture by a hot surface to the temperature slightly below the threshold Tvalue.
The critical energy densityrequired to initiate burning was determined by numerical integration of the dependence of radiation intensity vs.time from 0 toat>or from 0 toat≤.This way, the radiation losses coming from reflection from the cover glass (≈8%) were taken into account.
Several frames from the high-speed video record of the whole laser initiation process are shown in Fig.6.The same experiment,the radiation time-histories of which are presented in Fig.5 was recorded at the video.The interval between frames is 50 μs; the exposure time is 44 μs.The numbers written at the frames here and below in the text correspond to time intervals (in microseconds)from the beginning of the TTL-pulse that triggers the video camera.
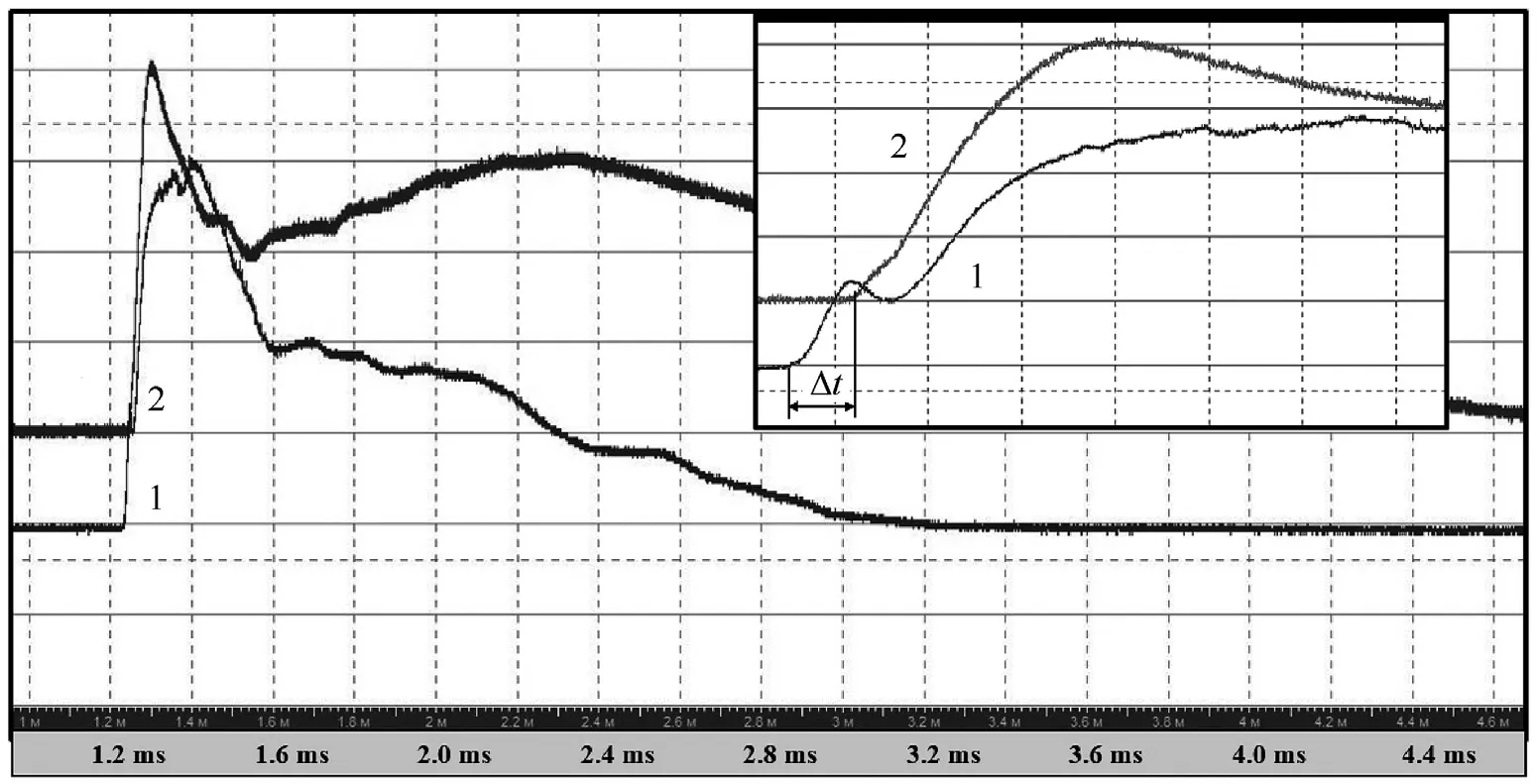
Fig.5.Typical radiation time-histories at the ignition of Al/CuO.
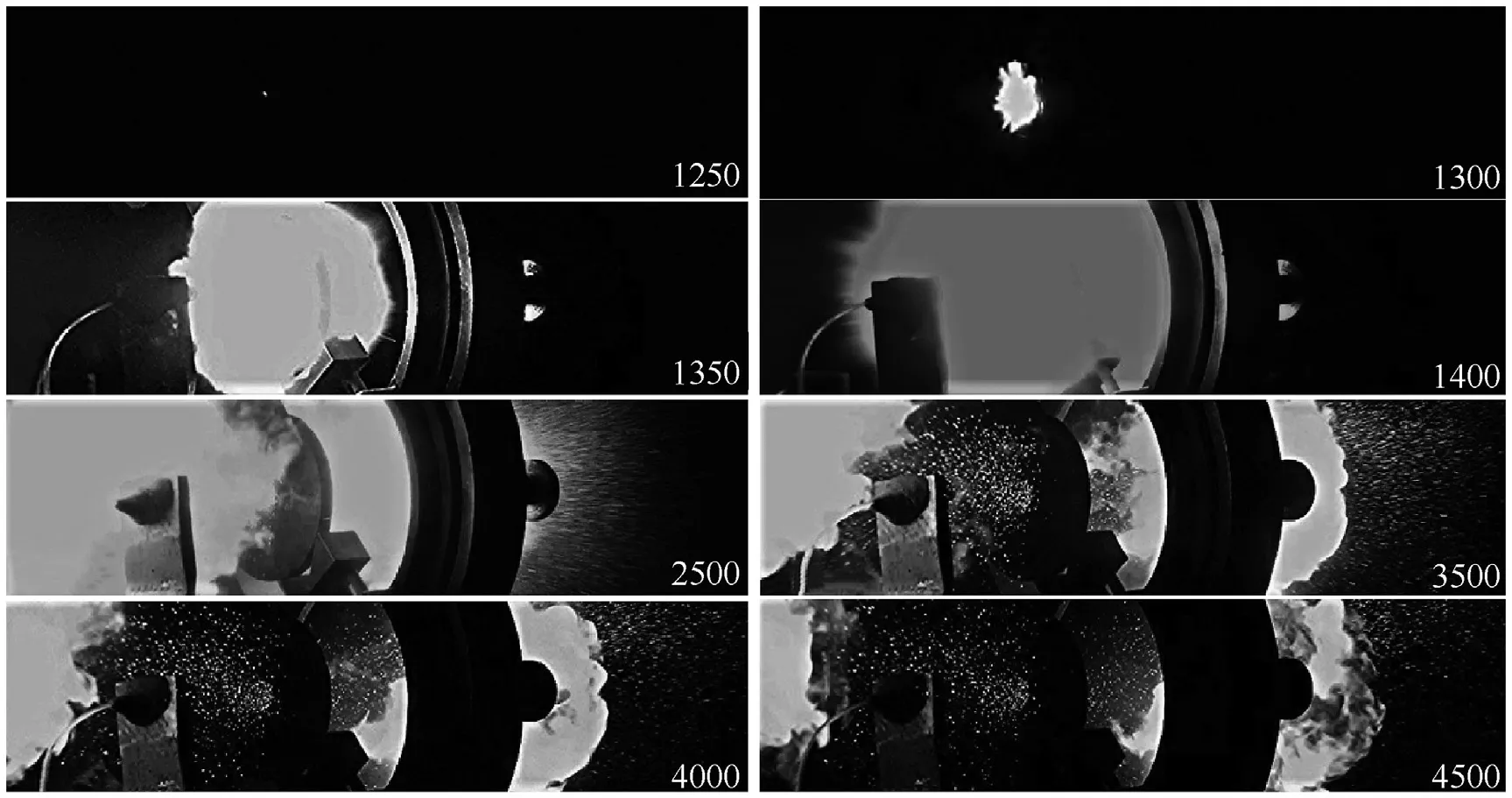
Fig.6.Cinematic footage of the development of the Al/CuO initiation process.
A luminous point seen at the 1st frame (1250 μs) is a sign of ignition of the frontal surface of the sample.After 50 μs,the onset of the injection of the reaction products out of the duct of the target is recorded with subsequent afterburning of the residues of the mixture accompanied by less bright light (see further several frames).The last three frames(3.5-4.5 ms)clearly show the scatter of molten copper droplets.After 5 ms, the glow of the reaction products disappears completely; this fact is also confirmed by the pyrometer records(see Fig.5).
The effect of the laser beam on the velocity of propagation of chemical reaction after ignition (at>) has been evaluated experimentally.In the test with Al/CuO composition(ε=90%),the burning rate was measured both along the axis of the laser beam()in the duct of the target and across the axis in the groove(3 mm of width)made at the front surface of the target().The velocities were measured as follows=(510±10)m/s and=(520±10)m/s.Nearly identical reaction propagation rates measured both along laser beam and perpendicular to it suggests that, being already initiated, the reaction propagates irrespectively of the presence of laser radiation energy supply.
3.Results and discussion
3.1.Optical pyrometer measurements
The key results of studies of characteristics of Al/CuO, Al/BiOand Al/MoOnanothermites’ ignition and burning obtained under the initiation by a laser pulse are presented in Table 3.The presented data is based on average values derived from 10 to 15 tests.Measurement error characterizes the maximum deviation from the average.
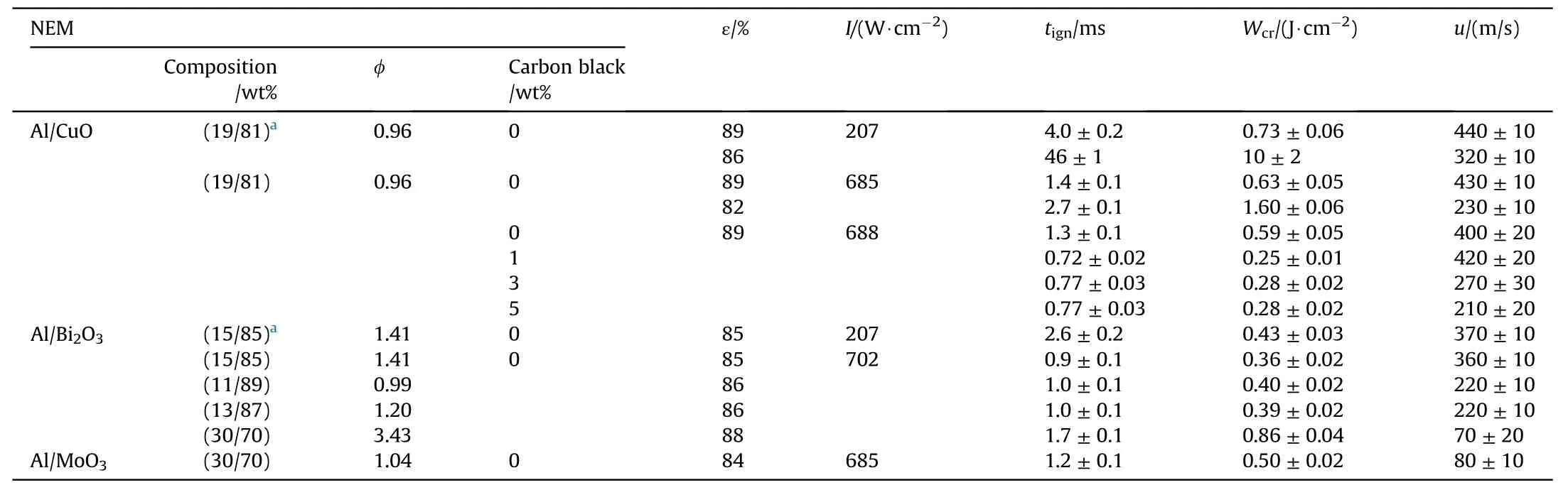
Table 3The key results of studies of characteristics of laser ignition and burning of NEM.
The porosity of the mixture as well as its chemical content has a strong influence on the threshold values of both theand the burning rate of NEM.For highly porous nanothermites, the minimum laser pulse energy=×, required for initiation,varies from 1 to 7 mJ.
The power of laser radiation begins to noticeably affect the initiation energy when the ignition delay (t) becomes comparable with the characteristic time τ estimated below.The data obtained at a lower laser power density[13]confirms this conclusion.
One can approximately estimate the characteristic time τ,whenheat conductivity becomes noticeable from the condition of equality of the heat flux from laser radiation heating() and the radial heat losses ().As it is shown in Ref.[17], in the steady state one-dimensional approximation and cylindrical geometry radial heat flux obeys Fourier’s law:
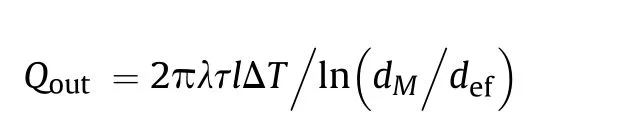
where λ is heat conductivity of the mixture,=/is the thickness of the layer heated by Δdegrees,is the volume of this layer,- is the ratio of the diameters of the hole in the target and the effective laser spot,is the area of the laser spot.On the other hand,the energy required for heating the volumeby Δdegrees can be estimated as:
Cinderella, indeed, expected well such answer, and was very glad of the refusal; for she would have been sadly put to it if her sister had lent her what she asked for jestingly.
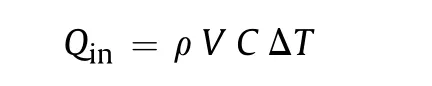
where ρ is density of the mixture andis its specific heat.
From the condition=, the characteristic time can be estimated by the formula:
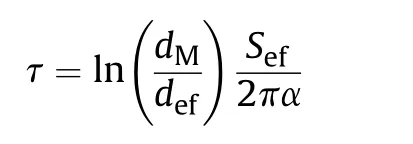
where α = λρis the coefficient of thermal diffusivity of the mixture.
The values of density,heat capacity,and thermal conductivity of the mixture were calculated according to the additivity rule.Thus,in the process of laser initiation, energy losses due to thermal conductivity can be neglected at<τ ≈3 ms.
Under certain conditions, heat loss into the target material can also affect the development of the initiated reaction (the burning rate).Indeed, when replacing a textolite target (thermal conductivity coefficient λ=0.3 W/(m×K))with a copper one(λ=401 W/(m × K)), the burning rate of Al/CuO (ε = 89%) decreases from 440 m/s to 340 m/s, while the threshold values ofremain the same.From the independence offrom the target material, it follows that the radial dimensions of the reaction volume are small.In Ref.[18]it is shown that the radial size of the reaction volume is much smaller than the radius of the laser beam and depends on the critical ignition temperature,the activation energy of the chemical reaction (for Arrhenius kinetics) and the radius of the light beam.
The ignition delayas well as the threshold energy have been more than halved when 1%of carbon black was added to the Al/CuO(φ = 1) mixture.This, apparently, suggests that carbon black particles, which have absorption ability close to one, act as additional active hot spots, from which additional reaction centers can arise.At the same time,an increase in the carbon black content up to 5%leads to a decrease in the burning rate at the same.
In our opinion,the issue of hot spots,the appearance of which is usually only considered for structural inhomogeneities and inclusions (like soot), etc.requires a separate discussion and study.For example, when the wavelength of a coherent light source is commensurate with the size of nanoparticles,diffraction can cause the appearance of diffuse maxima, which can arise when the light interacts with the sample’s inhomogeneities, which can be considered as “irregular” diffraction grating.
In all experiments, the radiation recorded by the pyrometer from the frontal surface of the sample has a clear two-peak profile(see curve 1 in Figs.5 and 7).
It is, thus,possible to assume that the reaction proceeds in two stages[6].In the first stage,the reaction is initiated and spread over the surface of the sample,and in the second,it is developed in inner layers of the NEM with subsequent dispersion of the particles.Since radiation on the rear surface of the sample appears before the first stage is completed,we can speak of the simultaneous development of the reaction both on the surface and in the bulk of the sample.
As mentioned before,in the event of failure of initiation during the first irradiation, a pulse with significantly higher energy is required to re-initiate the sample.As a result of the first irradiation,some of the active centers of the reaction are“annealed”.Therefore,repeated irradiation requires notably more energy for the reaction on the surface of the sample to be started.Since a part of the mixture on the surface has already reacted,the intensity of the first peak decreases after re-initiation.The energy released at the first stage of the reaction goes to the development of the second stage,which proceeds in the volume of the sample.It is recorded as the second, longer, radiation peak.Going forward the nature of the glow is determined by the afterburning and scattering of hot reaction products.
Four Al/BiOmixtures with different component ratio(φ varied from 0.99 to 3.43) were studied.The optimal composition with φ = 1.41 has the maximum burning rate (360 m/s) and minimum threshold values of the initiating laser.A close value of the burning rate (400 m/s) for a similar mixture was obtained in Ref.[5]at φ = 1.3.
The porosity of nanothermites has a strong influence on both the combustion rate and the initiation characteristics.One can assume that with the decrease in porosity of the composition, the bulk energy density would increase, but simultaneously the conditions for the convective combustion mode deteriorate.For tested mixtures, even insignificant pressing destroys the “macroscopic pores” in the sample and thereby significantly deteriorates the conditions for convective combustion(see Fig.2a and b).In order to confirm the leading role of convective mechanism and the role of the“macroscopic pores”in this combustion mode,the burning rate of Al/CuO mixture was measured for porosity ε varied from 50%to 90%, see Fig.8.The dependence of the combustion rate of the Al/CuO mixture on porosity shown in the Fig.by solid line is approximated with high accuracy by the function:= exp(4.57-0.0609ε + 0.000881ε), where burning rateis in m/s and the porosity ε is in %.
In Fig.8,the dotted line shows extrapolation of the dependence to higher porosity values.As can be seen from the Fig., the experimental values taken from Refs.[5,10]correlate with the results of the present work.Unfortunately, the lack of accurate information on the porosity of mixtures studied by other researchers[4,6,7,11,19]makes it impossible to perform unambiguous comparison of their data with the results of this work.Moreover, the differences in content of mixtures and experimental conditions also complicate the comparison.From the graph, one can see that an almost four-fold decrease in the combustion rate occurs with a decrease in porosity by only 16%(from 94 to 78%).This may indicate a noticeable rate of propagation of the reaction through the channels of macroscopic pores in-between porous granules.
3.2.High-speed imaging of laser ignition and burning
Several video frames of the initial stage of laser initiation of the reaction are shown in Fig.9 (in this test the registration was done without the neutral light filters).The time interval between the frames is 4.54 μs and the exposure time is 0.96 μs.In the first frame,only the spot of the laser beam is seen;after 4.54 μs(second frame)one can observe the light emitted by the reaction’s hot spots,which is still located within the laser spot in its center.In 9 μs the reaction still covers the laser spot (frames 3 and 4) and it then starts to spread over the surface (frames 5 and 6).A similar picture was observed in several tests, the formation of reaction centers occurs“inside”the laser radiation spot.It can appear both in the center of the beam or be displaced towards the edge.This fact indicates that reaction nucleation process is influenced not only by the energy distribution over the cross section of the laser beam, but also by local conditions on the surface and the presence of “favorable”points for initiation.In our opinion, the above mentioned points(i.e.potentially possible centers of the reaction’s ignition) are annealed if the energy of the initiating pulse is insufficient.
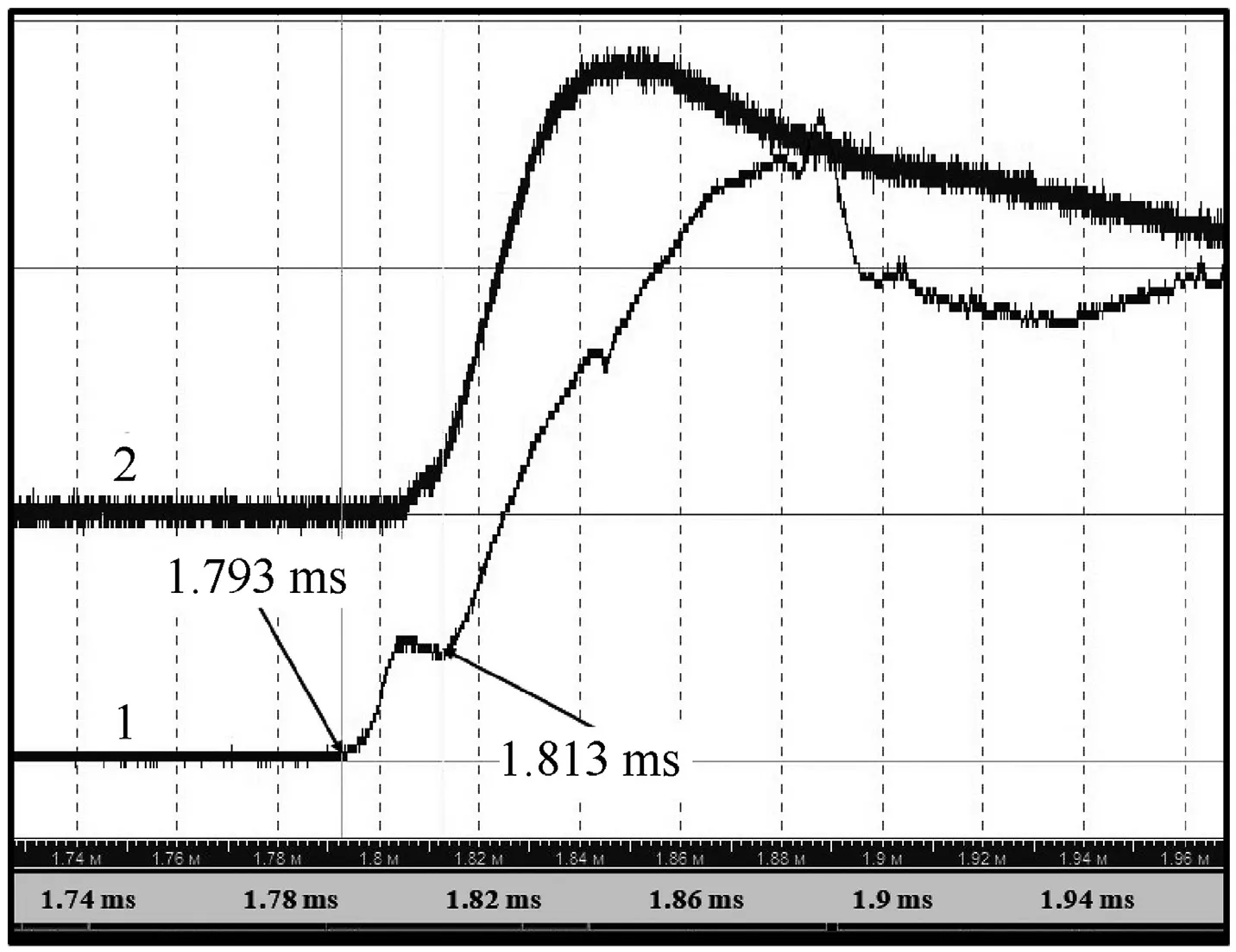
Fig.7.Radiation time-histories registered by pyrometer.
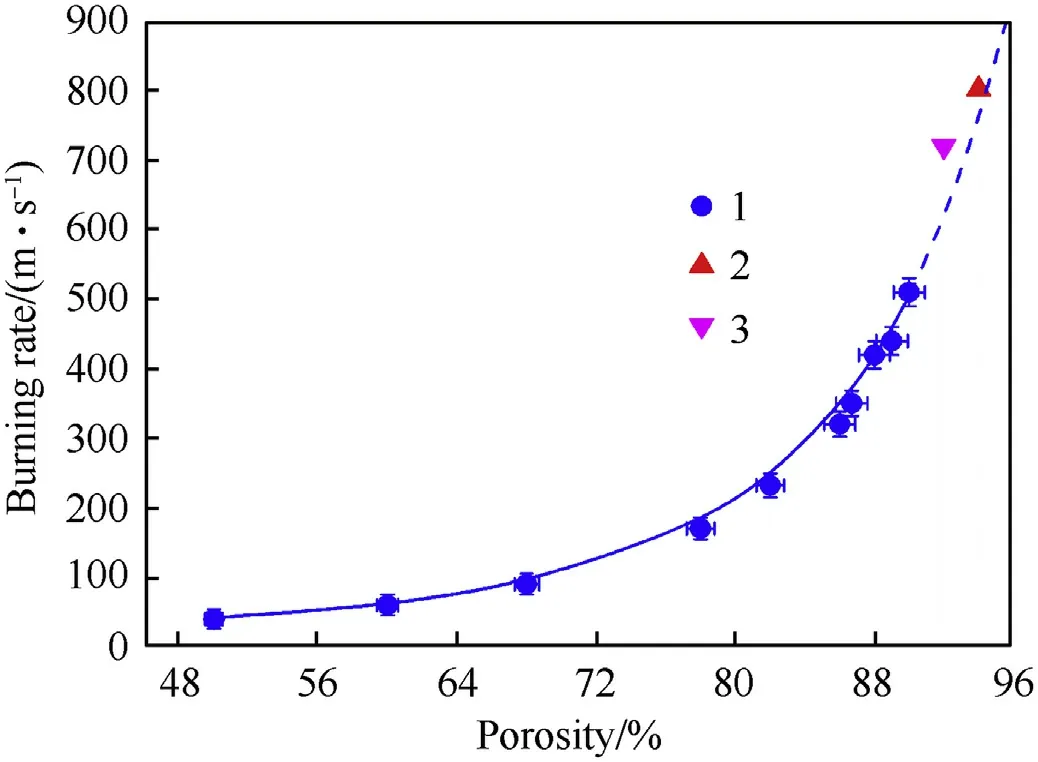
Fig.8.Burning rate vs.porosity for Al/CuO.
In Fig.10, the successive frames of high-speed video of the experiment are given, while the radiation time-histories of which are shown in Fig.7.In the test,light intensity was weakened using neutral light filter NS-8, which made it possible to detail the process under study.The frames demonstrate a complete picture of the development of the reaction at initiation of Al/CuO thermite, as well as its propagation through the macroscopic pores.
The video recording rate was 301,075 frame/s(interval 3.34 μs)at a frame size of 64 × 64 pix and an exposure time of 0.75 μs? In the frame,the dotted line indicates the boundary of the test sample,6.4 mm in diameter.The completion of the reaction on the surface occurred at 1805 μs from the start of the camera trigger by a TTLpulse or 20 μs after the ignition onset.With the accuracy of a microsecond, this corresponds to the time of the first peak of the curve denoted as“1”in Fig.7.The radial speed of the reaction along the surface changes drastically from 80 m/s at the initial stage to 400 m/s at a distance of 1 mm from the lateral surface of the sample.Attention should be paid to 1)a certain displacement of the bright point, i.e.the “center” of the reaction, from the initial position (compare frames 1 and 5), 2) the area of the sample surface that gets burnt, which one can be judged by the presence of concentric dark zone.We believe that details of the picture of the reaction seen in these frames evidence the jet mechanism of reaction propagation over the surface.
All the experimental data on burning rate were obtained under the conditions of scattering of the reaction products.However,the experimental data and thermodynamic calculations [5]show that in a closed volume, the efficiency of the NEM reaction can be significantly influenced by gas formation accompanied by pressure increase.To assess this effect,we carried out a series of experiments for combustion of Al/CuO mixtures(ε=88%)placed into glass tubes(3.5 mm of inner diameter and wall thickness of 1 mm) with lengthsvaried from 14 to 45 mm.Mixtures were initiated by laser pulse with intensity of 690 W/cm.The obtained results are provided in Table 4.
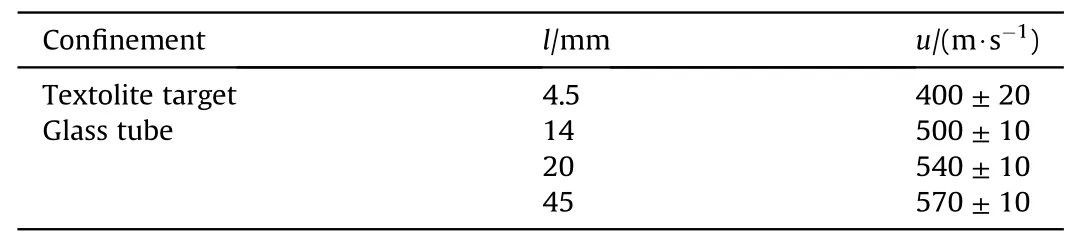
Table 4Burning rate Al/CuO (ε = 88%).
The process of reaction propagation in a tube of 20 mm long is shown in Fig.11.The video recording rate was 220472 frame/s(interval 4.54 μs), exposure time of 0.96 μs and frame size of 128×64 pix.A thin line shows the initial position of the tube and its dimensions.The visible length of the tube is 15.7 mm.

Fig.9.Initial stage of laser initiation of NEM.
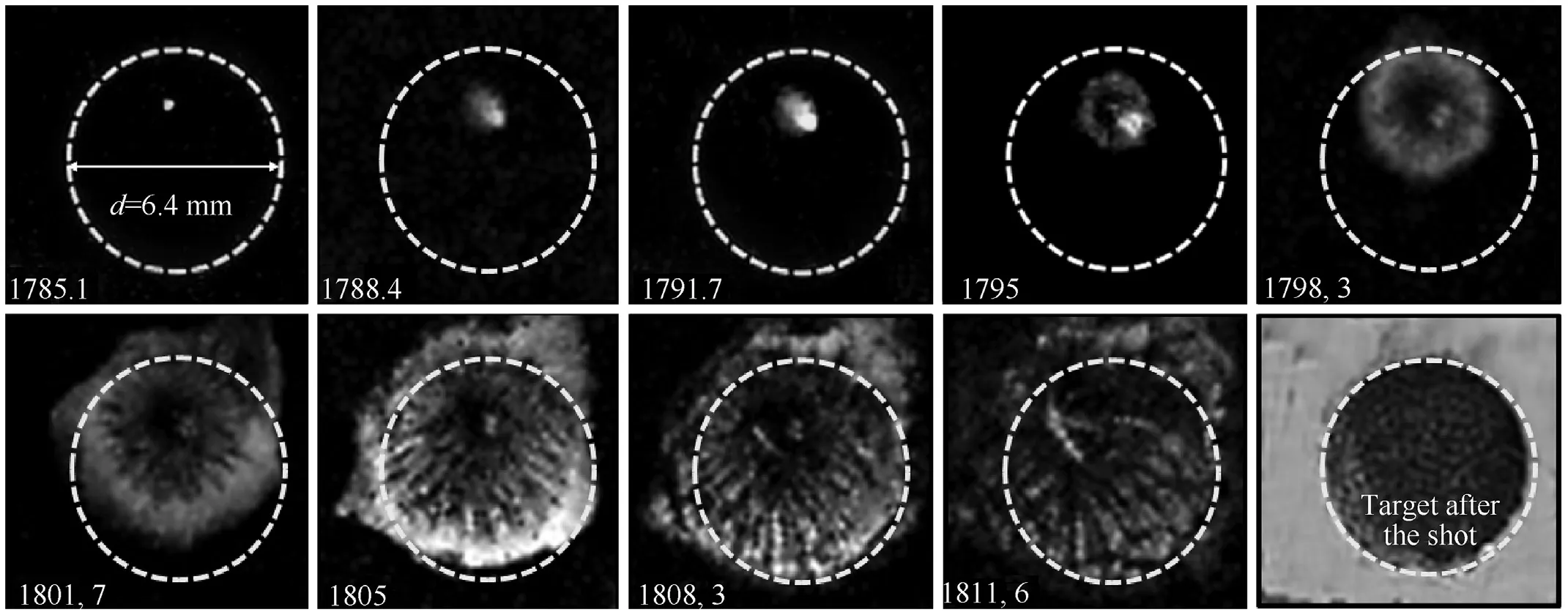
Fig.10.Successive frames of reaction propagation over the sample surface at laser initiation of Al/CuO (ε = 89%).
In the first frame at the top left,one can see a bright point,which indicates the reaction onset.After 9 μs an oblique glowing combustion front with“tongues”of jets is already formed.It is followed by a less bright zone in which one can assume that afterburning processes occur.It should be noted that in a tube placed horizontally, the steady-state combustion front has a similar shape,regardless of the place where the reaction starts.This effect may be a result of a small vertical density gradient due to gravity.Therefore,since the burning rate depends on the density,the upper,less dense layers of powder,burn a little faster than the lower ones.According to the pyrometer data, the average burning rate in the glass tube 20 mm of length was measured to be 540 ± 10 m/s.At the same time the processing of video records gave the following results:on the tube axis and below the burning rate was 530 ± 10 m/s and closer to the upper wall of the tube it was 540±10 m/s.Moreover,the velocity is virtually does not change, at least after the front passes a 2.5-3 mm path from the left end of the tube.About 45 μs after the reaction onset,the destruction of the tube is commenced(the last three frames in Fig.11).It has not yet been possible to find out whether the burning rate continues to grow following the increase of the tube length,since it is necessary to perform additional experiments.
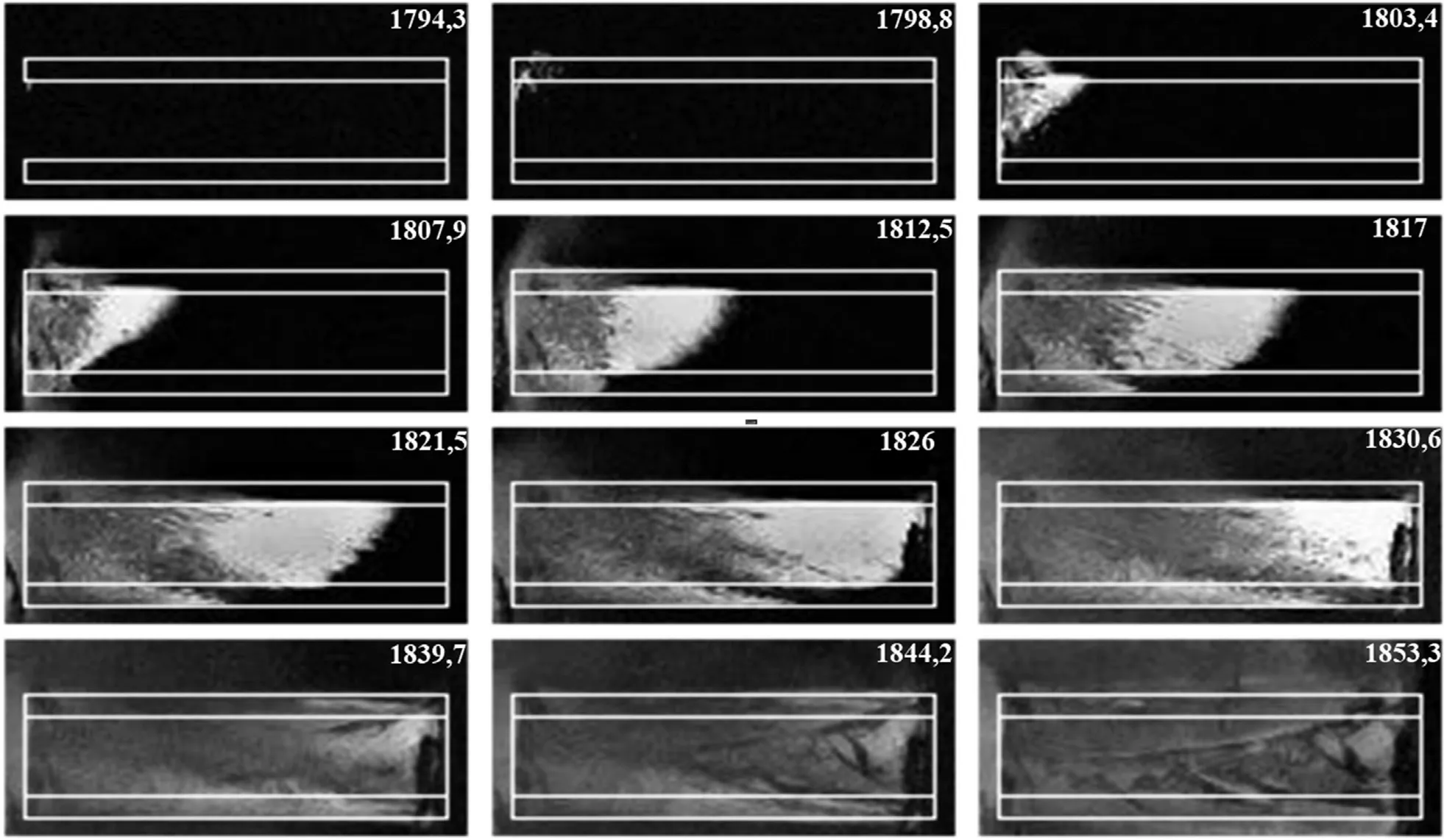
Fig.11.Burning process in a glass tube (Al/CuO, ε = 88%).
4.Conclusions
A study of the initiation of Al/CuO, Al/BiOand Al/MoOnanothermites by a laser pulse with wavelength 808 nm using a small-sized laser diode module has been carried out.The experimental set-up made it possible to create a pulse on the sample surface with a power density up to 770 W/cm,with the rise time to about 600 μs The effective cross-sectional area of the beam was 0.426 mm.
For nanothermites of various densities and component mass ratio, the delay times as well as the critical energy densityof ignition were measured.It was found that porosity of the initial mixture has an exclusive influence onFor samples with porosity from 82 to 89%, the initiation delays varied from 1.0 to 2.7 ms, and thefrom 0.36 to 1.60 J/cm, which corresponds to the minimum required laser pulse energyof (1-7) mJ.
The sensitivity of nanothermites to laser radiation can be increased by introducing a small amount of carbon black.An addition of 1% soot to Al/CuO nanothermite made it possible to almost halve both the delay and critical initiation energy.
For Al/BiO, both minimal ignition delay and critical energy,were obtained for a mixture of equivalent ratio φ = 1.41.
Analysis of the radiation time-histories recorded from the frontal surface of the samples allowed one to assume that the reaction is developed in two stages.At the first stage,the reaction is initiated on the surface, and at the second, it develops into inner layers followed by subsequent scattering of products.
The measurements Al/CuO nanothermite burning rates showed a strong dependency on porosity.Burning rate drops sharply with a decrease in porosity, which was varied from 90% to 50%.The analysis of the experimental data confirms the key role of convective burning mechanism at low densities, while one can observe a smooth transition to a slower conductive burning mode at higher densities.
Laser radiation itself does not affect the burning rate, i.e., the reaction, after having been initiated, propagates regardless of the presence or absence of laser radiation.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The work was carried out using the equipment of the USF“Sphere”of the Moscow Regional Explosive Center of Joint Institute for High Temperatures, Russian Academy of Sciences (JIHT RAS).The preparation and analysis of experimental samples was carried out at the Joint Institute for High Temperatures, Russian Academy of Sciences (JIHT RAS).It was supported by a grant for large scientific projects in priority areas of scientific and technological development No.13.1902.21.0035.The work on testing nanothermites was carried out at Federal Research Center for Chemical Physics, Russian Academy of Sciences (FRC CP RAS), Russian Academy of Sciences (RAS) financially supported by subsidies for the implementation of the state assignment on the topic No.0082-2019-0016.We are also thankful our colleagues Konstantin Monogarov and Igor Shamshin for their help in conducting the experiments.
- Defence Technology的其它文章
- Effect prediction of stiffened-ring cylindrical shells subjected to drop mass impact
- Study on the influence of armature on the efficiency of reluctance accelerator
- Research on a combinatorial control method for coaxial rotor aircraft based on sliding mode
- An optimization method for passive muzzle arc control devices in augmented railguns
- Numerical and experimental investigation on aluminum 6061-Vgrooved stainless steel 304 explosive cladding
- A capture probability analytic model for the electromagnetic launched anti-torpedo torpedo

