Nanomagnet-facilitated pharmaco-compatibility for cancer diagnostics:Underlying risks and the emergence of ultrasmall nanomagnets
Divy S.Primi,Ymini Gupt,Sreekr Mrpu,Chndr S.Bhtt,c,Thrun K.Bollu,Anil K.Suresh,*
aBionanotechnology&Sustainable Laboratory,Department of Biological Sciences,School of Engineering and Applied Sciences,Sri Ramaswamy Memorial(SRM)University,Amaravati,522503,India
bDepartment of Chemistry,University of North Texas,Denton,TX,76201,USA
cDepartment of Biotechnology,Faculty of Science and Humanities,Sri Ramaswamy Memorial(SRM)Institute of Science and Technology,Chennai,603203,India
ABSTRACT
Cancer therapy is a fast-emerging biomedical paradigm that elevates the diagnostic and therapeutic potential of a nanovector for identification, monitoring, targeting, and post-treatment response analysis.Nanovectors of superparamagnetic iron oxide nanoparticles (SPION) are of tremendous significance incancer therapy because of their inherited high surface area, high reactivity, biocompatibility, superiorcontrast, and magnetic and photo-inducibility properties. In addition to a brief introduction, we summarizevarious progressive aspects of nanomagnets pertaining to their production with an emphasis on sustainablebiomimetic approaches. Post-synthesis particulate and surface alterations in terms of pharmaco-affinity,liquid accessibility, and biocompatibility to facilitate cancer therapy are highlighted. SPION parametersincluding particle contrast, core-fusions, surface area, reactivity, photosensitivity, photodynamics, andphotothermal properties, which facilitate diverse cancer diagnostics, are discussed. We also elaborate on theconcept of magnetism to selectively focus chemotherapeutics on tumors, cell sorting, purification of bioentities, and elimination of toxins. Finally, while addressing the toxicity of nanomaterials, the advent ofultrasmall nanomagnets as a healthier alternative with superior properties and compatible cellular interactions is reviewed. In summary, these discussions spotlight the versatility and integration of multitasking nanomagnets and ultrasmall nanomagnets for diverse cancer theragnostics.
Keywords:
Nanomagnets
Biomimetics
Utrasmall-SPIONs
Magnetization
Core-fusion
Cancer theragnostics
1.Introduction
Nanotechnology refers to the utilization of materials with dimensions less than 100 nm that possess distinct physicochemical and optoelectronic properties compared to their macroscopic counterparts[1].These inherited novel properties of nanomaterials enable their use in various biomedical applications[1].The term“nanoparticle”,though,encompasses various forms of nanomaterials;therefore,researchers must be cautious while choosing the appropriate nanoparticle for their specific research.The properties of nanoparticles vary greatly based on the species attaining distinct characteristics[2].For instance,gold nanoparticles,owing to their surface plasmon resonance,elicit sizedependent absorbance at around 520 nm for application in biosensors,diagnostics,imaging,and targeted delivery[3].Likewise,silver nanoparticles with potent bactericidal properties are used as additives in wound dressing ointments to combat multidrug resistant microorganisms[4].Similarly,superparamagnetic iron oxide nanoparticles(SPIONs)with contrasting photothermal and magnetic properties are used for magnetic resonance imaging(MRI), focused drug delivery, thermal therapies, and magnetization-based cellular and molecular separations[5,6].
Moreover,capping agents that are used to stabilize nanoparticles possessing the desired morphological and surface characteristics can also alter the surface properties[6].Surface modifications,involving ligands,cap-exchangers,therapeutic moieties,and biomolecules,can also tune the overall surface characteristics of nanoparticles[6].Keeping in mind the distinct features that nanoparticles can attain,scientists continuously thrive in their development of novel production routes with attention towards sustainable techniques by minimizing hazardous repercussions[1,7].
Among the broad spectrum of nanomaterials,magnetic nanoparticles have multiple unique characteristics such as magnetic perturbation of dipolar fields,magnetophoretic mobility,generation of thermal energy upon exposure to an alternative magneticfield,anisotropy,shifted loops after field coding,long plasmatic half-life,superior contrast nature,and photoinducible properties[6-11](Figs.1 and 2).Consequently,SPION-based nanomedicine has led to advancements in cancer therapy and aids in early diagnosis,cures,metastasis prevention,molecular identification and trafficking,imaging,targeted drug delivery,separation of cells and toxins,magneto-thermotherapy,computed tomography(CT)scan,and MRI[6-11].In addition to being stable and biocompatible,nanomagnets are the only magnetic nanoparticles approved by the United States Food and Drug Administration for clinical purposes[10,11].
The most superior feature of nanomaterials is their superparamagnetic behavior,which can be evidenced when particle sizes are less than 30 nm.Superparamagnetism allows instantaneous alignment of magnetic dipoles in ferromagnetic and femtomagnetic substances under a magnetic field,where the thermal energy overrides the anisotropic energy of each SPION,resulting in zero net coercivity caused by random magnetization fluctuations[12].Despite being tiny magnets,they do not behave like magnets and remain separated,facilitating dispersion,surface alterations,and additive incorporations.However,when subjected to an external strong magnet(Fig.2),they are attracted to the magnet,thereby allowing modulations and enhancing magnetization-based biomedical applications[10,11].
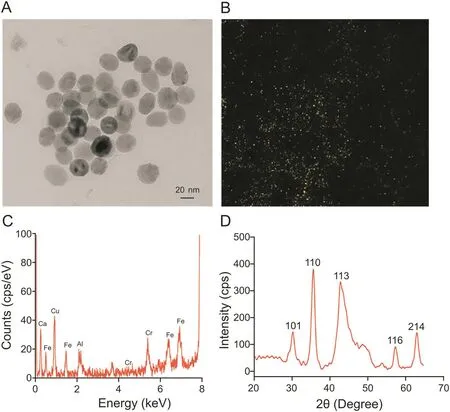
Fig.1.Physical characterizations of nanomagnets.(A)Transmission electron microscopy(TEM)depicting their morphological distributions.(B)Dark-field microscopy showing hyperspectral reflectance from the particles.(C)Elemental analysis revealing the presence of iron and other elementals.The peak for Cu is from the copper grid on which the sample was prepared.(D)X-ray diffractometry confirming the crystalline nature of the nanomagnets.

Fig.2.Scheme representing superparamagnetic behavior of nanomagnets.Stable nanomagnets(top)and their unique attractive ability towards strong external magnets(bottom).SPIONs:superparamagnetic iron oxide nanoparticles.
Numerous articles and perspectives have discussed various facets of nanomaterials related to cancer implications underlying morphological and surface properties[6-11,13-21].For example,SPION-doxorubicin(dox)-polyethylene glycol(PEG)nanocarrier cross-linked to pH-sensitive acyl hydrazone linkages,when exposed to an acidic environment in the tumor region under an external magnetic field,not only amplified the release of the drug but also produced high-resolution MRI images[22].Nanomagnets trapped inside a recombinant human heavy-chain ferritin protein shell with specific binding to transferrin receptor 1 overexpressed on tumor cells,were designed.The SPION core could catalyze the oxidation of peroxidase in the presence of diaminobenzidine substrate and hydrogen peroxide,imparting a brown color to the reaction mixture and thereby enabling visualization of tumor tissues using defined wavelengths[23].
Similarly,cytosine-guanine-rich membrane antigen aptamerconjugated thermally cross-linked nanomagnets have been reported as prostate cancer-specific nanotheragnostic agents.The nanomagnets accommodated specificity towards prostate specific membrane antigen overexpressed on prostate cancer cells for in vivo detection using MRI.Nuclease-enabled intercalation of the anticancer drug dox linked to nanomagnets via an aptamer was designed for the slow release of dox near the tumor.The ease and facile nature of the adapted targeting strategy can be implemented to target other prostate cancers by replacing dox with necessary chemotherapeutics[24].
However,our article uniquely introduces nanomagnets and their unique characteristics and summarizes various production techniques with a focus on nature-adapted sustainable strategies.Obligatory,post-synthesis alterations in terms of chemo-affinity,aqueous solubility,and systemic biocompatibility to facilitate cancer therapeutics are highlighted.Physicochemical parameters of nanomagnets such as particle contrast,core-fusion,surface reactivity,photo-inducibility,and intrinsic core magnetism to improvise cancer theragnostics are discussed.Next,exploitation of the magnetization feature of nanomagnets for targeted“tumor-therapeutics”and cell and molecular separations are discussed.Finally,the fate and transformation of nanomagnets under in vitro cellular and in vivo systemic conditions,with an emphasis on the advent of ultra-small(US)nanomagnets with superior properties and minimal immunogenic interactions,are highlighted.
2.Synthesis of nanomagnets
To accommodate a wide range of applications of nanomaterials,various compositions of nanomagnets enabling dispersion in both polar and non-polar solvents are synthesized[7].First,chemical syntheses include co-precipitation,in which ferrous and ferric ions are precipitated at extreme temperatures under alkaline conditions and inert nitrogen to produce monodispersed nanomagnets with microminiature diameters and ameliorated aqueous solubility[7].Second,thermal decomposition occurs when organometallic compounds that lack the inherent property of magnetism are heated in the presence of organic surfactants of aliphatic amines and fatty acids under non-oxygenated conditions.This method allows the synthesis of monodispersed nanomagnets using precursors such as iron oleate,iron pentacarbonyl,iron oxyhydroxide,and oleic acid or 1-octadecene,as surfactants.As the reaction is oxygen-free,post-synthesis oxidation is crucial for attaining single nano-crystallinity and avoiding oxygen deficiency [25].The generated nanomaterials will hydrophobically restrict their use in biomedicine or compel cap-exchange to attain solubility.Third,the microemulsion method involves the dissipation of two heterogeneous suspensions with a surfactant to form a monolayer on their interfaces,eliciting decreased tension and upgraded thermodynamic favor[7].This is often performed by blending two immiscible suspensions,one containing precursors,surfactant,and solvent,and the other containing inorganic bases,surfactants,and solvents prepared in deoxygenated water,producing micellar structures that assemble into nanoparticles[7].Variable size distributions can be produced using this technique,with no control over particle dispersity and crystallinity,which depend on the micelle size and other reaction parameters[7].Fourth,the hydrothermal/solvothermal method uses aqueous suspensions,organic solvents,and high temperature to produce hydrophilic nanomagnets,making them suitable for biomedical applications.Highly crystalline nanoparticles with the desired size distributions can be produced by manipulating the experimental parameters[26].Additional SPION syntheses include vapor deposition,electrodeposition,sedimentation techniques,sonochemical methods,microwave assistance[6-11],and emerging sustainable biomimetic techniques,which are detailed below.
Elevated concerns regarding biotic ecosystems have prompted researchers to explore sustainable syntheses of nanomagnets to avoid hazardous consumables[1,7].The first observation of magnetic nanoparticles within bacteria occurred while understanding the motility of magnetotactic bacteria in marine sediments.Evenly distributed bacteria under mud layers parallel to the Earth’s geographical orientations were observed,because of the alignment of magnetic particles as thin plates within cellular membrane vesicles[27].Mechanistic studies involved the breakage of genome complexity by identifying a minimal set of genes responsible for the biomineralization of highly oriented magnetic nanostructures[28].
Since then,natural organisms have been explored as biomachineries to produce nanomagnets.The predominant magnetite phase of soluble magnetic nanoparticles was produced using Fusarium oxysporum incubated with ferric and ferrous salts[29].Seaweed(Sargassum muticum)were exploited to produce hydrophilic SPIONs,where the sulfated polysaccharide-rich weed extract acted as an ion-reducer as well as a stabilizer[30].Very recently,chemically stable and non-toxic hematite nanoparticles were biosynthesized using the leaf extract of Salvadora perisca[31].
Interestingly,the occurrence of magnetic nanoparticles within mesenchymal stem cells was observed.Magnetism-based fingerprint analysis revealed the degradation of nanoparticles during“chondrogenesis,”whereas during the rest of the differentiation pathways,degradation followed by re-magnetization occurred,correlating to neo-synthesis in a cellular system[32].As insights into the analytics to determine the morphological and physicochemical characteristics,transmission electron microscopy imaging shows the size and shape of nanoparticles(Fig.1A),dark-field microscopy measures the reflectance from nanoparticles(Fig.1B),energy dispersive X-ray spectroscopy shows elemental compositions(Fig.1C),and X-ray diffraction demonstrates the crystalline nature(Fig.1D).However,the hunt for fabricating nanomagnets with better properties is a never-ending paradigm for scientists,by articulating the method,varying the combinations of elements,and en-capping molecules.
3.Solubilizing the insoluble:Decorating nanomagnets
As most of the syntheses produce aqueously insoluble nanomagnets,the solubilization of a nanocarrier is crucial to dictating their utilization in vivo.A drug is always administered in a hydrophilic environment,and insoluble drugs systemically accompany non-specific interactions with blood constituents and protein corona,induce cytotoxicity upon phagocytosis,and aggregate to lose activity[33].To circumvent this and provide dispersion and stability,several electrostatic molecules are conjugated,which are used in various biomedical applications[6,7].
Routinely used cap-exchange surface modifications include:1)ligand exchange,where the amphiphilic functional group is exchanged with an aquaphobic facial ligand on the surface of the nanoparticle,resulting in exposure of the hydrophilic constituent,while the hydrophobic component sticks to the nanoparticle;2)substitution dissolution:surface assimilation of amphiphilic functional groups by nanoparticles sustaining solubility in aqueous facial ligands;3)coating SPIONs with inorganic materials using gold and silica,which exclusively ameliorates aqueous stability and dissipation.
For example,thermal decomposition of an iron precursor using polyethylene glycol and polyethylenimine(PEI)produced highly stable nanoparticles soluble in aqueous and physiological suspensions,offering prolonged blood circulation and biocompatibility.The sizes were tuned by varying the reaction temperature and PEI concentration to obtain diverse contrasts for capturing intensely varied magnetic resonance images of a brain tumor[34].Likewise,the aquaphilic biocompatible polymer,dodecanethiolpolymethacrylic acid,was used to attain water-soluble,biocompatible,monodispersed nanomagnets.Encapment with thioether and carboxylic groups retained longer blood circulation,increased drug-loading,and amplified toxicity towards HepG2 cancer cells[35].Unlike usual solubilizing agents,a facile method for converting hydrophobic nanomagnets from chloroform via gelatin encapsulation based on hydrophobic-hydrophobic interactions was developed.Large quantities of active amine groups on gelatin allowed the engraftment of fluorescein isothiocyanate and anticancer platinum with carboxyl groups.Specific killing of MCF-7 cancer cells was achieved because of the reducing environment of the tumor that converted Pt(IV)to Pt(II)with marginally high toxicity[36].Subsequently,oleic acid-coated hydrophobic SPIONs were modified using triblock copolymers containing polybutylacrylate,polyethylacrylate,and polymethacrylic acid to attain solubility.The exposed carboxylic groups were conjugated with Anti-HER2/neu specific to the surface protein overexpressed on human breast cancer cells for efficient separation under magnetization[37].
Likewise,nanomagnets capped with mPEG polyamidoamine,a dendrimer containing amide and amine groups,provided water dispersity owing to pH-triggered release of dox into cancerous cells via a pH-sensitive hydrazine linker[24].Similarly,hyaluronic acid(HA)-encapsulated oleic acid-coated nanomagnets exhibited selective targeting against several human cancers,mainly colon adenocarcinoma.Selective cancer cell inhibition was observed even when the conjugates were combined with co-cultured cancer and normal cells,as confirmed using MRI and histopathological assays of tumor-induced rats[38].Concomitantly,the non-covalent binding of amphiphilic peptides onto nanomagnets accorded water solubility and biocompatibility while supplementing cell growth in vitro.The hydrophobic end of the peptide was noncovalently associated with the nanomagnets,whereas the hydrophilic end rendered solubility.The nanoparticles demonstrated virtuous magnetic resonance(MR)contrasts and allowed the tangibility of drug entities for targeting cancer[39].
Highly magnetized water-soluble nanomagnets were synthesized using a combination of surface functionalizers such as 2-amino terephthalic acid,trimesic acid,pyromellitic acid,1,4-diaminobenzene,4-aminobenzoic acid,terephthalic acid,and 3,4-diaminobenzoic acid(3,4-DABA).3,4-DABA exhibited faster thermal response and higher heating efficiencies under an amplified magnetic field(AMF),presumably owing to the amplified π-π conjugation of 3,4-DABA and anisotropy[40].A polymeric starch matrix with about 6 nm particles rendered high aqueous stability and systemic biocompatibility.However,H2O2/NaOH was injected into the striatum at an approximate depth of 4 mm,which reduced agglomeration by cleaving the glycosylic bonds of starch molecules,inducing reactive oxygen species(ROS)-mediated apoptosis[41].Simple,yet economical,and viable synthesis of water-soluble nanomagnets was accomplished by crushing iron precursors with sodium citrate using a ceramic pestle and motor.Hydrophilicity was due to the carboxylic groups of sodium citrate,and the produced nanoparticles likely exhibited high T2contrast,aiding better MRI[42].
4.Attainment of biocompatibility
Biocompatibility refers to the existence of a drug moiety within biotic systems,triggering the necessary maximum host response with no adverse impact.Biocompatible molecules tend to maintain stability with blood constituents,stimulating efficient responses without interrupting metabolic and physiological processes.SPIONs are adjugated with substances that impart stability,solubility,and biocompatibility and render in vitro and in vivo nonlethality.
PEGylation,the attachment of PEG to nanocarriers,is a commonly adopted surface modification to attain biocompatibility,processability,prolonged circulation by limiting clearance by mononuclear phagocytes,and guidance for renal clearance.In addition to enabling prolonged blood circulation,PEGylation tends to retain molecular and thermal stability within aquaphilic suspensions owing to steric hindrance.PEG can further prevent aggregation by masking the charge through a conformational cloud[33].Despite its advantages,the use of PEG has been limited recently owing to reported controversies such as anti-PEG immunogenic reactions fostering accelerated blood clearance,complement activation leading to hypersensitivity,repeated usage-mediated side-effects,time-driven desorption of PEG,gastrointestinal assimilation during oral administration,dermal allergies,non-biodegradability,cytotoxicity,and deterioration of PEG within the system under stress[33].
Alternatively,the operationalization of nanovectors with PEG-like characteristics has emerged as a successor to replace PEG[33],including poly(vinyl pyrrolidine),poly(vinyl alcohol),poly(amino acid)s, poly(glycerol)s, poly(2-oxazoline)s, poly(acrylamide)s,porphyrin,chitosan,gelatin,dextran,heparin,bovine serum albumin,HA,and polyoxazoline[33].To identify a highly stable self-assembled monolayer of the peptide on the surface of nanomagnets,86 types of peptides were screened for stability against electrolyte-induced aggregation under physiological conditions,preventive non-specific binding to cells,non-cytotoxicity,and enhanced contrast ability.The bis-phosphorylated peptide,2 PGS*VVVT-PEG4-ol,was identified to be the most biocompatible with no cytotoxicity or cell-adhesion and exhibit enhanced MRI with T1and T2relaxivities of 2.4 mM-1s-1and 217.8 mM-1s-1,respectively[43].Similarly,the complementary properties of SPIONs and porphyrin derivatives,including high biodegradability,low cytotoxicity,and high biocompatibility,allowed their conjoint assemblage into molecular therapeutic nanovectors.Porphyrin,a heterocyclic sensitizer composed of four pyrrole subunits interconnected at their α-carbon,was linked to SPIONs using methine bridges connected via stable covalent bonds.The non-cytotoxic nature of the nanovector endocytosed by HeLa cells was demonstrated by fluorescence microscopy upon excitation using yellow light exhibiting red emission at a wavelength of 610 nm[44].Likewise,oleic acid-coated hydrophobic SPIONs were made biocompatible using polysiloxane amphiphilic diblock copolymer poly(ethylene oxide)-block-poly(α-methacryloxypropyltrimethoxysilane).The hydrophobic portion of the polymer acted as a particle surface-anchoring moiety,whereas the exposed hydrophilic segment rendered solubility and biocompatibility[45].Fig.3 illustrates the functionalization of nanomagnets with PEG for biocompatibility and CpG(an oligonucleotides containing an unmethylated CpG motif)to stimulate the immune response and suppress brain glioblastoma.
To further safeguard nanoformulates,minimize opsonization,and prolong retention,human blood constituents have been reported as camouflage magneto-responsive nanocarriers.Red blood corpuscle(RBC)membrane-derived vesicles encumbering SPION nanoclusters to mask recognition by mononuclear phagocytic cells enhanced tumor aggregation with increased photothermal and imaging properties[46].RBC membrane vesicles were obtained via hypotonic treatment,followed by extrusion through variable polycarbonate membranes fused with magnetic nanoclusters(MNCs)using sonication.Ultrastealth RBC-embedded MNCs with prolonged blood retention,lowered liver uptake,and high tumor accumulation were demonstrated when introduced intravenously into mice[46].Likewise,RBC membrane-MNC was synthesized through electroporation inside a microfluidic device to create pores that facilitated the entry of SPIONs for enhanced tumor MRI and photothermal therapy(PTT).RBC-MNCs prepared using microfluidic electroporation exhibited meritorious treatment over conventional extrusions[47].Distinct from RBCs,cell membrane vesicles derived from macrophages in turn embedded with SPIONs incurred stealth,profound biocompatibility,and low immune evasion with light-to-heat conversion abilities for efficient photodynamic therapy of breast cancer.This biomimetic model could be used as a novel nanoplatform for implementation in translational medicine[48].A creative and facile strategy for producing nanomagnets involves the transfection of human mesenchymal stem cells with mms6,a gene from a magnetotactic bacterium,resulting in cytoplasmic accumulation of nanomagnets[49].
5.SPION characteristics facilitate cancer theragnostics:A hope for a cure
The biomedical implications of nanomaterials due to their contrasts,surface reactivity,photodynamics,photothermalization,hyperthermia,core-magnetism,and fused-cores are discussed in the following sections.Table 1[50-78]illustrates the compilation of various surface-modified nanomagnets/US-nanomagnets and their mechanisms of delivery for various theragnostics.To better illustrate SPION property-dependent applications,a scheme depicting the potential properties of nanomaterials and their representative uses in cancer theragnostics is shown in Fig.4.
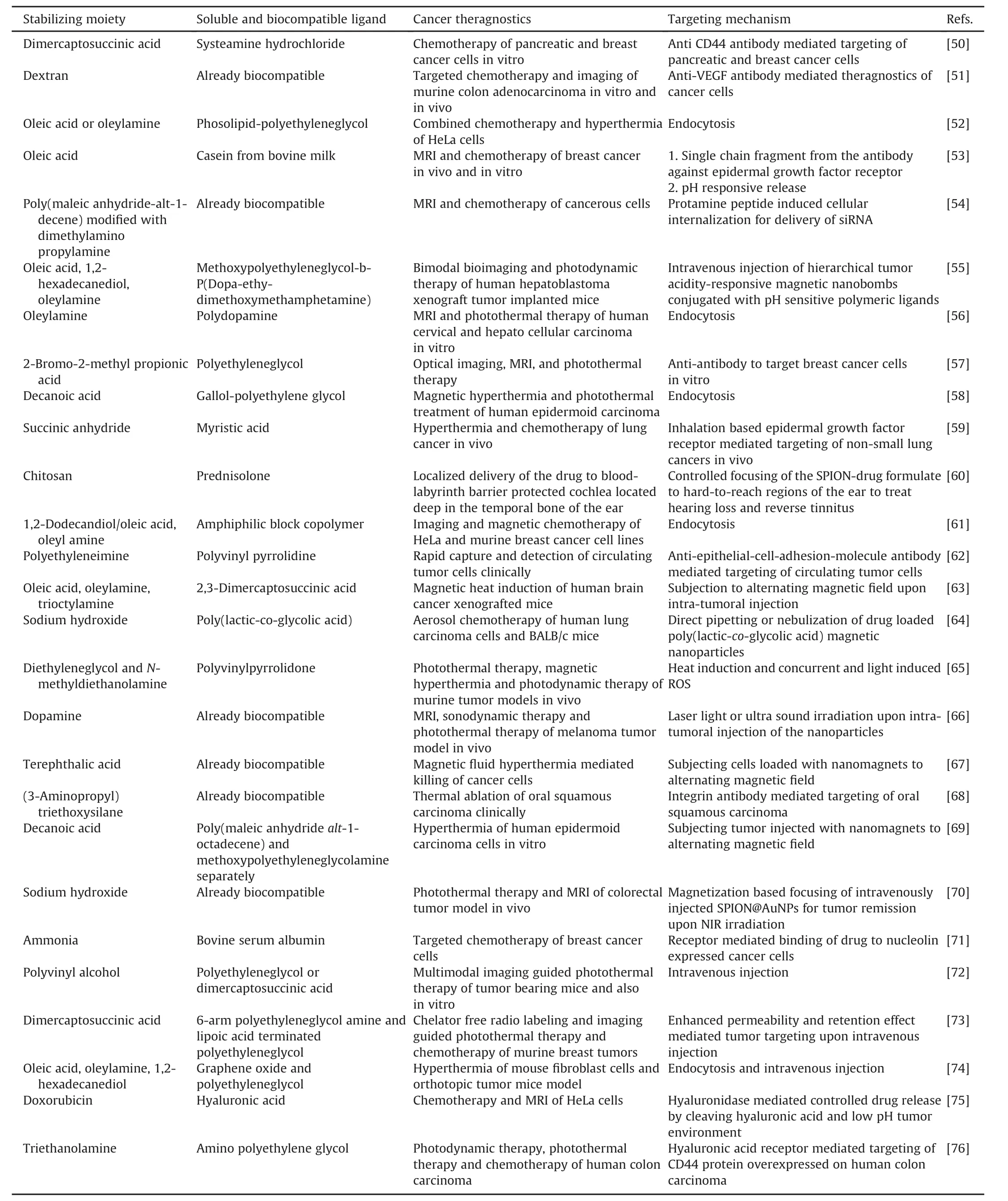
Table 1Nanomagnets and the emerging ultra-small-nanomagnets in various cancer theragnostics and their targeting mechanism.

Table 1(continued)

Fig.3.(A)Nanomagnets functionalized with polyethylene glycol(PEG)to attain biocompatibility via an oligonucleotide CpG to activate an immune cascade in microglia for the selective apoptosis of glioblastoma cells.(B)Live-dead cell-stained microscopy image showing the non-cytotoxicity of the above conjugates against macrophages.LC-SPDP:(sulfosuccinimidyl 6-[3′-(2 pyridyldithio)propionamido]hexanoate;HS-CpG:5′-H-C6-S-C6-TAAACGTTATAACGTTATGACGTCAT-3′;RSS-CpG:5′-HO-C6-SS-C6-TAAACGTTATAACGTTATGACGTCAT-3′;APTMS:(3-aminopropyl)trimethoxysilane;IONP:iron oxide nanoparticle.
5.1.Contrast-based imaging implications
Contrast agents(CA)are used as additives to improve the visibility of organs,tissues,or cells under the influence of external MR in the form of high-resolution images.Common contrast enhancement agents for MRI are lanthanide(gadolinium)or transition metals(manganese).Contrast agents may be administered orally or intravenously and,as per the biodistribution,have been developed to selectively distinguish disease conditions,organs,inflammation,and tumors.Contrast agents can shorten the T1or T2relaxation time,resulting in increased signal intensity on T1-weighted images or reduced signal intensity on T2-weighted images.The efficiency of contrast agents depends on their swift total relaxation processes,defined as relaxivity ri,according to the following equation:
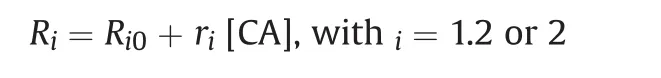
where Ri=1/Tiis the relaxation rate observed in the presence of CA,Ri0is the baseline tissue relaxation rate,riis the CA relaxivity,and[CA]is the concentration of CA.
MRI is a non-invasive,non-ionization technique that provides imaging of biological specimens with greater spatial resolution.MRI uses a strong magnetic field to produce images by recording radio signals generated by systemic water.Radio signals are produced by fluctuating orientations of the spin magnetic moment of hydrogen protons in water molecules.Once turned off,the spins realign to the original state,imparting radio signals to form a highresolution image of the exact location of the proton.Since re-alignment occurs at different rates for different molecules,organs,and tissues,they can be captured distinctly[79].
Nanomagnets are well established for MRI over conventional contrast agents and possess excellent properties suitable for various biomedical applications such as molecular and cellular identification,tracking,and identification of progression and migration.Recently,the proliferative and migratory abilities of transplanted stem cells loaded with nanomagnets to track stem cell migration,repair,proliferation,and aggregation at the site of the lesion have made innovative avenues available.However,the inability of MRI to distinguish iron loaded within transplanted stem cells and iron released during programmed cell death is a constraint[80].
Similarly,carbon-coated nanomagnets produced using the hydrothermal method exhibited both T1spin-lattice and T2spin-spin relaxation process relaxivities of hydrogen protons,contrary to the usual T2negative contrast ability for both T1and T2contrasts in MRI[81].Correspondingly,immune clearance evasion,in vivo tumor targeting,and MRI were possible upon administering protein or cell-resistant polymer(poly(TMSMA-r-PEGMA))-coated nanomagnets into tumor-prone mice intravenously[82].Despite the lack of targeting ligands,the tumors could be detected in vivo using MRI,presumably owing to the high stability-permitted enhanced permeability and retention effect and,therefore,the modified nanomagnet could be established as a potent cancer detection probe[82].
Eight-angled,size-controllable octopod nanomagnets were fabricated under vacuum using iron precursors,oleic acid,and sodium chloride.The evident role of chloride ions in the formation of such octuplets bound to the exposed iron ions on the high-index facet{311}was observed.Octopod nanomagnets exhibited an ultrahigh T2transverse relaxivity of 679.3±30 mM-1s-1owing to the increased effective radius and local field inhomogeneity of the magnetic core and were utilized for the early diagnosis of small tumors[83].
5.2.Surface reactivity-based drug delivery implications
The surface reactivity of nanomagnets can accommodate combinations of theragnostic particles with high intensities owing to their high surface area.Multifunctional formulations can address complex issues of cancer treatments,while the small size can invade the immune system,allowing enzymatic degradation in the liver or renal endothelial clearance.Several strategies have been adapted to covalently attach ligands and nanocarriers such as amide,carboxyl,thioether,disulfide,acetyl-hydrazone,and polycyclic linkages along with R-X-R “click chemistry”to couple small elements and heteroatoms[84].Moreover,depending on the molecular weight and structure of the drug,drug interactive abilities,and particle surface area,each nanoparticle can house a few to thousands of drug molecules[85].This drastically reduces the systemic payloads to deplete non-specific interactions,thereby intensely lowering side effects.A schematic diagram of the utilization of surface reactivities of nanomagnets to target therapeutic entities for tumors is shown in Fig.4.Likewise,to overcome thephysical barrier of the tumor,urokinase plasminogen activator(uPAR)-mediated targeting of nanomagnets carrying gemcitabine tagged with amino-terminal fragment(ATF)of the uPAR-binding domain overexpressed on tumor and stromal cells was accomplished.A lysosomally cleavable tetrapeptide linker was used to enhance the intracellular release of the drug,following receptormediated endocytosis,allowing examination of the tumor using MRI[86].Using a similar concept,PEGylated nanomaterials tagged with ATF,loaded with cisplatin and dox,reduced the viability of orthotropic mouse pancreatic tumors with no systemic lethality.A three-fold increase in the accumulation of the nanoformulation was observed upon intratumoral injection compared to that in intravenous delivery.Moreover,the efficient penetrability of nanoformulation into deep vicinities of the tumor and the metastasized tumor was high[87].
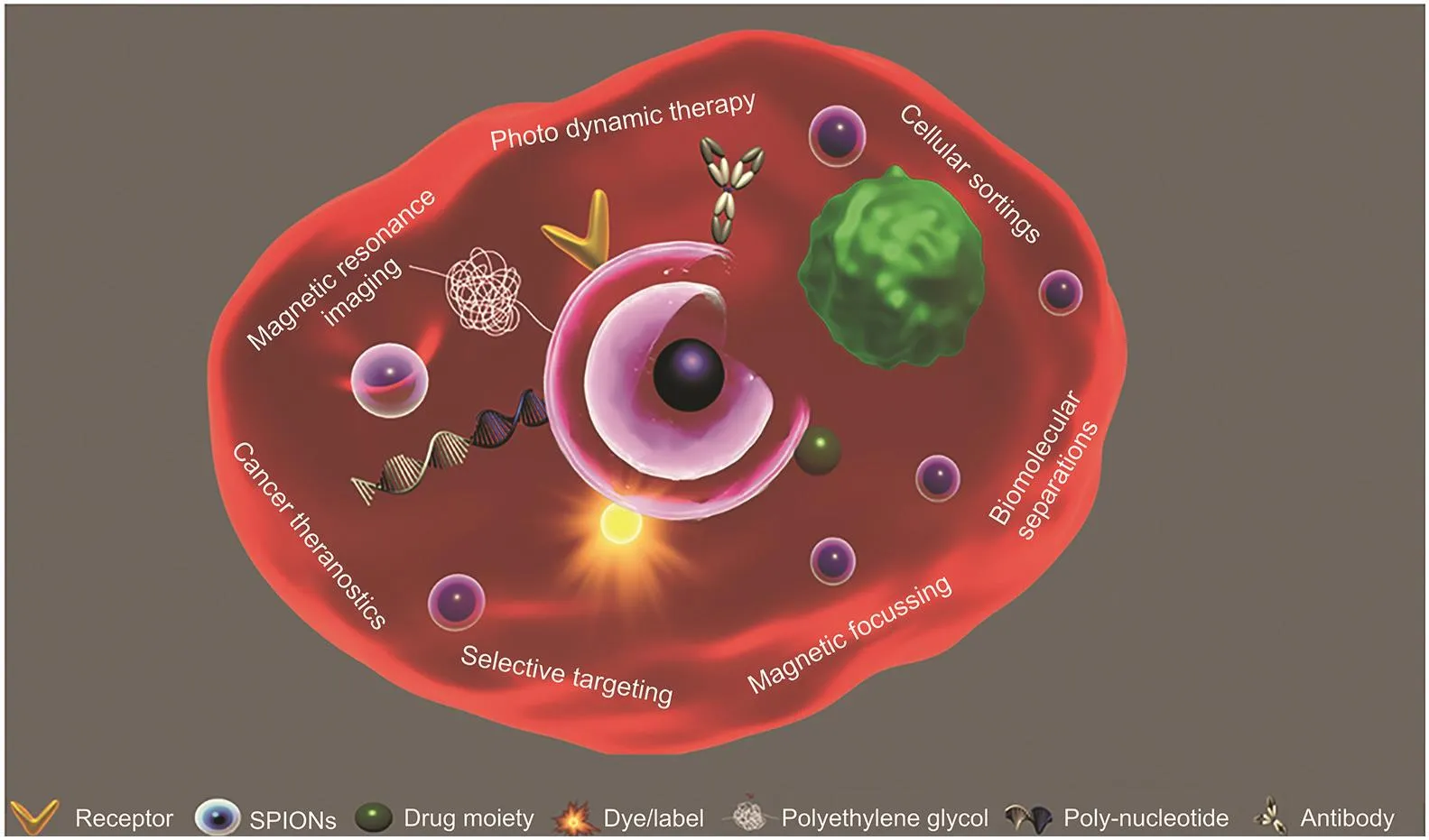
Fig.4.Schematic illustration of nanomagnet-mediated diagnosis and selective targeting to tumors.Surface alterations of nanomagnets for selective targeting of cancer and utilization of the nanomagnets’inherent core magnetic property for selective focusing of a nanoformulate to the tumor.
Likewise,pH-responsive ester bond cleavage through hydrolysis for sustained release of paclitaxel,eliminating tumors under in vivo and in vitro conditions,was demonstrated[50].Similarly,SPIONs were activated with anti-CD44 against lymphocyte receptor CD44 overexpressed in wide tumors including circulating tumor cells to deliver gemcitabine to multiple organ cancers and were verified using ultrastructural characterizations and downregulation of CD44 expression[50].
When anti-vascular endothelial growth factor(VEGF)-functionalized SPIONs coated with dextran were introduced into human and mouse colon cancers in vivo,binding of nanocarriers to VEGF overexpressed on cancer cells was distinguished from healthy cells.The expression of VEGF in CT26 cells and colon tumors was confirmed using analytical techniques,immunohistochemistry,and Western blotting[51].Similarly,dextran-coated SPIONs were linked to azademethylcolchicine using a custom-designed peptide βAla-Cys-Arg-Ser-Cit-Gly-HPhe-Tyr-Leu-Tyr,which can be cleaved by matrix metalloproteinase-1 overexpressed in the tumor environment.In vitro and in vivo conditions demonstrated specific scissoring of the peptide bond between the amino acids glycine and homophenylalanine,allowing drug accumulation in tumor cells to induce cytotoxicity[88].
Likewise,phospholipid-PEG-coated nanomaterials,to concurrently deliver the covalently linked dox,propitiously enhanced in vitro cancer suppression.Kinetics revealed the slow release of dox up to 72 nanomagnets,and interestingly,this could be controlled by optimizing the length of PEG[52].In another study,a novel class of enhanced MRI contrast agents exhibiting a T2transverse relaxivity of 273 mM-1s-1at 3 tesla using 15 nm nanomagnets coated with casein was produced.pH-responsive reversible structural and conformational modifications of casein led to clumping-mediated endocytosis of the conjugated epidermal growth factor receptor(EGFR)to target breast tumor cells[53].
The after-effects of nanoformulates can lead to non-specific interactions,agglomeration,and decreased sensitivity.To improve the dismal prognosis of brain cancer,SPION nanovectors coated with chitosan,PEG,and PEI and conjugated to a green fluorescent protein(GFP)-encoding DNA and chlorotoxin were developed.Upon intravenous injection into C6 glioma mice,gene targeting to glioma was observed,as evidenced by higher gene expression levels[89].
RNA interference can silence mRNA expression by forming dsRNA and inhibit translation and protein expression through gene silencing.Cell-penetrating magnetic nanoparticles conjugated to eGFP siRNA,surface-modulated with amphipol polymer and protamine peptide allow the conjugates to serve as dual transmembrane carriers for targeted drug delivery and MRI contrast.The above nanoformulation not only prevented siRNA from degradation by nucleases,accommodating low permeation,but also silenced eGFP expression within cancerous cells[54].
Likewise,post-translational modifications through protein and gene regulation for tumor suppression,detection,modulation,and delivery are significant for combating cancer through RNA nanotechnology.Decoding microRNAs to deregulate proteins within tumorous cells were exploited by coating magneto-beads with a complementary sequence of miR-198.A five-fold increase in the capturing of miR-198 compared to that in non-operationalized controls was observed[90].
5.3.Thermality-based therapeutic implications
Present-day conventional multi-potent cancer theragnostic systems such as photodynamic therapy,MRI,photothermal therapy,and magnetic hyperthermal therapy can evaluate,assess,diagnose,and remediate cancer and are supported by nanomagnets,superparamagnetism,and biocompatibility.The above-listed thermality-based cancer diagnostic techniques are discussed in the following subsections.
5.3.1.Photodynamic therapy
Photodynamic therapy(PDT)is a non-intrusive cancer therapy that uses a photosensitizing agent to produce cancer cell-destructive free radicals upon bombardment with a specific spectral wavelength window.Photosensitizers are inactive in the absence of light,enabling selective elimination of cancerous cells by apoptosis,necrosis,or destruction of the cell membrane due to an exogenous increase in cellular ROS levels by being subjected to light of a specific near-infrared spectrum.ROS are highly reactive free oxygen radicals with unpaired electrons in the outermost shell.Superoxide,hydrogen peroxide,and hydroxyl radicals are most prevalent in cancers,inducing oxidative stress-mediated irreparable breakdown of cellular proteins,lipids,and DNA,causing apoptosis and preventing parturition of blood vessels within tumors[91].
Although PDT is the preferred treatment and has undergone many successful clinical trials minimizing deleterious effects when compared to conventional radiation and surgery,its use is still limited owing to poor solubility and decreased ability to elicit excitation[92].To overcome this,photosensitizers are often used in combination with SPIONs to achieve a synergistic effect and precise cancer ablation[92].For example,a photosensitizer,protoporphyrin IX,loaded with magneto liposomes,was designed to reduce the viability of breast tumors under the influence of nearinfrared radiation(NIR)[93].To encapsulate and deliver photosensitizers for enhanced PDT,a smart magnetite nanocage was reported as a reliable wagon to the tumor[94].A protein(Cys-Asp-Cys-Arg-Gly-Asp-Cys-Phe-Cys)-based nanocage could encapsulate and deliver large amounts of zinc hexadecafluropthalocyanine to human glioblastoma with prominent tumor inhibition[94].
To target head and neck squamous cell carcinoma(HNCC),PDT uses silicon phthalocyanine,SPIONs,and a tumor-targeting ligand,fibronectin mimetic peptide,that can bind to integrin β1.The nanoformulation that piled up in the HNCC xenograft resulted in efficient reduction of tumor size upon laser irradiation and was meritorious over undesired damage caused by the free drug[95].Likewise, bimodal anticancer nanoformulations for PDT and hyperthermal therapy have been demonstrated.The nanoformulation holding the photosensitizer diaminoporphyrinbridged SPIONs upon endocytosis into HeLa cells and illumination with yellow light(545-580 nm)resulted in a distorted cell membrane,leading to apoptosis[44].The intracellular Fenton reaction utilizing hydrogen peroxide responsiveness was contrived by electrostatically labeling SPIONs with indocyanine green and HA-generated hydroxyl radicals,inducing apoptosis of HCT116 cancer cells and tumor inhibition in nude mice[76].
Recently,tumor pH-responsive magnetic nano-bombs were developed for PDT and imaging by self-assembling chlorin e6(Ce6)-functionalized polypeptide,methoxy PEG-block-poly(dopamine-ethylenediamine-2,3-dimethyl maleic anhydride)-L-glutamate-Ce6,and nanomagnets.The acidic tumor environment reversed the surface charge of the nano-bombs,allowing passive tumor accumulation and cellular uptake to explode the nanobombs liberating the photosensitizer to activate ROS-mediated cancer apoptosis[55].
5.3.2.Photothermal therapy
PTT is an advancement to PDT and uses photoabsorbers instead of photosensitizers to generate sufficient heat capable of destroying tumors by absorbing light[96].However,to avoid damage to healthy cells and to exactly locate and kill malignant cells,this technique requires tracking agents.For example,a theragnostic nanoplatform based on manganese-doped nanomagnets functionalized with bovine serum albumin was developed.In vivo and in vitro experiments revealed better T1-weighted capacity with an r1of 8.24 mM-1s-1and r2/r1of 2.18 mM-1s-1when irradiated with NIR of 808 nm in an induced 4T1 tumor model[97].
Similarly,core-shell nanomagnets clustered through sodium dodecyl sulfate emulsification were attached with poly(dopamine)(PDA)to attain magnetic field targetability and the combined potentiality of MRI and photothermal cancer therapy.The photothermal conversion feature of PDA under an NIR 808 nm laser could induce cancer cell death in cervical and hepatocellular carcinomas.Moreover,the cluster design of nanomagnets could enhance the r2and r2*relaxivities,with r2*values three-fold more efficient than r2[56].In another study,microcapsules of poly(lactic acid)loaded with SPIONs surface-functionalized with graphene oxide as a multipotent theragnostic vector were fabricated using a double emulsion strategy.The microcapsules not only served as contrast agents to enhance ultrasound,MR,and photoacoustic imaging,but also photoabsorbed NIR for photothermal ablation of HeLa cancer at 808 nm[98].
SPIONs coated with gold nanoshells conjugated to anti-HER2/neu antibody show targeted binding to cancer cells,where nanomagnets aid high-resolution magnetic imaging of cancer cells,enabling detection.Gold nanoshells,upon exposure to lowpowered femtosecond laser pulses with an NIR wavelength of 800 nm,produced adequate heat for selective destruction of cancer cells[57].Similarly,SPION nanocubes exhibiting optical absorption at 808 nm,by being subjected to NIR radiation,produced heatmediated tumor cell death[58].Likewise,highly crystalline nanomagnets coated with polysiloxane polymer upon intratumoral injection in mice subjected to 885 nm diode laser,completely destroyed tumors at 33°C,unlike commercial magnetic nanoparticles that require 38-42°C,presumably owing to the crystalline lattice structure of SPIONs [99].A two-dimensional nanoassemblage of dimercaptosuccinic acid-modified SPIONs on MoS2nanosheets laminated using double-layered PEG was synthesized.The nanoconjugate maintained a perfect balance in brine suspension containing glutathione for boosting radiolabeled imaging and PTT of cancer upon irradiation with 808 nm NIR[73].
5.3.3.Hyperthermal therapy
Electromagnetic heat treatment,defined as hyperthermia,is emerging as a tumor ablative modality to cure various solid tumors by subjecting them to elevated temperatures(40-45°C)to destroy cancer cells globally and locally.Hyperthermia can either necrotize cancer cells by causing irreparable denaturation of proteins and other biological molecules or can directly char cancer cells,leading to instantaneous cell death[11].For example,magnetic nanoparticles alone,without anticancer drugs,were efficient against subcutaneous mouse melanoma.The particles were modified with organic dopamine-oligo ethylene glycol ligands attached to 4-tetracarboxyphenyl porphyrin,shielding them from corrosion.Intratumoral injection into murine melanoma revealed a 50% reduction in tumor size within an initial 10 min exposure to AMF[100].Likewise,to enhance hyperthermality and to increase the superparamagnetization,nanomagnets encapsulated in gold nanoshells treated with breast carcinoma and cardiomyoblast cells resulted in 4-to 5-fold elevated hyperthermia using low-frequency oscillating magnetic fields of 44 Hz[101].Similarly,thermosensitive nanomagnet-loaded nanocapsule hydrogels were synthesized using poly(organophospahzene)as an outer coat to increase the retention time of nanomagnets in systemic circulation to facilitate efficient hyperthermality.Unlike typical magnetic hyperthermia at 38-42°C,this study uniquely utilized AMF to accomplish anticancerous activity at 34.4°C through apoptosis-induced necrosis after four cycles of magnetic hyperthermia[102].
A custom-built four-port chamber was designed as a convenient nasal-inhalation route of administration to inhale dried EGFRSPION aerosol steam through nostrils of non-small cell lung cancer-bearing orthotopic mice to target epithelial tissues of tumors.Aerosolized EGFR-nanomagnets were directed to the lung periphery by controlling the aerodynamic diameter of the SPION agglomerates,resulting in enhanced in vitro accumulation compared to that of non-targeted particles.A reduction in tumor size of the lung cancer after magnetic hyperthermal treatment under 6 kA/m and 386 kHz of AMF was observed,as confirmed using bioluminescence imaging[59].Furthermore,nanomagnetembedded hydroxyapatite nanocrystals(HAIO)were synthesized using co-precipitation to enable self-controlled hyperthermia when subjected to AMF and enhanced contrast MRI.HAIO under a 33.8 mT and 275 kHz magnetic field,when assorted with HeLa cells,conferred 75% apoptosis due to a tunable rise in temperature with an increase in the concentration of nanocrystals and the intensity of the magnetic field[103].
In addition to employing magnetic targeting,the hyperthermal capability of the nanocomposite was used to excavate the cancer cells.Nanomagnets functionalized with the nucleolin antagonist multivalent pseudo peptide Nucant(N6L),dox,or both,were administered into breast adenocarcinoma for targeted focusing via an external alternating magnetic field by placing the nude mice inside the AMF coil.Specific magnetic guidance of the nanomagnets to nucleolin-receptor overexpressed tumor cells to accumulate at the tumor site imparted dox-mediated toxicity.Simultaneously,AMF-mediated heating of nanomagnets resulted in a 40% reduction in breast adenocarcinoma tumor size upon intratumoral injection[104].
5.4.Core magnetism-based implications
Among various magnetic materials,magnetism pertains to only ferromagnetic and ferrimagnetic materials because of controlled miniaturization to form single-domain SPIONs.This is because of the tendency of unpaired electrons in the orbital of the nanomagnets to elicit high magnetization under the influence of a strong magnetic field[60,105].Details on supermagnetic behavior and its mechanism are elaborated in Section 1.The utilization of the core magnetism feature of SPIONs to focus on a therapeutic entity and the separation of cells and molecules is described below.
5.4.1.Magnetization-based controlled focusing of therapeutics
The magnetic susceptibility and superparamagnetic properties of nanomaterials play a prominent role in magnetization-based selective drug and gene targeting,drug trafficking,cell migration and cellular interactions,minimized unintended interactions,and understanding cellular and molecular functions for safe and efficient treatments.These tasks can be accomplished by computationally manipulating the frequency,intensity,magnet strength,and magnetic fields using a strong external magnetic field(EMF)[60].A schematic diagram illustrating the superparamagnetic behavior of nanomagnets to focus a targeted moiety on cancer using an external strong magnet is shown in Fig.5.
The concept of magnetization-based focusing of nanomagnets has been a boon for treating glioblastoma and other hard-to-reach deep organ tumors[106].To cross the blood-brain barrier and treat glioblastoma,microglia cells phagocytosed with SPIONs functionalized to an unmethylated CpG oligonucleotide as an immunostimulator to maximize its efficacy were administered(Fig.6).Trafficking was achieved[5]using targeted magnetic delivery to demonstrate magnet shape-induced in vitro migration(Fig.7)of SPION-CpG-loaded microglia using EMF,with the prediction that the CpG motif will trigger an immune response by activating tolllike receptor-9,followed by enhanced T1-helper secretion leading to apoptosis of glioblastoma-associated microglia[5].

Fig.5.Schematic illustration of nanomagnet-mediated selective targeting to tumors.Surface alterations of nanomagnets for selective targeting of cancer(left)and utilization of the magnetic property to selectively focus nanomagnet-formulate to the tumor(right).NC:normal cells;TC:tumor cells.

Fig.6.(A)TEM image of microglia cells loaded with nanomagnets.(B)A magnified version to visualize the nanomagnets.
Similarly,a minimally invasive drug delivery platform to passively reach glioblastoma using a clinically translatable method was validated[107].Intravenously injected magneto-responsive nanomagnets could be actively retained by applying a local EMF in a 9 L-glioma-bearing rat with a 3.6-fold target accumulation index over non-targeted glioma.MRI of prior and post accumulation of nanoparticles based on T2contrast revealed a time-dependent uptake by brain tumor cells[107].
Nanomagnets have also been used in regenerative medicine to regenerate and replace damaged tissues under various circumstantial losses.Mesenchymal progenitors labeled with SPIONs and a fluorescent moiety elicited a magneto response under the influence of an EMF to study seeding patterns in 2-and 3-dimensional models to understand selective targeting,retention within required tissue,localization,and migration patterns[108].
Upconversion nanoparticles were utilized for image-guided therapy by encapsulating with nanomagnets using an amphiphilic block co-polymer,polystyrene-block-allyl-alcohol-adapting microemulsion technique.To foster a tri-modal,down-conversion nanocarrier integrating dox,a UC-nanomagnets@polymer-dox complex was formed enabling targeted delivery,along with fluorescence and MRI under in vitro and in vivo conditions[61].
Multifunctional hybrid nanocomposites of 2-dimensional graphene functionalized SPIONs tagged with biocompatible pH-sensitive 6-arm amine PEG and dox were established[109].For the controlled release of dox,in vivo analysis of tumor destruction was performed using MRI in mice bearing murine breast cancer on a clinical MRI scanner[109].Multicombinatorial porous kaolinite scaffolds loaded with ferrous platinum nanoparticles adsorbed with dox were injected intravenously into magnetically target hepatocarcinoma.Analysis revealed tumor suppression due to the generation of hollow apertures within the tumor.Moreover,the differential killing of carcinoma by varying the temperature and proportions of kaolinite was observed with a seven-fold reduction in tumor size[110].
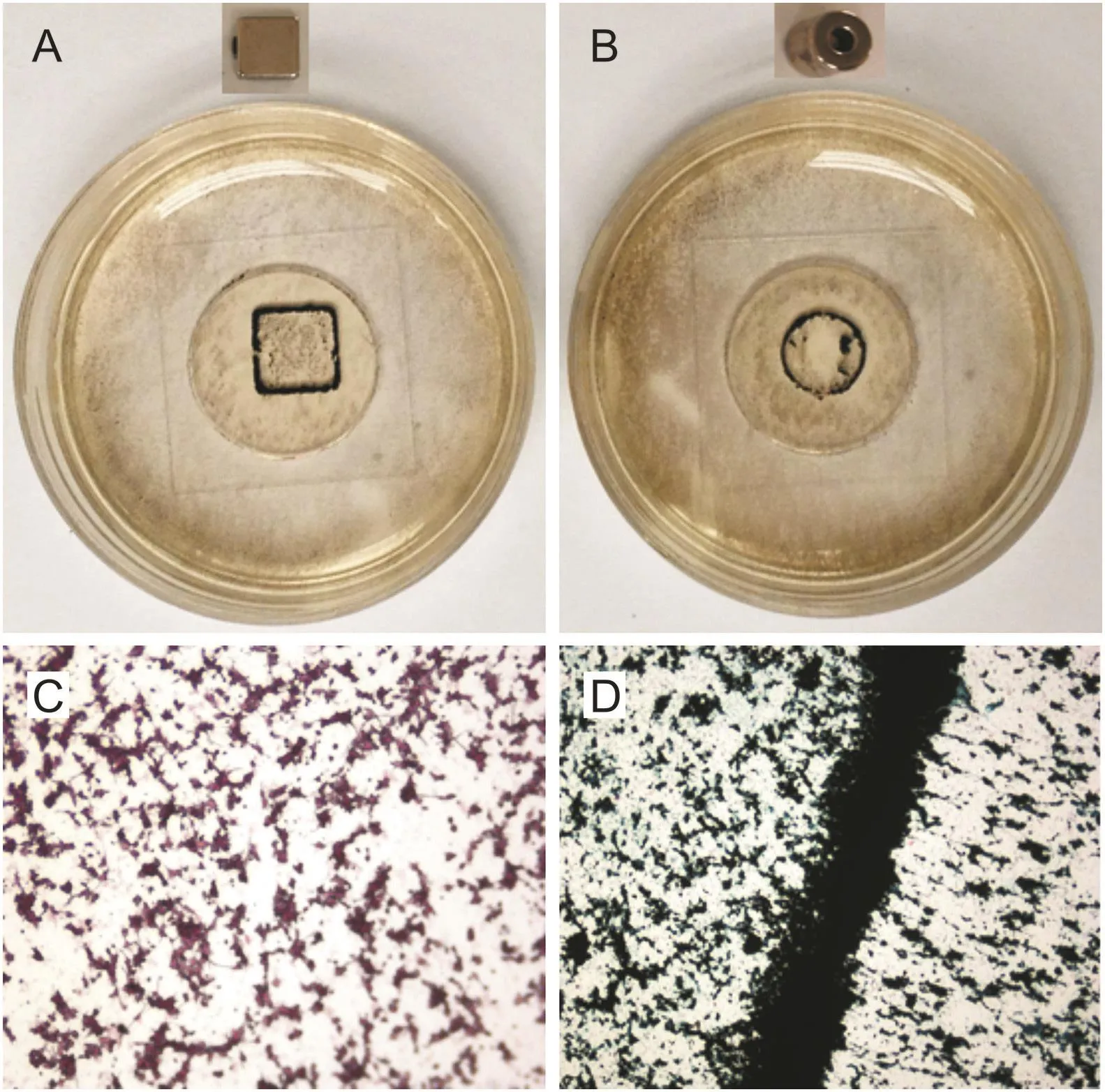
Fig.7.(A and B)Phone-captured images of cultured microglia cells loaded with nanomagnets to demonstrate magnetic focusing.The shape of the magnet used is shown on top of the representative image.(C and D)Epifluorescence microscopy images of the same cells at 20× and 40× magnifications.
5.4.2.Magnetization-based cellular and molecular separations
SPIONs,upon appropriate surface manipulations,are successfully used to restore analytes(toxicants and inflammatory and septic substances)to sublethal concentrations and eliminate systemic disease factors magnetically.Segregation of biomolecules is a holistic approach for the extraction of analytes,minimizing nonproductive interactions for efficient diagnostics[111].The development of quantitative and robust techniques for analyzing blood markers is of great significance for disease diagnostics.Cancer diagnosis demands simple,affordable,reliable,point-of-care diagnosis and minimal blood volumes.Moreover,low secretion of antigens and receptors at concentrations lower than the detectable concentrations is a challenge for the early diagnosis of cancer.Conventional detection assays that depend on small-molecule radioisotopes and fluorophores have poor stability,low sensitivity,and difficulty in processing and can be carcinogenic[112].Techniques such as MRI,CT scan,endoscopy,and tissue biopsy cannot be performed frequently because they are expensive and timeconsuming and expose patients to harmful radiation[112].
By injecting stable nanomagnets into the blood,extracorporeal elimination of harmful compounds(lead,steroids(digoxin,cardiac drug),proteins(interleukin-6),and an inflammatory mediator),while monitoring the integrity of the blood to sustain clinical parameters,was demonstrated.The high viscosity of blood forged the use of strong external magnets with a magnetization of 140 emu/g[113].Likewise,efficient immunomagnetic separation of breast cancer cells from the blood was developed using amphiphilic polymer-coated SPIONs modified with anti-HER2 antibody against cell membrane proteins.The results showed a 1:10,000,000 enriched removal of cancer cells over normal cells at a magnetic gradient of 100 T/m[37].
Circulating tumor cells(CTCs)present in the peripheral blood of cancer patients can lead to cancer metastasis by detaching themselves from the tumor into the lymphatic system to settle distantly to form a new tumor.Disclosure,high-sensitivity detection,and capture of CTCs can be effective means of treatment and prognostication of early cancer.To facilitate this,immunomagnetic nanospheres functionalized with anti-epithelial cell antibody,biotin,and streptavidin resulted in expedited responsiveness and lowered loss rates while capturing CTCs.Immunomagnetic nano-beads could successfully capture tumor cells within the peripheral blood with a purification competence of 94%,facilitating their culture,reverse transcription-polymerase chain reaction,and immunocytochemistry[62].
Similarly,magnetic polymicrospheres conjugated with zinc ions were used to separate bovine hemoglobin from bovine serum albumin using a defoamer technique involving adsorption,washing,and desorption under a gas-assisted low magnetic field(GAMS).GAMS is cost-efficient,aiding continuous magnetic bioseparation,and amicable large-scale separation and avoiding protein denaturation during separation[114].Similarly,nickel-charged iminodiacetate-immobilized silica-coated nanomagnets were used to purify histidine-tagged recombinant proteins from cell lysates.Magnetic properties permitted their separation using a magnetic separator,and the attached protein was detached from the beads using affinity chromatography[115].In an interesting study,letrozole,a nonsteroidal aromatase inhibitor which slows the production of estrogen to inhibit breast tumor,was recovered from human samples using thermolabile isopropyl acrylamide and chitosan-grafted trimethoxysilane-coated magnetic nanoparticles[116].
5.5.Particle core-fusion based theragnostics
Particle species combinations,an amalgam of metal species infused together giving rise to novel properties,have tremendous potential.First,they function as individual metals and exhibit species properties.Furthermore,property exchanges lead to novel properties.For instance,by swapping a magnetic core of CoFe2O4and soft shell MnFe2O4,the loss power values increased to a higher magnitude[63].Similarly,SPIONs fused with AuNPs encapsulated with PEG-polycaprolactone acquired tumor detection by the T2contrast imaging ability of SPIONs and AuNP-mediated free radical DNA damage in tumors[117].
Likewise,a plasmonic gold shell-magnetite core-driven approach for targeted photothermal destruction of cancer cells upon S6-aptamer functionalization was demonstrated.This was performed through the targeting,imaging,and destruction of a cancer when co-cultured with other cancers.The killing was accomplished by subjecting the cells to 670 nm light at 2.5 nanomagnets/c2for 10 min,leading to irreparable tumor damage[77].
Aerosol inhalation targeting lung cancer via nebulization was customized using magnetized core-shell nanomagnets grafted with poly(DL-lactic-co-glycolic acid).Poorly suspended quercetin was chosen because of its potent growth inhibition against human cancers[64],inferred based on automated image acquisition and real-time impedance sensing.Fabrication of SPIONs with nanoflowers as the core and spiky CuS shell consolidated to tritherapeutics including magnetic hyperthermia and PTT and PDT was performed[65].
Recently,nanocarpets of graphene oxide-iron oxide-dox for hyperthermality,chemotherapy,and MRI,have been fabricated.Graphene oxide enhanced the heating capacity of the nanocarpets under an EMF,promoting apoptosis of murine fibroblasts and colon carcinoma cells[118].In contrast,pH-triggered dox release exhibited a tumoricidal effect that increased considerably owing to drug sensitization through repeated hyperthermia.In an interesting study,selective quashing of oral squamous cancer cells using iron-core gold-shell nanoparticles triggered irreversible loss in mitochondrial membrane function,leading to mitochondrial autophagy-mediated cell death.The cancer cells were unrecoverable even upon introduction of ROS scavengers;enzymes that neutralize free radicals demonstrated irreversibility[119].Very recently,monodispersed carbon-coated manganese SPION nanocomposites with cracked surfaces having a magnetization of 13.250 emu/g,a coercivity of 13.204 G,and a magnetic moment of 0.55 tesla were fabricated.Cell histological analysis revealed that combinatorial therapy using laser and sonodynamic therapy led to 20-fold enhanced deep tumor necrosis[66].
6.Interactions of nanomagnets with cells and cellular systems
Nanomagnets are exponentially being applied beyond our understanding with regard to their fate,interactions,transport,and transformation within biotic systems[120,121].Nanomagnets predominantly used in biomedicine,initially believed to be noncytotoxic,showed lethal impacts when investigated later.The toxicity impact of nanomagnets on cells and organisms is distinguished by in vitro and in vivo toxicity studies.In vitro toxicity determines the interaction of nanomagnets with cells in terms of cellular viability and cellular localization,including cell proliferation,cell membrane damage,oxidative stress and apoptosis,necrosis,DNA damage,and metabolomic,proteomic,and genomic responses. In vitro toxicity assesses the whole system to analyze the fate,transport,interactions,transformation,biodistribution,and impacts on various organs and non-specific binding to cellular bioreceptors,proteins,enzymes,and other significant biomolecules[122].In vitro and in vivo toxicity assessments of several cellular systems,along with the mode of interactions,have recently been reviewed(Fig.8)[120,123].
7.Advent of US-nanomagnets:An alternative remedy
To compensate for the limitations of nanomagnets,the emergence of US-nanomagnets of less than 5 nm has received immense interest in cancer therapy.This is mainly because of their potential as positive T1MRI contrast agents,unlike SPIONs,which act as negative T2MRI contrast agents.The merits of US nanomagnets include:1)although nanomagnets and US-nanomagnets can be endocytosed,the small size of US-nanomagnets makes them more biocompatible and less likely to be phagocytosed by immune cells,aiding clearance through the renal endothelial system and avoiding timely accumulation in the liver.2)There is a difference in the halflife of US-nanomagnets in animals and humans,with about 3-10 times higher half-life in humans accommodating extra-flexibility.3)SPIONs have negative contrasts and can be confused with calcification,metal deposition,and hemorrhage,unlike US-nanomagnets with positive contrast because of their short T2relaxation time,basic T1-weighted acquisition,and dephasing gradient effect on slice axis[122].4)Nanomagnets tend to aggregate and show decreased dissolution rates in biofluids and under magnetic fields,whereas US-nanomagnets are stable.5)The small size and high surface area of US-SPIONs make them amenable to surface functionalization with enhanced complexity,reactivities,adsorption,and drug loading potential.6)US-nanomagnets have longer intracellular retention time than SPIONs and the ability to transmigrate to the capillary wall.

Fig.8.Distribution and degradation of intravenously injected 59 Fe-labeled nanomagnets in mice. (Reprint with permission from Ref. [120]). PVEC: peripheral vascular endothelial cell;MPS: mononuclear phagocyte system; LSEC: liver sinus endothelial cell.
For example,polydopamine-encapsulated magnetite stuffed in mesoporous silica nanocomposites,in turn coated with polydopamine,possessed colloidal stability,r1relaxivity,and NIR sensitivity for trimodal therapy against breast and metastatic lymphatic tumors,presumably owing to the clustering compactness of US-nanomagnets within the shrunken spaces of mesoporous silica[124].Likewise,agglomeration-induced shuffling of T1and T2relaxivities to affect the contrast abilities of US-nanomagnets using various molecular weights of HA(12000-14000)was demonstrated.By increasing the molecular mass of HA,various combinations of self-assembled US-nanomagnets@HA:Fe3O4@HA120,Fe3O4@HA280,Fe3O4@HA520 and Fe3O4@HA750 were synthesized via degradation of HA by an enzyme hyaluronidase overexpressed in the tumor environment[125].Similarly,clarification of size relationship between the r1and r2/r1ratios of seven sizes of US-nanomagnets including 1.9,2.6,3.3,3.6,4.2,4.8,and 4.9 nm was assessed showing US-nanomagnets 3.6 nm with decreased r1value of 12.66±1.55 mM-1s-1and increased r2/r1ratio of 1.9±0.1 mM-1s-1,allowing them to be most suitable as T1-weighted MR contrast agents[126].
Similarly,PEI-based hybrid nanogels(NGs)incorporated with US-nanomagnets and dox for T1-weighted MR image-guided chemotherapy of tumors were created.The conjugates possessed favorable water dispersibility and colloidal stability,with an excellent dox loading efficiency of 51.4%.The pH-dependent release profile of dox revealed an accelerated discharge rate under acidic pH that displayed appreciable therapeutic activity and greater r1relaxivity of 2.29 mM-1s-1than that of free US-nanomagnets at 1.15 mM-1s-1[127].Likewise,nanohybrids of double-layered hydroxide,US-nanomagnets,HA,and dox were constructed for image-guided cancer therapy.The nanohybrid possessed greater colloidal stability and cytocompatibility and displayed enhanced r1relaxivity(4.38 mM-1s-1)by 10-fold over that of pristine US-nanomagnets(0.42mM-1s-1).Moreover,the nanohybrids could efficiently release the drug into the nucleus in a pH-responsive manner to specifically target B16 cells overexpressing CD44 receptors in vivo,mediated by HA[128].Recently,alginate NGs embedded with PEI-coated US-nanomagnets were loaded into mesenchymal stem cells for tumor directing and enhanced MRI.US-nanomagnets were loaded into bone mesenchymal stem cells(BMSCs)without compromising the cell viability and characteristics of tumor tropism,allowing the BMSCs to facilitate enhanced delivery of nanomagnet-NGs to tumors upon intravenous administration,enabling strengthened MRI of tumors compared to that of free NGs[129].
US-nanomagnets were combined with an imidazopyridinebased translocator protein(TP)ligand and a fluorescent NIR-dye cyanine 5.5 to target glioblastoma multiforme.TP is an 18 kDa protein overexpressed on the mitochondrial membrane as a multiprotein complex named mitochondrial permeability transition pore,which is involved in various cellular functions of organ tumors.Optical and structural characteristics revealed that they were highly stable colloidally in physiological media,preserving NIR response.Cellular internalization of their selective receptor binding was confirmed using confocal microscopy,and the ability to recognize the intracellular receptor was confirmed in vivo using U87-MG xenograft models,demonstrating the effectiveness of visualizing the tumor[130].
Very recently,the effects of US-nanomagnets on the uptake,cytotoxicity,proliferation,and multipotency of primary neural stem cells (NSCs) were demonstrated. US-nanomagnets were functionalized with various biocompatible and biologically significant molecules,such as gluconic acid(GA),tannic acid(TA),and HA to label the NSCs.Direct functionalization with TA and HA conjugates promoted NSC proliferation and boosted cellular uptake in a dose-dependent manner compared to those of the conjugates with GA.Furthermore,the combined functionalization of TA and HA coating enhanced cell proliferation,cellular uptake,and labeling efficiency,which depended on the molecular weight of HA.Highmolecular weight HA-TA-US-SPIONs remained on the cell surface,indicating the utility of physiologically relevant extracellular matrix molecules to guard and improve NSC survival upon transplantation[131].
In a promising effort to determine whether tartaric/adipic acidcoated US-nanomagnets can be used as a potent iron supplement for the oral management of Fe-deficient anemia,an array of in vitro and in situ evaluations was performed.Initially,a partial reduction of the core size by 40% was noted after 90 min of treatment at intestinal pH(about 3)due to gastric secretions.In vivo absorption experiments through the small intestine by intestinal perfusion using nanoparticles in Wistar rats before and after perfusion demonstrated remarkable Fe absorption by 79%.Moreover,such high absorption levels did not affect cell viability as assessed in enterocyte cytotoxicity,ROS production,genotoxicity,and lipid peroxidation tests.Moreover,regional differences in Fe concentration,including in the small intestine,duodenum,jejunum,and ileum,showed intact particles around the intestinal microvilli with no significant tissue damage,providing a better alternative for oral management of anemia[132].
8.Conclusion and future perspectives
The enormous biomedical implications of engineered nanomagnets are exponentially developing owing to multi-potent properties inherent in nanomaterials such as contrasts,superparamagnetism,surface reactivity,and photo-thermality.In this review,while introducing nanomagnets,we have summarized various progressive aspects of nanomagnets,including their synthesis,surface modifications,and drug encapsulation for aiding cancer diagnosis and therapeutics.
First,to implement nanomagnets in biomedicine,they need to be fabricated.Owing to scientific innovations,old-school alarming chemistry is eventually turning safe and sustainable by avoiding toxic and combustible reactants.Even though various core composites and phases of nanomagnets have been biofabricated using nature-inspired materials,it is still a challenge to attain dispersible and stable nanoparticles essential for biomedical purposes,which are amenable to hassle-free surface alterations.This is because of the highly evolved biological machinery involved in a cascade of complex reactions.Nevertheless,further analysis to gain a thorough understanding of the mechanistic insights into the synthetic processes opens the possibility of replicating the reactions in vitro for complete control over the particle size,shape,dispersions,and magnetic strength to accommodate in situ systemic implications.
Among the various unique properties possessed by SPIONs,magnetism is extensively utilized for concentrating tumor-specific therapeutic entities to the targeted site,to remove accumulated toxins from the system under medical conditions,and to separate cells.This appealing magnetism feature can further be extended in the future to separate cancerous cells or CTCs from the blood to prolong the life expectancy of cancer patients and minimize the side effects that cause excruciating pain.Therefore,achieving a novel targeting strategy using permanent magnets to control and remove multidrug-resistant cancerous cells can enable immediate treatment options for the treatment of leukemia.
The concept of magnetization can also be extended to amplify or concentrate cancer-specific markers and receptors in the blood to a detectable range and to simultaneously make use of the chromogenic property of nanomagnets to develop a novel sensitive assay for the early diagnosis of cancer and other hard-to-diagnose diseases.Curative therapies are fruitful when a disease is diagnosed and treated at an early stage.
Finally,nanomagnets with such unique properties have shown great potential in various engineering and theragnostic applications.The behavior of nanomagnets in the presence of a conjugant is another area that can exploit the co-behavioral properties of nanomagnets fused with other significant nanoparticles.There is a good possibility for the emergence of smart new materials by varying the elemental compositions to produce better and nonexistent material properties.
CRediT author statement
Divya S.Parimi:Writing-Reviewing and Editing,Data curation,Data presentation,Literature review;Yamini Gupta:Writing-Reviewing and Editing,Literature reviewing;Sreekar Marpu:Writing-Reviewing and Editing;Chandra S.Bhatt and Tharun K.Bollu:Writing-Reviewing and Editing;Anil K.Suresh:Conceptualization,Methodology,Investigation,Data curation,Writing-Original draft preparation,Supervision.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Anil K.Suresh would like to thank the Department of Biotechnology,Government of India,for financial support through Ramalinga Swami Fellowship Award,and the Science Education and Research Board,Department of Science and Technology,Government of India,New Delhi,for financial support through Early Career Research Award(Grant No.:ECR/2017/000339).The authors would like to thank Saptarshi Mazumder and Rajarshi Mazumder for making the 3-dimensional animations in the figures.
 Journal of Pharmaceutical Analysis2022年3期
Journal of Pharmaceutical Analysis2022年3期
- Journal of Pharmaceutical Analysis的其它文章
- Highly sensitive electrochemical determination of rutin based on the synergistic effect of 3D porous carbon and cobalt tungstate nanosheets
- Characterization of multiple chemical components of GuiLingJi by UHPLC-MS and 1H NMR analysis
- Modifying current thin-film microextraction(TFME)solutions for analyzing prohibited substances:Evaluating new coatings using liquid chromatography
- Discussion on the dimerization reaction of penicillin antibiotics
- A strategy of screening and binding analysis of bioactive components from traditional Chinese medicine based on surface plasmon resonance biosensor
- MIL-53-based homochiral metal-organic framework as a stationary phase for open-tubular capillary electrochromatography
